Myocardial Infarction in Children after COVID-19 and Risk Factors for Thrombosis
Abstract
:1. Introduction
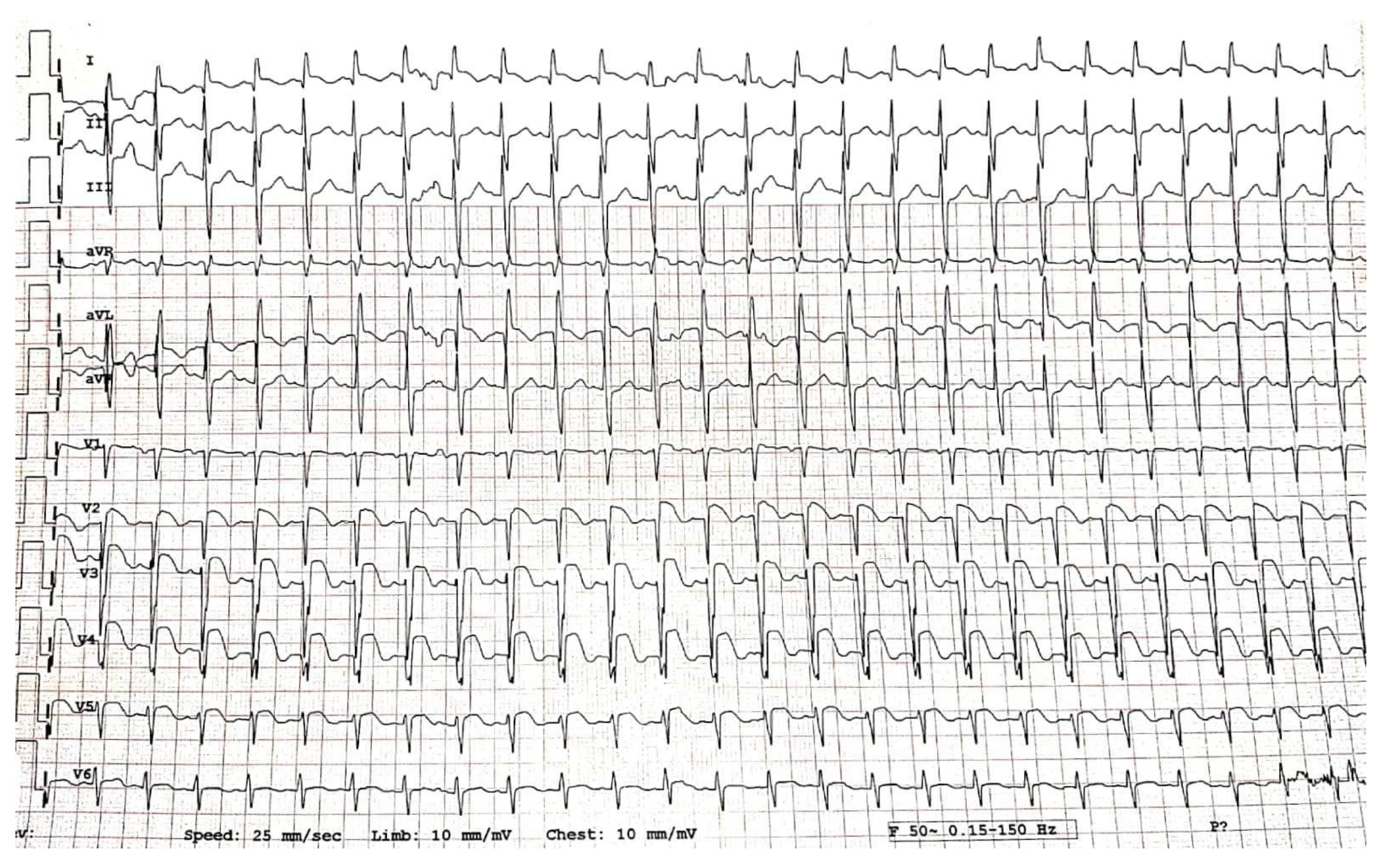
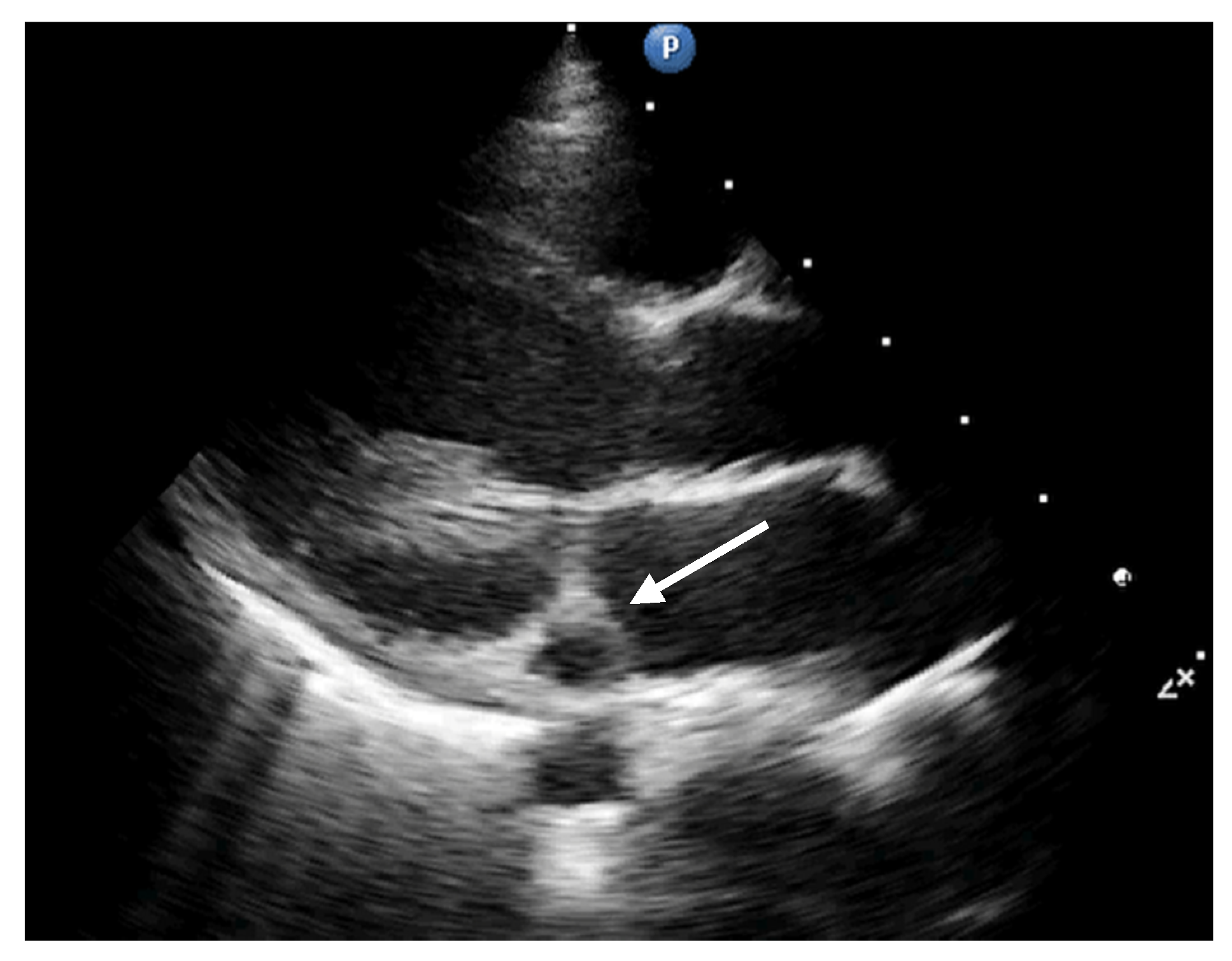
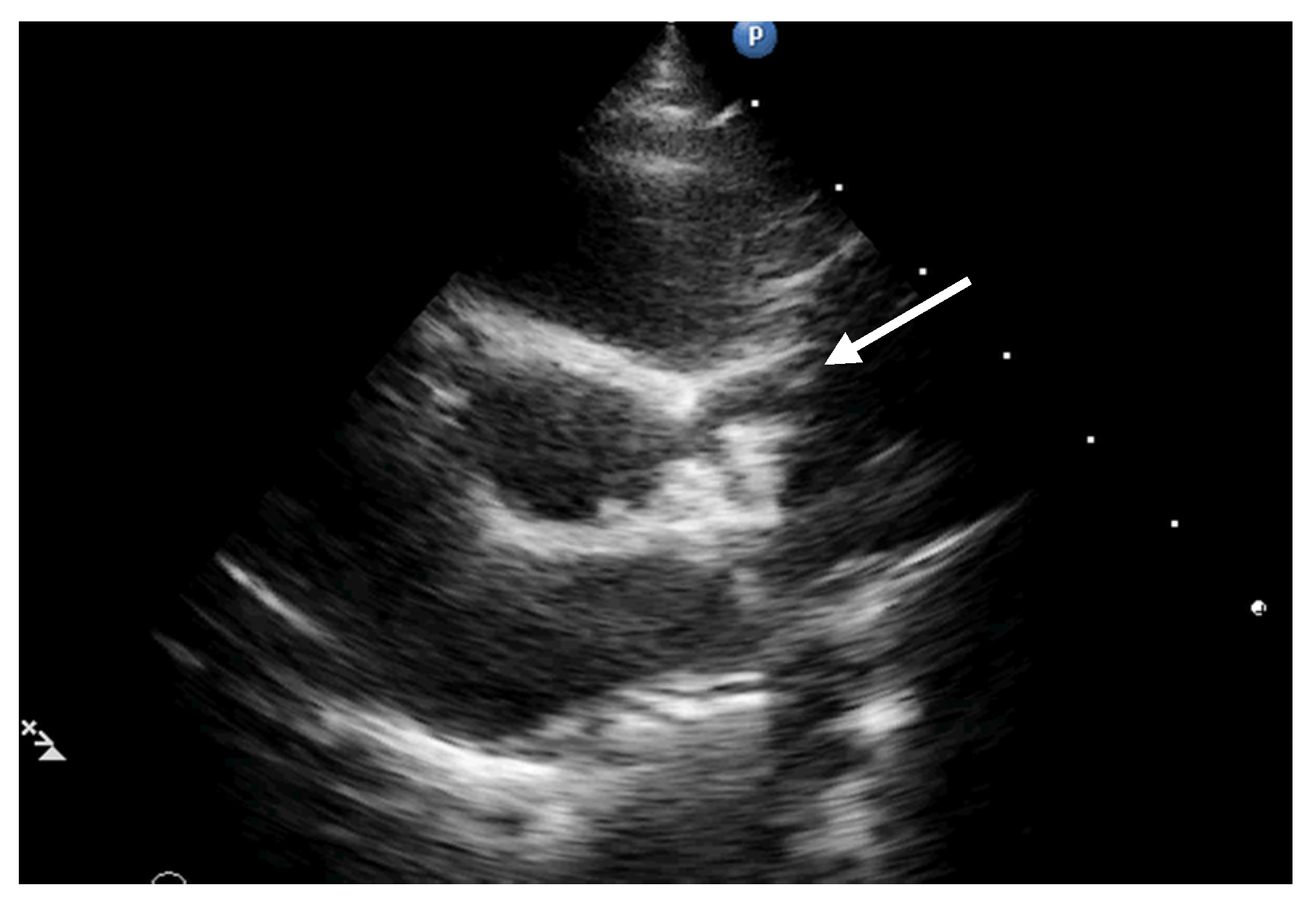
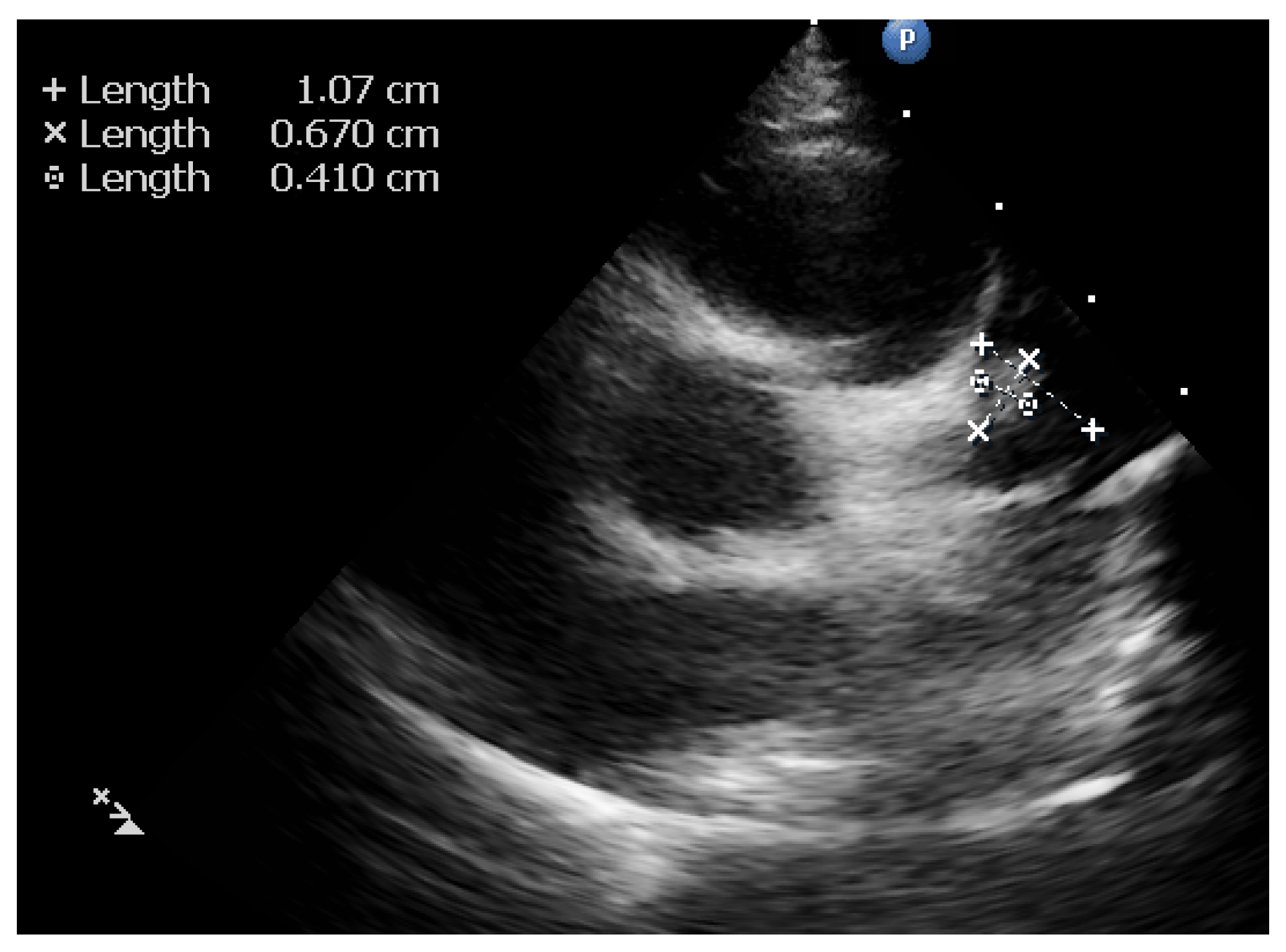
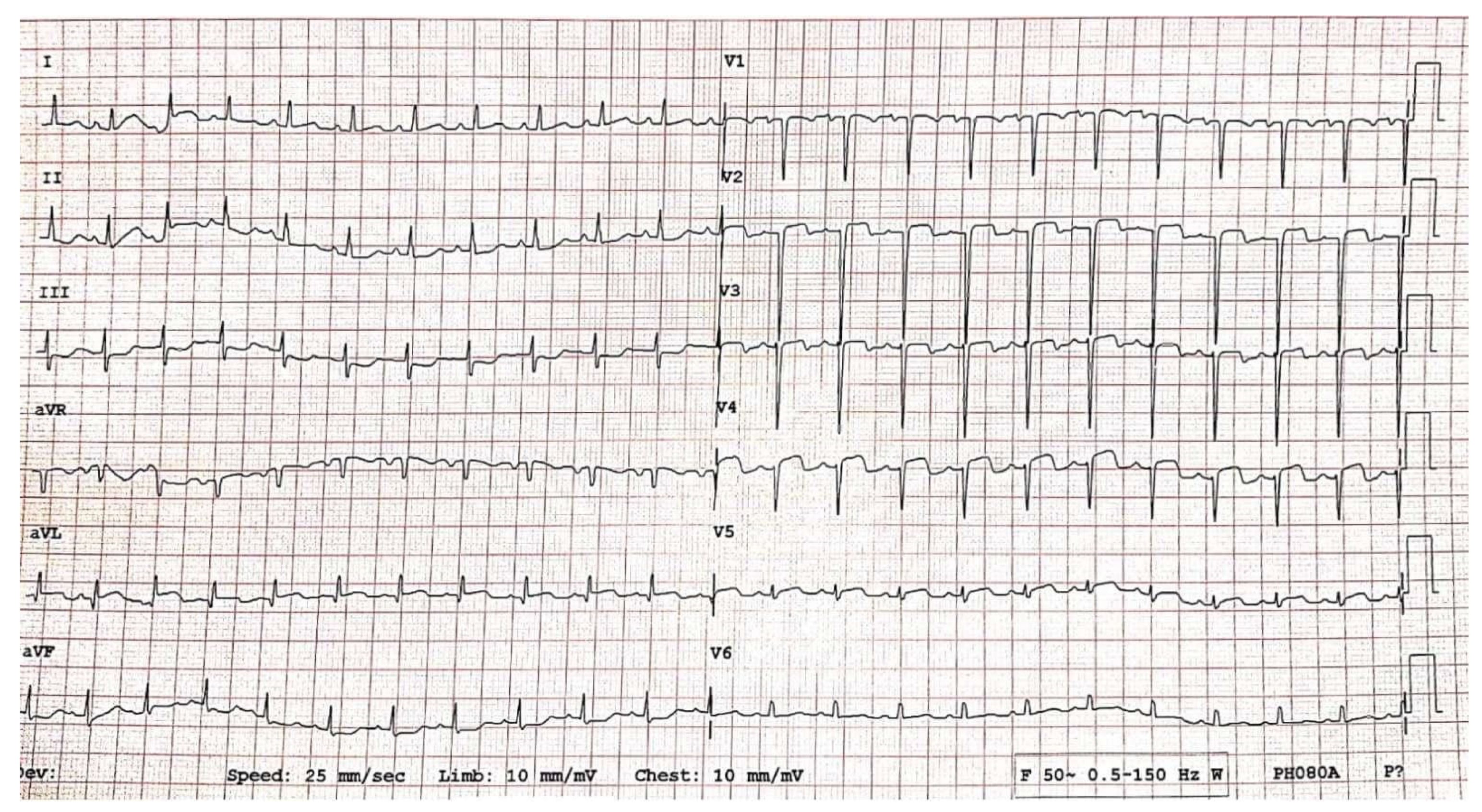
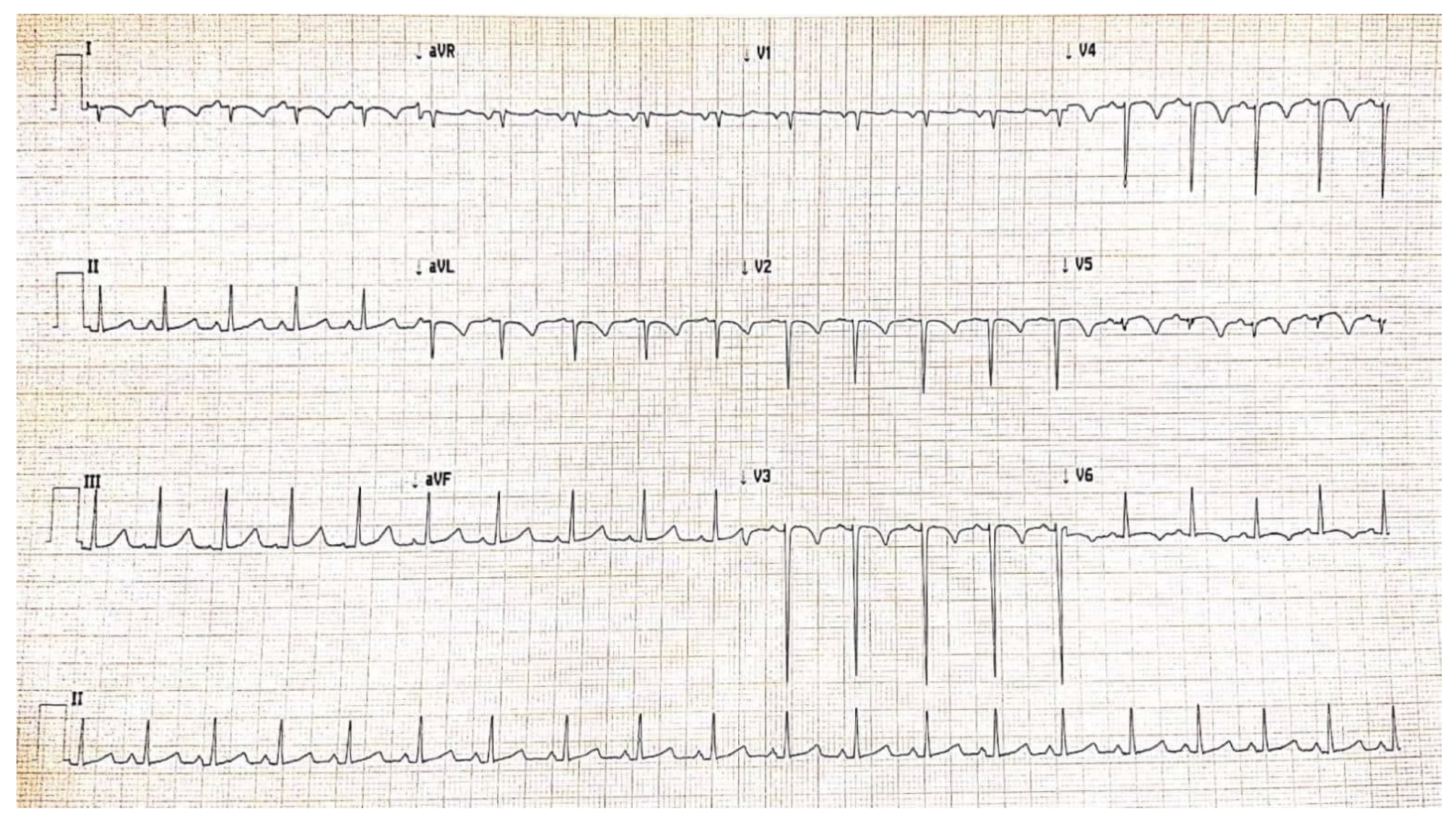
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 14 February 2022).
- Chow, E.J.; Englund, J.A. SARS-CoV-2 Infections in Children. Infect. Dis. Clin. N. Am. 2022. advance online publication. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, M.; Castellano-Martinez, A.; Cascales-Poyatos, H.M.; Perez-Reviriego, A.A. Cardiovascular impact of COVID-19 with a focus on children: A systematic review. World J. Clin. Cases 2020, 8, 5250–5283. [Google Scholar] [CrossRef] [PubMed]
- Cinteza, E.; Voicu, C.; Grigore, C.; Stefan, D.; Anghel, M.; Galos, F.; Ionescu, M.; Balgradean, M.; Nicolescu, A. Cardiac Implication in Pediatric Multisystemic Inflammatory Syndrome—Three Case Reports and Review of the Literature. Rom. J. Cardiol. 2021, 31, 885–892. [Google Scholar] [CrossRef]
- Ionescu, M.D.; Balgradean, M.; Cirstoveanu, C.G.; Balgradean, I.; Popa, L.I.; Pavelescu, C.; Capitanescu, A.; Berghea, E.C.; Filip, C. Myopericarditis Associated with COVID-19 in a Pediatric Patient with Kidney Failure Receiving Hemodialysis. Pathogens 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021, 323, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Bellino, S.; Punzo, O.; Rota, M.C. COVID-19 Disease Severity Risk Factors for Pediatric Patients in Italy. Pediatrics 2020, 146, e2020009399. [Google Scholar] [CrossRef]
- Royal College of Paediatrics and Child Health. Guidance: Paediatric Multisystem Inflammatory Syndrome Temporally Associated with COVID-19. Available online: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem inflammatory-syndrome-temporally-associatedcovid-19 (accessed on 22 May 2020).
- Voicu, C.; Grigore, C.; Stefan, D.; Filip, C.; Duica, G.; Nicolae, G.; Balgradean, M.; Nicolescu, A.; Cinteza, E. Overlaping syndromes: Kawasaki-like disease in pediatric multisystem inflammatory syndrome vs. atypical kawasaki disease. British or American? One case, many possibilities. Rom. J. Cardiol. 2021, 31, 897–902. [Google Scholar] [CrossRef]
- WHO. Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-infl ammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 15 May 2020).
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Pahl, E. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, 927–999. [Google Scholar] [CrossRef]
- Whittaker, E.; Bamford, A.; Kenny, J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020, 324, 259–269. [Google Scholar] [CrossRef]
- Verdoni, L.; Mazza, A.; Gervasoni, A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Ionescu, M.D.; Taras, R.; Dombici, B.; Balgradean, M.; Berghea, E.C.; Nicolescu, A. The Challenging Diagnosis of Pediatric Multisystem Inflammatory Syndrome Associated with SARS-CoV-2 Infection-Two Case Reports and Literature Review. J. Pers. Med. 2021, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Tsoucalas, G.; Sgantzos, M. Primary Asthenic Gout by Augustin-Jacob Landre-Beauvais in 1800: Is this the first description of Rheumatoid Arthritis? Mediterr. J. Rheumatol. 2017, 28, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, T. Kawasaki disease. Int. J. Rheum. Dis. 2014, 17, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Singh, S. Kawasaki disease—The journey over 50 years: 1967–2017. Int. J. Rheum. Dis. 2018, 21, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Thomas, E.; Wright, J.G.; Vettukattil, J.J. Congenital coronary artery dilatation. Heart 2003, 89, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ohyanagi, M.; Ikeoka, K. Clinical course of patients with coronary ectasia. Cardiology 1999, 91, 145–149. [Google Scholar] [CrossRef]
- Befeler, B.; Aranda, J.M.; Embi, A. Coronary artery aneurysms. Study of their etiology, clinical course and effect on left ventricular function and prognosis. Am. J. Med. 1977, 62, 597–607. [Google Scholar] [CrossRef]
- Ae, R.; Makino, N.; Kosami, K.; Kuwabara, M.; Matsubara, Y.; Nakamura, Y. Epidemiology, Treatments, and Cardiac Complications in Patients with Kawasaki Disease: The Nationwide Survey in Japan, 2017–2018. J. Pediatr. 2020, 225, 23–29. [Google Scholar] [CrossRef]
- Ae, R.; Abrams, J.Y.; Maddox, R.A.; Schonberger, L.B.; Nakamura, Y.; Kuwabara, M.; Makino, N.; Matsubara, Y.; Matsubara, D.; Kosami, K.; et al. Outcomes in Kawasaki disease patients with coronary artery abnormalities at admission. Am. Heart J. 2020, 225, 120–128. [Google Scholar] [CrossRef]
- Masuda, H.; Ae, R.; Koshimizu, T.A.; Matsumura, M.; Kosami, K.; Hayashida, K.; Makino, N.; Matsubara, Y.; Sasahara, T.; Nakamura, Y. Epidemiology and Risk Factors for Giant Coronary Artery Aneurysms Identified After Acute Kawasaki Disease. Pediatr. Cardiol. 2021, 42, 969–977. [Google Scholar] [CrossRef]
- Ae, R.; Shibata, Y.; Kosami, K.; Nakamura, Y.; Hamada, H. Kawasaki Disease and Pediatric Infectious Diseases during the Coronavirus Disease 2019 Pandemic. J. Pediatr. 2021, 239, 50–58.e2. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataria, V.; Wang, H.; Wald, J.W.; Phan, Y.L. Lisinopril-Induced Alopecia: A Case Report. J. Pharm. Pract. 2017, 30, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Afford, R.; Leung, A.K.C.; Lam, J.M. Pediatric Alopecia Areata. Curr. Pediatr. Rev. 2021, 17, 45–54. [Google Scholar] [CrossRef]
- Namazi, M.R.; Ashraf, A.; Handjani, F.; Eftekhar, E.; Kalafi, A. Angiotensin converting enzyme activity in alopecia areata. Enzym. Res. 2014, 2014, 694148. [Google Scholar] [CrossRef]
- Shin, J.W.; Kang, T.; Lee, J.S.; Kang, M.J.; Huh, C.H.; Kim, M.S.; Kim, H.J.; Ahn, H.S. Time-Dependent Risk of Acute Myocardial Infarction in Patients with Alopecia Areata in Korea. JAMA Dermatol. 2020, 156, 763–771. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sholler, G.F.; Howman-Giles, R.; Celermajer, J.M. Myocardial infarction in childhood: Clinical analysis of 17 cases and medium term follow up of survivors. Br. Heart J. 1991, 65, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Keshavarz, P.; Yazdanpanah, F.; Azhdari, S. Coronavirus disease 2019 (COVID-19): A systematic review of 133 Children that presented with Kawasaki-like multisystem inflammatory syndrome. J. Med. Virol. 2021, 93, 5458–5473. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Randolph, A.G. Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Grimaud, M.; Starck, J.; Oualha, M. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann. Intensive Care 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Moraleda, C.; Serna-Pascual, M.; Tagarro, A. Multi-Inflammatory Syndrome in Children related to SARS-CoV-2 in Spain. Clin. Infect. Dis. 2021, 72, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Méot, M.; Bonnet, D. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Schranz, D. COVID-19 in children: Acute endotheliopathy, but forgotten prostacyclin replacement? Cardiol. Young 2021, 1–2, published online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ichinose, E.; Kawasaki, T. Myocardial infarction in Kawasaki disease: Clinical analyses in 195 cases. J. Pediatr. 1986, 108, 923–927. [Google Scholar] [CrossRef]
- Tsuda, E.; Hirata, T.; Matsuo, O.; Abe, T.; Sugiyama, H.; Yamada, O. The 30-year outcome for patients after myocardial infarction due to coronary artery lesions caused by Kawasaki disease. Pediatr. Cardiol. 2011, 32, 176–182. [Google Scholar] [CrossRef]
- Zaffanello, M.; Piacentini, G.; Nosetti, L. Thrombotic risk in children with COVID-19 infection: A systematic review of the literature. Thromb. Res. 2021, 205, 92–98. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, 438–440. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, 362–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Kawasaki Disease (KD) | Incomplete (or Atypical) KD | Pediatric Multisystemic Inflammatory Syndrome (Required all 6 Criteria) |
|---|---|---|
Fever, and 4/5 criteria:
|
|
|
| Initial | 4 h after Starting tPA | 8 h Post-tPA | 12 h Post-tPA | 24 h Post-tPA | 7 Days after Admission | At Discharge | |
|---|---|---|---|---|---|---|---|
| Troponin T (ng/mL) | >2000 | >2000 | >2000 | >2000 | >2000 | 611 | <40 |
| Fibrinogen (mg/dL) | 270 | 232 | 266 | 249 | 270 | normal | normal |
| CK (IU/L) | 2.563 | 1.937 | 1654 | 1446 | 1437 | 34 | 72 |
| CK-MB (IU/L) | 463 | 338 | 221 | 174 | 140 | 20 | 34.8 |
| NT-proNBP (pg/mL) | 7.857 | - | - | 7.078 | - | 7.837 | 8.030 |
| TGO (IU/L) | 288.8 | 258 | 234 | 210 | 161 | normal | normal |
| Author | Feldstein | Verdoni | Whittaker | Grimaud | Moraleda | Belhadjer |
|---|---|---|---|---|---|---|
| Country | USA | Italy | UK | France | Spain | Switzerland/France |
| Number of Patients | 186 | 10 | 58 | 20 | 31 | 35 |
| Age | 8.3 | 7.5 | 9 | 10 | 7.6 | 10 |
| Shock | NR | 5 (50%) | 27 (46%) | 20 (100%) | 15 (48%) | 28(80%) |
| Myocardial Dysfunction | 70 (38%) | 5 (50%) | 18 (31%) | 20 (100%) | 15 (48%) | 35 (100%) |
| Coronary Artery Involvement | 15 (8%) | 2 (20%) | 8 (14%) | 0 (0%) | 3 (10%) | 6 (17%) |
| Coronary Sequelae | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Death | 4 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (3%) | 0 (0%) |
| 1. | Factors That Increase the Vascular Turbulence in Coronary Arteries
|
| 2. | Factors Associated with Reduced Myocyte Supply
|
| 3. | Primary Factors That Increase the Risk for Thrombosis
|
| 4. | Secondary Factors That Increase the Risk for Thrombosis
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinteză, E.; Voicu, C.; Filip, C.; Ioniță, M.; Popescu, M.; Bălgrădean, M.; Nicolescu, A.; Mahmoud, H. Myocardial Infarction in Children after COVID-19 and Risk Factors for Thrombosis. Diagnostics 2022, 12, 884. https://doi.org/10.3390/diagnostics12040884
Cinteză E, Voicu C, Filip C, Ioniță M, Popescu M, Bălgrădean M, Nicolescu A, Mahmoud H. Myocardial Infarction in Children after COVID-19 and Risk Factors for Thrombosis. Diagnostics. 2022; 12(4):884. https://doi.org/10.3390/diagnostics12040884
Chicago/Turabian StyleCinteză, Eliza, Cristiana Voicu, Cristina Filip, Mihnea Ioniță, Monica Popescu, Mihaela Bălgrădean, Alin Nicolescu, and Hiyam Mahmoud. 2022. "Myocardial Infarction in Children after COVID-19 and Risk Factors for Thrombosis" Diagnostics 12, no. 4: 884. https://doi.org/10.3390/diagnostics12040884
APA StyleCinteză, E., Voicu, C., Filip, C., Ioniță, M., Popescu, M., Bălgrădean, M., Nicolescu, A., & Mahmoud, H. (2022). Myocardial Infarction in Children after COVID-19 and Risk Factors for Thrombosis. Diagnostics, 12(4), 884. https://doi.org/10.3390/diagnostics12040884








