Artificial Intelligence in Digestive Endoscopy—Where Are We and Where Are We Going?
Abstract
:1. Introduction
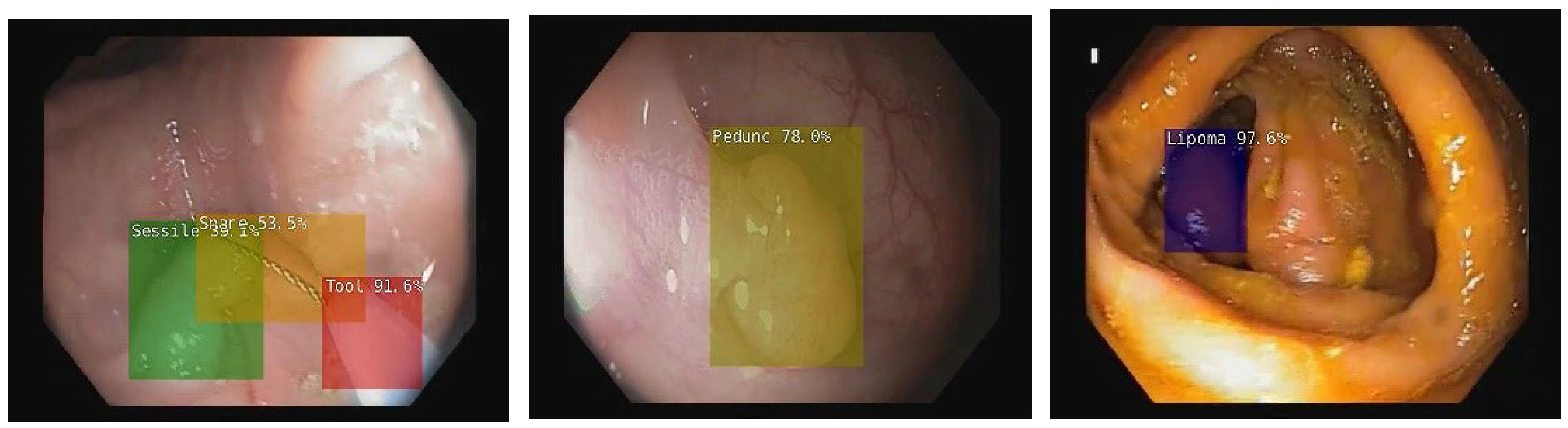
2. Artificial Intelligence in Colonoscopy
3. Artificial Intelligence in Upper Digestive Endoscopy
4. Challenges in Implementing AI Systems in Healthcare
5. AI Systems Currently Approved for Use in Gastroenterology
6. Future Potential of AI in Digestive Endoscopy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.G.; Shortliffe, E.H. Rule-Based Expert Systems: The MYCIN Experiments of the Stanford Heuristic Programming Project; Addison-Wesley: Reading, MA, USA, 1984; ISBN 0201101726. [Google Scholar]
- Shortliffe, E.H.; Buchanan, B.G. A model of inexact reasoning in medicine. Math. Biosci. 1975, 23, 351–379. [Google Scholar] [CrossRef]
- Madiajagan, M.; Raj, S.S. Parallel Machine Learning and Deep Learning Approaches for Bioinformatics. In Deep Learning and Parallel Computing Environment for Bioengineering Systems; Academic Press: Cambridge, MA, USA, 2019; pp. 245–255. [Google Scholar]
- GLOBOCAN 2020: New Global Cancer Data|UICC. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 7 February 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Siersema, P.D. Colorectal Cancer Awareness Issue 2019. Endoscopy 2019, 51, 207–208. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, J.; Chen, Y.; Sun, H.; Li, B.; Zhang, Q.; Sun, K.; Wang, Z.; Qian, X.; Zhan, T.; et al. Negative Effects of Endoscopists’ Fatigue on Colonoscopy Quality on 34,022 Screening Colonoscopies. J. Gastrointest. Liver Dis. 2021, 30, 358–365. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Thomas-Gibson, S.; Bugajski, M.; Bretthauer, M.; Rees, C.J.; Dekker, E.; Hoff, G.; Jover, R.; Suchanek, S.; Ferlitsch, M.; et al. Performance measures for lower gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2017, 49, 378–397. [Google Scholar] [CrossRef] [Green Version]
- Kaul, V.; Enslin, S.; Gross, S.A. History of artificial intelligence in medicine. Gastrointest. Endosc. 2020, 92, 807–812. [Google Scholar] [CrossRef]
- Rex, D.K.; Kahi, C.; O’Brien, M.; Levin, T.R.; Pohl, H.; Rastogi, A.; Burgart, L.; Imperiale, T.; Ladabaum, U.; Cohen, J.; et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest. Endosc. 2011, 73, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Houwen, B.B.S.L.; Hassan, C.; Coupé, V.M.H.; Greuter, M.J.E.; Hazewinkel, Y.; Vleugels, J.L.A.; Antonelli, G.; Bustamante-Balén, M.; Coron, E.; Cortas, G.A.; et al. Definition of competence standards for optical diagnosis of diminutive colorectal polyps: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2022, 54, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A.; et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020, 159, 512–520.e7. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Spadaccini, M.; Antonelli, G.; Correale, L.; Maselli, R.; Galtieri, P.A.; Pellegatta, G.; Capogreco, A.; Milluzzo, S.M.; Lollo, G.; et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut 2022, 71, 757–765. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, W.; Wang, Y.; Tan, Y.; Xi, C.; Ye, N.; Wu, D.; Xu, X. Comparison of diagnostic performance between convolutional neural networks and human endoscopists for diagnosis of colorectal polyp: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246892. [Google Scholar] [CrossRef]
- Hassan, C.; Spadaccini, M.; Iannone, A.; Maselli, R.; Jovani, M.; Chandrasekar, V.T.; Antonelli, G.; Yu, H.; Areia, M.; Dinis-Ribeiro, M.; et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 93, 77–85.e6. [Google Scholar] [CrossRef]
- Spadaccini, M.; Iannone, A.; Maselli, R.; Badalamenti, M.; Desai, M.; Chandrasekar, V.T.; Patel, H.K.; Fugazza, A.; Pellegatta, G.; Galtieri, P.A.; et al. Computer-aided detection versus advanced imaging for detection of colorectal neoplasia: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 793–802. [Google Scholar] [CrossRef]
- Van Der Zander, Q.E.W.; Schreuder, R.M.; Fonollà, R.; Scheeve, T.; Van Der Sommen, F.; Winkens, B.; Aepli, P.; Hayee, B.; Pischel, A.B.; Stefanovic, M.; et al. Optical diagnosis of colorectal polyp images using a newly developed computer-aided diagnosis system (CADx) compared with intuitive optical diagnosis. Endoscopy 2021, 53, 1219–1226. [Google Scholar] [CrossRef]
- Sakamoto, T.; Nakashima, H.; Nakamura, K.; Nagahama, R.; Saito, Y. Performance of Computer-Aided Detection and Diagnosis of Colorectal Polyps Compares to That of Experienced Endoscopists. Dig. Dis. Sci. 2021, 1–8. [Google Scholar] [CrossRef]
- Chen, P.J.; Lin, M.C.; Lai, M.J.; Lin, J.C.; Lu, H.H.S.; Tseng, V.S. Accurate Classification of Diminutive Colorectal Polyps Using Computer-Aided Analysis. Gastroenterology 2018, 154, 568–575. [Google Scholar] [CrossRef]
- Zachariah, R.; Samarasena, J.; Luba, D.; Duh, E.; Dao, T.; Requa, J.; Ninh, A.; Karnes, W. Prediction of Polyp Pathology Using Convolutional Neural Networks Achieves ‘Resect and Discard’ Thresholds. Am. J. Gastroenterol. 2020, 115, 138. [Google Scholar] [CrossRef] [PubMed]
- Song, E.M.; Park, B.; Ha, C.A.; Hwang, S.W.; Park, S.H.; Yang, D.H.; Ye, B.D.; Myung, S.J.; Yang, S.K.; Kim, N.; et al. Endoscopic diagnosis and treatment planning for colorectal polyps using a deep-learning model. Sci. Rep. 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, E.H.; Lee, D.; Bae, J.H.; Kang, H.Y.; Kwak, M.S.; Seo, J.Y.; Yang, J.I.; Yang, S.Y.; Lim, S.H.; Yim, J.Y.; et al. Improved Accuracy in Optical Diagnosis of Colorectal Polyps Using Convolutional Neural Networks with Visual Explanations; The American Gastroenterological Association: Bethesda, MD, USA, 2020; Volume 158, ISBN 8222072293. [Google Scholar]
- Parsa, N.; Byrne, M.F. Artificial intelligence for identification and characterization of colonic polyps. Ther. Adv. Gastrointest. Endosc. 2021, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Takemura, Y.; Yoshida, S.; Tanaka, S.; Onji, K.; Oka, S.; Tamaki, T.; Kaneda, K.; Yoshihara, M.; Chayama, K. Quantitative analysis and development of a computer-aided system for identification of regular pit patterns of colorectal lesions. Gastrointest. Endosc. 2010, 72, 1047–1051. [Google Scholar] [CrossRef] [Green Version]
- Misawa, M.; Kudo, S.E.; Mori, Y.; Nakamura, H.; Kataoka, S.; Maeda, Y.; Kudo, T.; Hayashi, T.; Wakamura, K.; Miyachi, H.; et al. Characterization of Colorectal Lesions Using a Computer-Aided Diagnostic System for Narrow-Band Imaging Endocytoscopy. Gastroenterology 2016, 150, 1531–1532.e3. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Kudo, S.-E.; East, J.E.; Rastogi, A.; Bretthauer, M.; Misawa, M.; Sekiguchi, M.; Matsuda, T.; Saito, Y.; Ikematsu, H.; et al. Cost savings in colonoscopy with artificial intelligence-aided polyp diagnosis: An add-on analysis of a clinical trial (with video). Gastrointest. Endosc. 2020, 92, 905–911.e1. [Google Scholar] [CrossRef]
- Takenaka, K.; Kawamoto, A.; Okamoto, R.; Watanabe, M.; Ohtsuka, K. Artificial intelligence for endoscopy in inflammatory bowel disease. Intest. Res. 2022. [Google Scholar] [CrossRef]
- Ozawa, T.; Ishihara, S.; Fujishiro, M.; Saito, H.; Kumagai, Y.; Shichijo, S.; Aoyama, K.; Tada, T. Novel computer-assisted diagnosis system for endoscopic disease activity in patients with ulcerative colitis. Gastrointest. Endosc. 2019, 89, 416–421.e1. [Google Scholar] [CrossRef]
- Stidham, R.W.; Liu, W.; Bishu, S.; Rice, M.D.; Higgins, P.D.R.; Zhu, J.; Nallamothu, B.K.; Waljee, A.K. Performance of a Deep Learning Model vs Human Reviewers in Grading Endoscopic Disease Severity of Patients With Ulcerative Colitis. JAMA Netw. Open 2019, 2, e193963. [Google Scholar] [CrossRef] [Green Version]
- Bossuyt, P.; Vermeire, S.; Bisschops, R. Scoring endoscopic disease activity in IBD: Artificial intelligence sees more and better than we do. Gut 2020, 69, 788–789. [Google Scholar] [CrossRef]
- Marchal-Bressenot, A.; Salleron, J.; Boulagnon-Rombi, C.; Bastien, C.; Cahn, V.; Cadiot, G.; Diebold, M.D.; Danese, S.; Reinisch, W.; Schreiber, S.; et al. Development and validation of the Nancy histological index for UC. Gut 2017, 66, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.P.L.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.F.; Feagan, B.G.; Hanauer, S.B.; Lichtenstein, G.R.; Marteau, P.R.; et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology 2013, 145, 987–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klang, E.; Barash, Y.; Margalit, R.Y.; Soffer, S.; Shimon, O.; Albshesh, A.; Ben-Horin, S.; Amitai, M.M.; Eliakim, R.; Kopylov, U. Deep learning algorithms for automated detection of Crohn’s disease ulcers by video capsule endoscopy. Gastrointest. Endosc. 2020, 91, 606–613.e2. [Google Scholar] [CrossRef] [PubMed]
- Barash, Y.; Azaria, L.; Soffer, S.; Margalit Yehuda, R.; Shlomi, O.; Ben-Horin, S.; Eliakim, R.; Klang, E.; Kopylov, U. Ulcer severity grading in video capsule images of patients with Crohn’s disease: An ordinal neural network solution. Gastrointest. Endosc. 2021, 93, 187–192. [Google Scholar] [CrossRef]
- Ding, Z.; Shi, H.; Zhang, H.; Meng, L.; Fan, M.; Han, C.; Zhang, K.; Ming, F.; Xie, X.; Liu, H.; et al. Gastroenterologist-Level Identification of Small-Bowel Diseases and Normal Variants by Capsule Endoscopy Using a Deep-Learning Model. Gastroenterology 2019, 157, 1044–1054.e5. [Google Scholar] [CrossRef]
- Wang, C.C.; Chiu, Y.C.; Chen, W.L.; Yang, T.W.; Tsai, M.C.; Tseng, M.H. A Deep Learning Model for Classification of Endoscopic Gastroesophageal Reflux Disease. Int. J. Environ. Res. Public Health 2021, 18, 2428. [Google Scholar] [CrossRef]
- Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; Gurudu, S.R.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359.e2. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, R.; Requa, J.; Dao, T.; Ninh, A.; Tran, E.; Mai, D.; Lugo, M.; El-Hage Chehade, N.; Chang, K.J.; Karnes, W.E.; et al. Artificial intelligence using convolutional neural networks for real-time detection of early esophageal neoplasia in Barrett’s esophagus (with video). Gastrointest. Endosc. 2020, 91, 1264–1271.e1. [Google Scholar] [CrossRef]
- De Groof, A.J.; Struyvenberg, M.R.; van der Putten, J.; van der Sommen, F.; Fockens, K.N.; Curvers, W.L.; Zinger, S.; Pouw, R.E.; Coron, E.; Baldaque-Silva, F.; et al. Deep-Learning System Detects Neoplasia in Patients With Barrett’s Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterology 2020, 158, 915–929.e4. [Google Scholar] [CrossRef]
- Hu, Z.; Zuo, Z.; Miao, H.; Ning, Z.; Deng, Y. Incidence, Risk Factors and Prognosis of T4a Gastric Cancer: A Population-Based Study. Front. Med. 2022, 8, 767904. [Google Scholar] [CrossRef]
- Wu, L.; He, X.; Liu, M.; Xie, H.; An, P.; Zhang, J.; Zhang, H.; Ai, Y.; Tong, Q.; Guo, M.; et al. Evaluation of the effects of an artificial intelligence system on endoscopy quality and preliminary testing of its performance in detecting early gastric cancer: A randomized controlled trial. Endoscopy 2021, 53, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Charow, R.; Jeyakumar, T.; Younus, S.; Dolatabadi, E.; Salhia, M.; Al-Mouaswas, D.; Anderson, M.; Balakumar, S.; Clare, M.; Dhalla, A.; et al. Artificial Intelligence Education Programs for Health Care Professionals: Scoping Review. JMIR Med. Educ. 2021, 7, e31043. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Baxter, S.L.; Xu, J.; Xu, J.; Zhou, X.; Zhang, K. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019, 25, 30–36. [Google Scholar] [CrossRef]
- Singh, R.P.; Hom, G.L.; Abramoff, M.D.; Campbell, J.P.; Chiang, M.F. Current Challenges and Barriers to Real-World Artificial Intelligence Adoption for the Healthcare System, Provider, and the Patient. Transl. Vis. Sci. Technol. 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Fonollà, R.; van der Zander, Q.E.W.; Schreuder, R.M.; Subramaniam, S.; Bhandari, P.; Masclee, A.A.M.; Schoon, E.J.; van der Sommen, F.; de With, P.H.N. Automatic image and text-based description for colorectal polyps using BASIC classification. Artif. Intell. Med. 2021, 121, 102178. [Google Scholar] [CrossRef] [PubMed]
- Taghiakbari, M.; Mori, Y.; von Renteln, D. Artificial intelligence-assisted colonoscopy: A review of current state of practice and research. World J. Gastroenterol. 2021, 27, 8103. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Neumann, H.; Misawa, M.; Kudo, S.E.; Bretthauer, M. Artificial intelligence in colonoscopy—Now on the market. What’s next? J. Gastroenterol. Hepatol. 2021, 36, 7–11. [Google Scholar] [CrossRef]
- Spadaccini, M.; De Marco, A.; Franchellucci, G.; Sharma, P.; Hassan, C.; Repici, A. Discovering the first FDA-approved computer-aided polyp detection system. Future Oncol. 2022, 18, 1405–1412. [Google Scholar] [CrossRef]
- Olympus Olympus Launches ENDO-AID, an AI-Powered Platform for Its Endoscopy System—Olympus Europe, Middle East and Africa. Available online: https://www.olympus-europa.com/company/en/news/press-releases/2020-10-09t08-30-00/olympus-launches-endo-aid-an-ai-powered-platform-for-its-endoscopy-system.html (accessed on 4 March 2022).
- Koo, C.S.; Dolgunov, D.; Koh, C.J. Key tips for using computer-aided diagnosis in colonoscopy-observations from two different platforms. Endoscopy 2021. [Google Scholar] [CrossRef]
- Lafeuille, P.; Yzet, C.; Rivory, J.; Pontarollo, G.; Latif, E.H.; Bartoli, A.; Pioche, M. Flat colorectal adenocarcinoma: A worrisome false negative of artificial intelligence-assisted colonoscopy. Endoscopy 2022. [Google Scholar] [CrossRef]
- Neumann, H.; Kreft, A.; Sivanathan, V.; Rahman, F.; Galle, P.R. Evaluation of novel LCI CAD EYE system for real time detection of colon polyps. PLoS ONE 2021, 16, e0255955. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.; Kudo, S.-E.; Mori, Y.; Hotta, K.; Ohtsuka, K.; Matsuda, T.; Saito, S.; Kudo, T.; Baba, T.; Ishida, F.; et al. Development of a computer-aided detection system for colonoscopy and a publicly accessible large colonoscopy video database (with video). Gastrointest. Endosc. 2021, 93, 960–967.e3. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kudo, S.; Misawa, M.; Hotta, K.; Kazuo, O.; Saito, S.; Ikematsu, H.; Saito, Y.; Matsuda, T.; Kenichi, T.; et al. Artificial intelligence-assisted colonic endocytoscopy for cancer recognition: A multicenter study. Endosc. Int. Open 2021, 9, E1004–E1011. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kudo, S.-E.; Mori, Y.; Misawa, M.; Ogata, N.; Sasanuma, S.; Wakamura, K.; Oda, M.; Mori, K.; Ohtsuka, K. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest. Endosc. 2019, 89, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, A.; Luca, M.; Barbu, T.; Drug, V.; Olteanu, A.; Vulpoi, R. Experimental Deep Learning Object Detection in Real-time Colonoscopies. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; Institute of Electrical and Electronics Engineers (IEEE): Iasi, Romania, 2021. [Google Scholar]
- Luca, M.; Ciobanu, A.; Barbu, T.; Drug, V. Artificial Intelligence and Deep Learning, Important Tools in Assisting Gastroenterologists. In Handbook of Artificial Intelligence in Healthcare; Lim, C.-P., Vaidya, A., Jain, K., Mahorkar, V.U., Jain, L.C., Eds.; Springer: Cham, Switzerland, 2021; Volume 211, pp. 197–213. [Google Scholar]
- Ciobanu, A.; Luca, M.; Oltean, A.; Barboi, O.; Drug, V. Cielab Automatic Colonoscopy Post-Evaluation. Endoscopy 2021, 53, S194–S195. [Google Scholar] [CrossRef]

| Product | Manufacturer | Place of Approval and Year | Computer System Used |
|---|---|---|---|
| EndoBRAIN | Cybernet System Corp./Olympus Corp. | Japan 2018 | CADx |
| EndoBRAIN-EYE | Cybernet System Corp./Olympus Corp. | Japan 2020 | CADe |
| EndoBrain-PLUS | Cybernet System Corp./Olympus Corp. | Japan 2020 | CADx |
| EndoBrain-UC | Cybernet System Corp./Olympus Corp. | Japan 2020 | CADx |
| GI Genius | Medtronic Corp. | Europe 2019 | CADe |
| United States 2021 | |||
| ENDO-AID | Olympus Corp. | Europe 2020 | CADe |
| CAD EYE | Fujifilm Corp. | Europe 2020 | CADe/ CADx |
| Japan 2020 | |||
| DISCOVERY | Pentax Corp. | Europe 2020 | CADe |
| WISE VISION | NEC Corp. | Europe 2021 | CADe |
| Japan 2021 | |||
| CADDIE | Odin Vision | Europe 2021 | CADe |
| ME-APDS | Magentiq Eye | Europe 2021 | CADe |
| EndoAngel | Wuhan EndoAngel Medical Technology Company | China 2020 | CADe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulpoi, R.-A.; Luca, M.; Ciobanu, A.; Olteanu, A.; Barboi, O.-B.; Drug, V.L. Artificial Intelligence in Digestive Endoscopy—Where Are We and Where Are We Going? Diagnostics 2022, 12, 927. https://doi.org/10.3390/diagnostics12040927
Vulpoi R-A, Luca M, Ciobanu A, Olteanu A, Barboi O-B, Drug VL. Artificial Intelligence in Digestive Endoscopy—Where Are We and Where Are We Going? Diagnostics. 2022; 12(4):927. https://doi.org/10.3390/diagnostics12040927
Chicago/Turabian StyleVulpoi, Radu-Alexandru, Mihaela Luca, Adrian Ciobanu, Andrei Olteanu, Oana-Bogdana Barboi, and Vasile Liviu Drug. 2022. "Artificial Intelligence in Digestive Endoscopy—Where Are We and Where Are We Going?" Diagnostics 12, no. 4: 927. https://doi.org/10.3390/diagnostics12040927
APA StyleVulpoi, R.-A., Luca, M., Ciobanu, A., Olteanu, A., Barboi, O.-B., & Drug, V. L. (2022). Artificial Intelligence in Digestive Endoscopy—Where Are We and Where Are We Going? Diagnostics, 12(4), 927. https://doi.org/10.3390/diagnostics12040927






