Patient Perception When Transitioning from Classic to Remote Assisted Cardiac Rehabilitation
Abstract
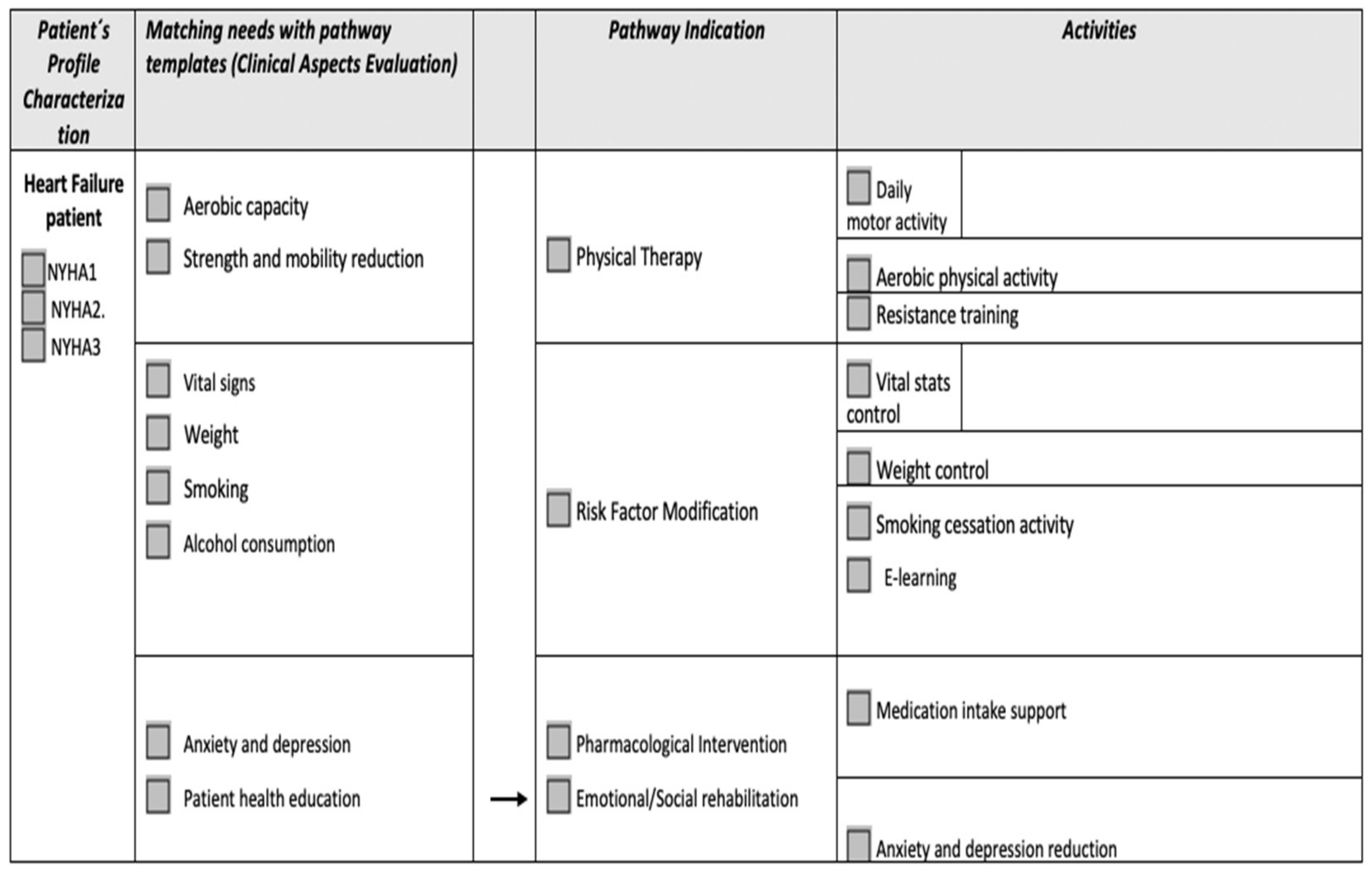
:1. Introduction
1.1. Cardiovascular Disease: Epidemiology, Economic Impact, and Management Strategies
1.2. Virtual Assistants: A Solution to Telerehabilitation Implementation Difficulties in Eastern European Countries
1.3. Study Goals
2. Materials and Methods
2.1. Participants and Procedures
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. Sample Description
3.2. Home Technology Assessment
3.3. System Usability Assessment
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balady, G.J.; Williams, M.A.; Ades, P.A.; Bittner, V.; Comoss, P.; Foody, J.M.; Franklin, B.; Sanderson, B.; Southard, D. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation and Prevention Committee. J. Cardiopulm. Rehabil. Prev. 2007, 27, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.M.; Wijaya, L.C.G. Cardiac rehabilitation via telerehabilitation in COVID-19 pandemic situation. Egypt. Heart J. 2021, 73, 31. [Google Scholar] [CrossRef] [PubMed]
- Dalal, H.M.; Doherty, P.; Taylor, R.S. Cardiac rehabilitation. BMJ 2015, 351, h5000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruano-Ravina, A.; Peña-Gil, C.; Abu-Assi, E.; Raposeiras, S.; Hof, A.V.; Meindersma, E.; Prescott, E.I.B.; González-Juanatey, J.R. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int. J. Cardiol. 2016, 223, 436–443. [Google Scholar] [CrossRef]
- Chłoń-Domińczak, A.; Kotowska, I.E.; Kurkiewicz, J.; Stonawski, M.; Abramowska-Kmon, A. Population Ageing in Europe: Facts, Implications and Policies; European Commission: Brussels, Belgium, 2014. [Google Scholar] [CrossRef]
- Maggioni, A.P.; Dahlstrom, U.; Filippatos, G.; Chioncel, O.; Leiro, M.C.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Metra, M.; et al. EURObservational Research Programme: The Heart Failure Pilot Survey (ESC-HF-Pilot). Heart Failure Association of ESC (HFA). Eur. J. Heart Fail. 2010, 12, 1076–1084. [Google Scholar] [CrossRef] [Green Version]
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S.M.; Ho, M.; Howard, V.; Kissella, B.; et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics- 2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008, 117, e25–e146. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; Alkatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Farmakis, D.; Parissis, J.; Lekakis, J.; Filippatos, G. Acute Heart Failure: Epidemiology, Risk Factors and Prevention. Rev. Esp. Cardiol. 2015, 68, 245–248. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, C.Y.; Lai, Y.H.; Liao, Y.C.; Wen, Y.K.; Chang, S.T.; Huang, J.L.; Wu, T.J. Home-based cardiac rehabilitation improves quality of life, aerobic capacity and readmission rates in patients with chronic heart failure. Medicine 2018, 97, e9629. [Google Scholar] [CrossRef]
- Bekfani, T.; Fudim, M.; Cleland, J.G.; Jorbenadze, A.; von Haehling, S.; Lorber, A.; Rothman, A.M.; Stein, K.; Abraham, W.T.; Sievert, H.; et al. A current and future outlook on upcoming technologies in remote monitoring of patients with heart failure. Eur. J. Heart Fail. 2021, 23, 175–185. [Google Scholar] [CrossRef]
- O’Doherty, A.F.; Humphreys, H.; Dawkes, S.; Cowie, A.; Hinton, S.; Brubaker, P.H.; Butler, T.; Nichols, S. How was technology been used to deliver cardiac rehabilitation during the COVID-19 pandemic? An interntional cross-sectional survey of healthcare professionals conducted by the BACPR. BMJ Open 2021, 11, e046051. [Google Scholar] [CrossRef]
- Powell, R.; McGregor, G.; Ennis, S.; Kimani, P.; Underwood, M. Is exercise based cardiac rehabilitation effective? A systematic review and meta-analysis to reexamine the evidence. BMJ Open 2018, 14, e019656. [Google Scholar] [CrossRef] [Green Version]
- Piotrowicz, E.; Baranowski, R.; Bilinska, M.; Stepnowska, M.; Piotrowska, M.; Wójcik, A.; Piotrowski, W. Telerehabilitation in heart failure patients: The evidence and the pitfalls. Int. J. Cardiol. 2016, 220, 408–413. [Google Scholar] [CrossRef] [Green Version]
- Mampuya, W.M. Cardiac rehabilitation past, present and future: An overview. Cardiovasc. Diagn. Ther. 2012, 2, 38–49. [Google Scholar] [CrossRef]
- Doimo, S.; Fabris, E.; Piepoli, M.; Barbati, G.; Antonini-Canterin, F.; Bernardi, G.; Maras, P.; Sinagra, G. Impact of ambulatory cardiac rehabilitation on cardiovascular outcomes: A long term follow-up study. Eur. Heart J. 2019, 40, 678–685. [Google Scholar] [CrossRef]
- Winnige, P.; Filakova, K.; Hnatiak, J.; Dosbaba, F.; Bocek, O.; Pepera, G.; Papathanasiou, J.; Batalik, L.; Grace, S.L. Validity and Reliabilty of the Cardiac Rehabilitation Barriers Scale in the Czeck Republic. Int. J. Environ. Res. Public Health 2021, 18, 13113. [Google Scholar] [CrossRef]
- Brouwers, R.W.M.; van Exel, H.J.; van Hal, J.M.C.; Jorstad, H.T.; de Kluiver, E.P.; Kraaijenhagen, R.A.; Kujipers, P.M.J.C.; van der Linde, M.R.; Spee, R.F.; Sunamura, M.; et al. Cardiac telerehabilitation as an alternative to centre-based cardiac rehabilitation. Neth. Heart J. 2020, 28, 443–451. [Google Scholar] [CrossRef]
- Paruchuri, K.; Finneran, P.; Marston, N.A.; Healy, E.W.; Andreo, J.; Lynch, R.; Blood, A.J.; Jones-O’Connor, M.; Lander, B.; Kelly, N.; et al. Outcomes of a smartphone-based application with live health-coaching post-percutaneous coronary intervention. eBioMedicine 2021, 72, 103593. [Google Scholar] [CrossRef]
- Kamali, M.E.; Angelini, L.; Caon, M.; Carrino, F.; Rocke, C.; Guye, S.; Rizzo, G.; Mastropietro, A.; Sykora, M.; Elayan, S.; et al. Virtual Coaches for Older Adults’ Wellbeing: A systematic review. IEEE Access 2020, 8, 101884–101902. [Google Scholar] [CrossRef]
- Kruse, C.S.; Krowksi, N.; Rodriguez, B.; Tran, L.; Vela, J.; Brooks, M. Telehealth and patient satisfaction: A systematic review and narrative analysis. BMJ Open 2017, 3, e016242. [Google Scholar] [CrossRef]
- Available online: https://vcare-project.eu/project/objectives/ (accessed on 7 September 2020).
- Nguyen, M.; Waller, M.; Pandya, A.; Portnoy, J. A review of patient and provider satisfaction with telemedicine. Curr. Allergy Asthma Rep. 2020, 20, 72. [Google Scholar] [CrossRef]
- Gioia, G.; Salducci, M. Medical and legal aspects of telemedicine in ophthalmology. Rom. J. Ophthalmol. 2019, 63, 197–207. [Google Scholar] [CrossRef]
- Clark, P.A.; Capuzzi, K.; Harrison, J. Telemedicine: Medical, legal and ethical perspectives. Med. Sci. Monit. 2010, 16, RA261–RA272. [Google Scholar]
- Ateriya, N.; Saraf, A.; Meshram, V.P.; Setia, P. Telemedicine and virtual consultations: The Indian perspective. Natl. Med. J. India 2018, 31, 215–218. [Google Scholar] [CrossRef]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An empirical evaluation of the system usability scale. Int. J. Hum.-Comput. Interact. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Anca, P.S.; Toth, P.P.; Kempler, P.; Rizzo, M. Gender differences in the battle against COVID-19: Impact of genetics, comorbidities, inflammation and lifestyle on differences in outcomes. Int. J. Clin. Pract. 2021, 75, e13666. [Google Scholar] [CrossRef]
- Nabutovsky, I.; Nachshon, A.; Klempfner, R.; Shapiro, Y.; Tesler, R. Digital cardiac rehabilitation programs: The Future of Patient-Centered Medicine. Telemed. e-Health 2020, 26, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Balsa, J.; Félix, I.; Cláudio, A.P.; Carmo, M.B.; Silva, I.C.E.; Guerreiro, A.; Guedes, M.; Henriques, M.A.; Guerreiro, M.P. Usability of an intelligent virtual assistant for promoting behaviour change and self-care in older people with type 2 diabetes. J. Med. Syst. 2020, 44, 130. [Google Scholar] [CrossRef]
- Haggerty, T.; Brabson, L.; Grogg, K.A.; Herschell, A.D.; Giacobbi, P., Jr.; Sedney, C.; Dino, G. Usability testing of an electronic health application for patient activation on weight management. Mhealth 2021, 7, 45. [Google Scholar] [CrossRef]
- Banach, M.; Gaita, D.; Haluzik, M.; Janez, A.; Kamenov, Z.; Kempler, P.; Lalic, N.; Linhart, A.; Mikhailidis, D.P.; Nocoń, A.; et al. Adoption of the ADA/EASD guidelines in 10 Eastern and Southern European countries: Physician survey and good clinical practice recommendations from and international expert panel. Diabetes Res. Clin. Pract. 2021, 172, 108535. [Google Scholar] [CrossRef]
- Brewer, L.C.; Kaihoi, B.; Schaepe, K.; Zarling, K.; Squires, R.W.; Thomas, R.J.; Kopecky, S. Patient-perceived acceptability of a virtual world-based cardiac rehabilitation program. Digit. Health 2017, 3, 2055207617705548. [Google Scholar] [CrossRef] [PubMed]
- Banner, D.; Lear, S.; Kandola, D.; Singer, J.; Horvat, D.; Bates, J.; Ignaszewski, A. The experiences of patients undertaking a ‘virtual’ cardiac rehabilitation program. Stud. Health Technol. Inform. 2015, 209, 9–14. [Google Scholar] [PubMed]




| Patient | Age | Sex | Smoker Status | AHT | Dyslipidemia | BMI | DM | PA Status |
|---|---|---|---|---|---|---|---|---|
| HF_P01 | 66 | M | No | Yes (grade III) | Yes | 25.7 kg/m2 | No | Sedentary |
| HF_P02 | 46 | M | No | Yes (grade II) | Yes | 32.4 kg/m2 | No | Active |
| HF_P03 | 72 | F | No | Yes (grade II) | Yes | 31.7 kg/m2 | Yes | Sedentary |
| HF_P04 | 65 | M | No | Yes (grade III) | No | 28.5 kg/m2 | No | Low active |
| HF_P05 | 41 | M | Yes | Yes (grade I) | No | 34 kg/m2 | No | Low active |
| Patient | Age | Sex | Smoker Status | AHT | Dyslipidemia | BMI | DM | PA Status |
|---|---|---|---|---|---|---|---|---|
| IHD_P01 | 41 | M | No | Yes (grade I) | No | 30.4 kg/m2 | Yes | Low active |
| IHD_P02 | 49 | M | Yes | No | Yes | 28.7 kg/m2 | No | Low active |
| IHD_P03 | 48 | F | No | Yes (grade III) | Yes | 33.2 kg/m2 | No | Sedentary |
| IHD_P04 | 56 | M | No | No | Yes | 27.8 kg/m2 | No | Low active |
| IHD_P05 | 24 | M | Yes | No | Yes | 26.2 kg/m2 | No | Sedentary |
| IHD_P06 | 34 | M | Yes | Yes (grade I) | No | 29 kg/m2 | No | Very active |
| Patient | Internet | Smart Phone | Smart TV | Tablet | Laptop | PC | Intelligent Bracelet | BP Monitor | Intelligent Weight Scale |
|---|---|---|---|---|---|---|---|---|---|
| HF_P01 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| HF_P02 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| HF_P03 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| HF_P04 | ✓ | ✓ | ✓ | ✓ | |||||
| HF_P05 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Patient | Internet | Smart Phone | Smart TV | Tablet | Laptop | PC | Intelligent Bracelet | BP Monitor | Intelligent Weight Scale |
|---|---|---|---|---|---|---|---|---|---|
| IHD_P01 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| IHD_P02 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| IHD_P03 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| IHD_P04 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| IHD_P05 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| IHD_P06 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busnatu, Ș.-S.; Pană, M.-A.; Lăcraru, A.E.; Jercălău, C.-E.; Paun, N.; Caprino, M.; Gand, K.; Schlieter, H.; Kyriazakos, S.; Andrei, C.L.; et al. Patient Perception When Transitioning from Classic to Remote Assisted Cardiac Rehabilitation. Diagnostics 2022, 12, 926. https://doi.org/10.3390/diagnostics12040926
Busnatu Ș-S, Pană M-A, Lăcraru AE, Jercălău C-E, Paun N, Caprino M, Gand K, Schlieter H, Kyriazakos S, Andrei CL, et al. Patient Perception When Transitioning from Classic to Remote Assisted Cardiac Rehabilitation. Diagnostics. 2022; 12(4):926. https://doi.org/10.3390/diagnostics12040926
Chicago/Turabian StyleBusnatu, Ștefan-Sebastian, Maria-Alexandra Pană, Andreea Elena Lăcraru, Cosmina-Elena Jercălău, Nicolae Paun, Massimo Caprino, Kai Gand, Hannes Schlieter, Sofoklis Kyriazakos, Cătălina Liliana Andrei, and et al. 2022. "Patient Perception When Transitioning from Classic to Remote Assisted Cardiac Rehabilitation" Diagnostics 12, no. 4: 926. https://doi.org/10.3390/diagnostics12040926
APA StyleBusnatu, Ș.-S., Pană, M.-A., Lăcraru, A. E., Jercălău, C.-E., Paun, N., Caprino, M., Gand, K., Schlieter, H., Kyriazakos, S., Andrei, C. L., & Sinescu, C.-J. (2022). Patient Perception When Transitioning from Classic to Remote Assisted Cardiac Rehabilitation. Diagnostics, 12(4), 926. https://doi.org/10.3390/diagnostics12040926









