What Is COVID 19 Teaching Us about Pulmonary Ultrasound?
Abstract
:1. Introduction
1.1. Historical Perspective of Ultrasound (US) Vertical Artifacts
1.2. An Introduction to the Clinical Use of Artifacts
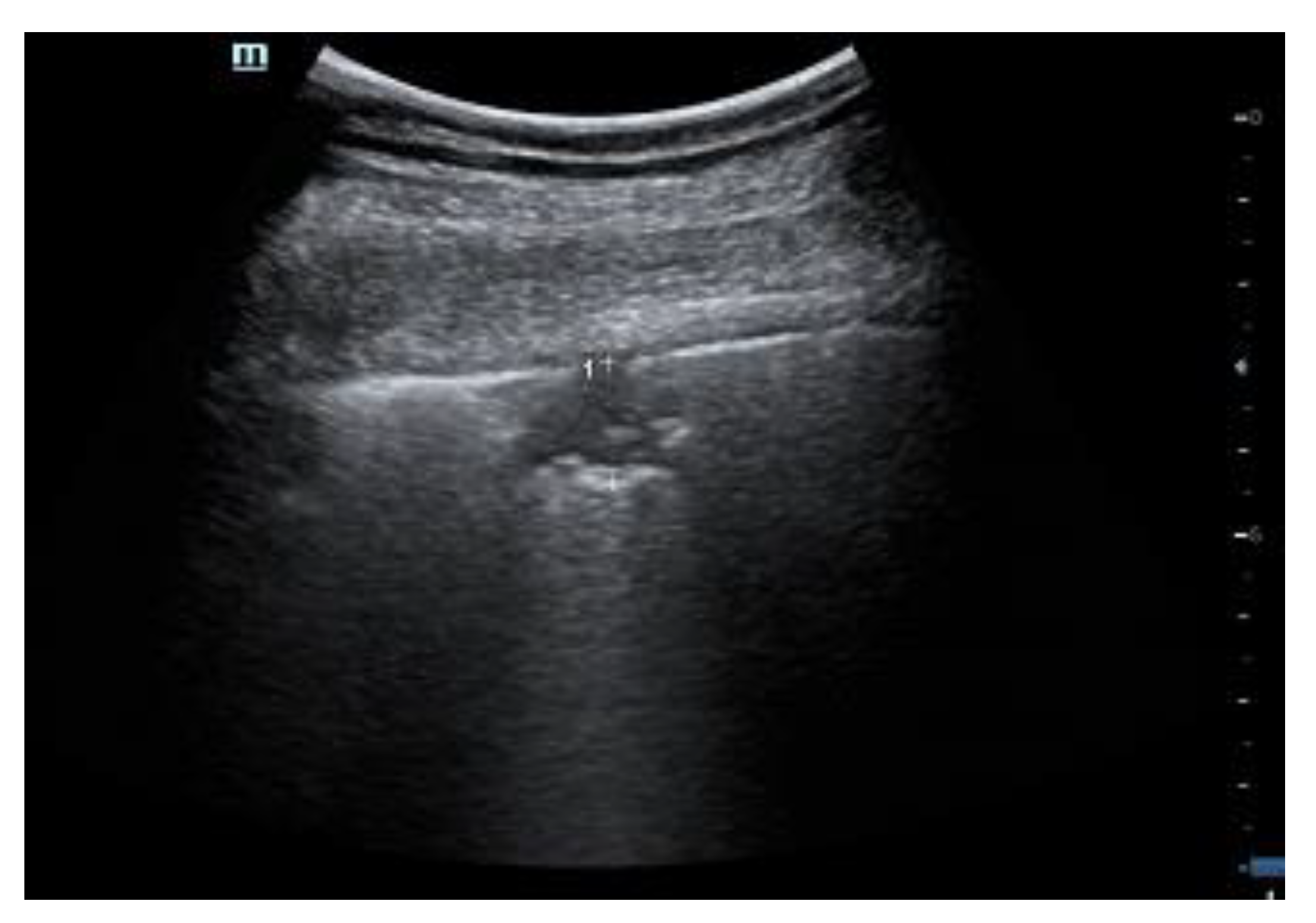
2. Clinical Interpretation of SIS
- Characteristics of the pleural line;
- Characteristics of the artifacts;
- Extension and distribution of SIS;
- Relationships with clinical data and integrated multi-district sonography.
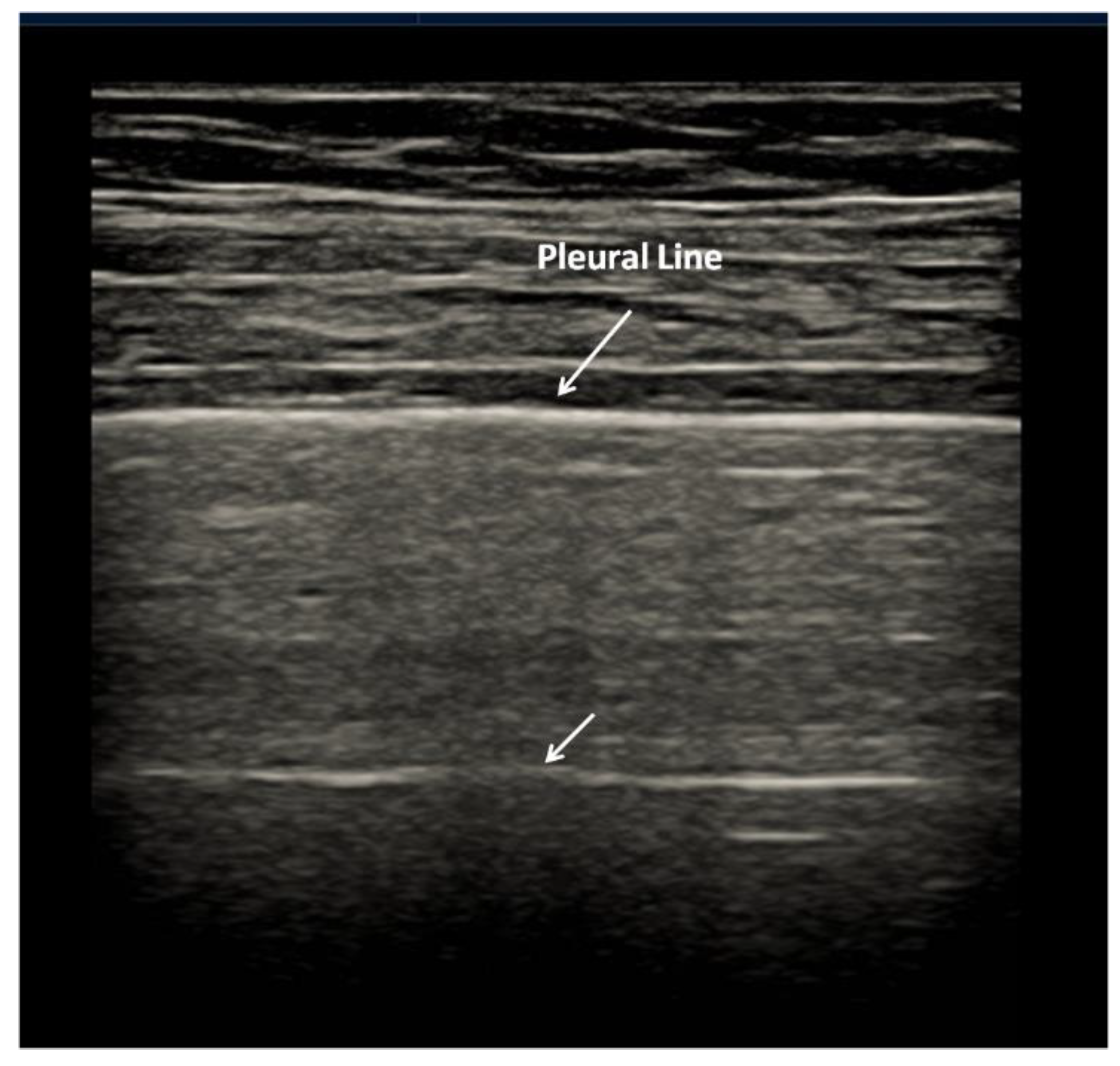
2.1. Characteristics of the Pleural Line
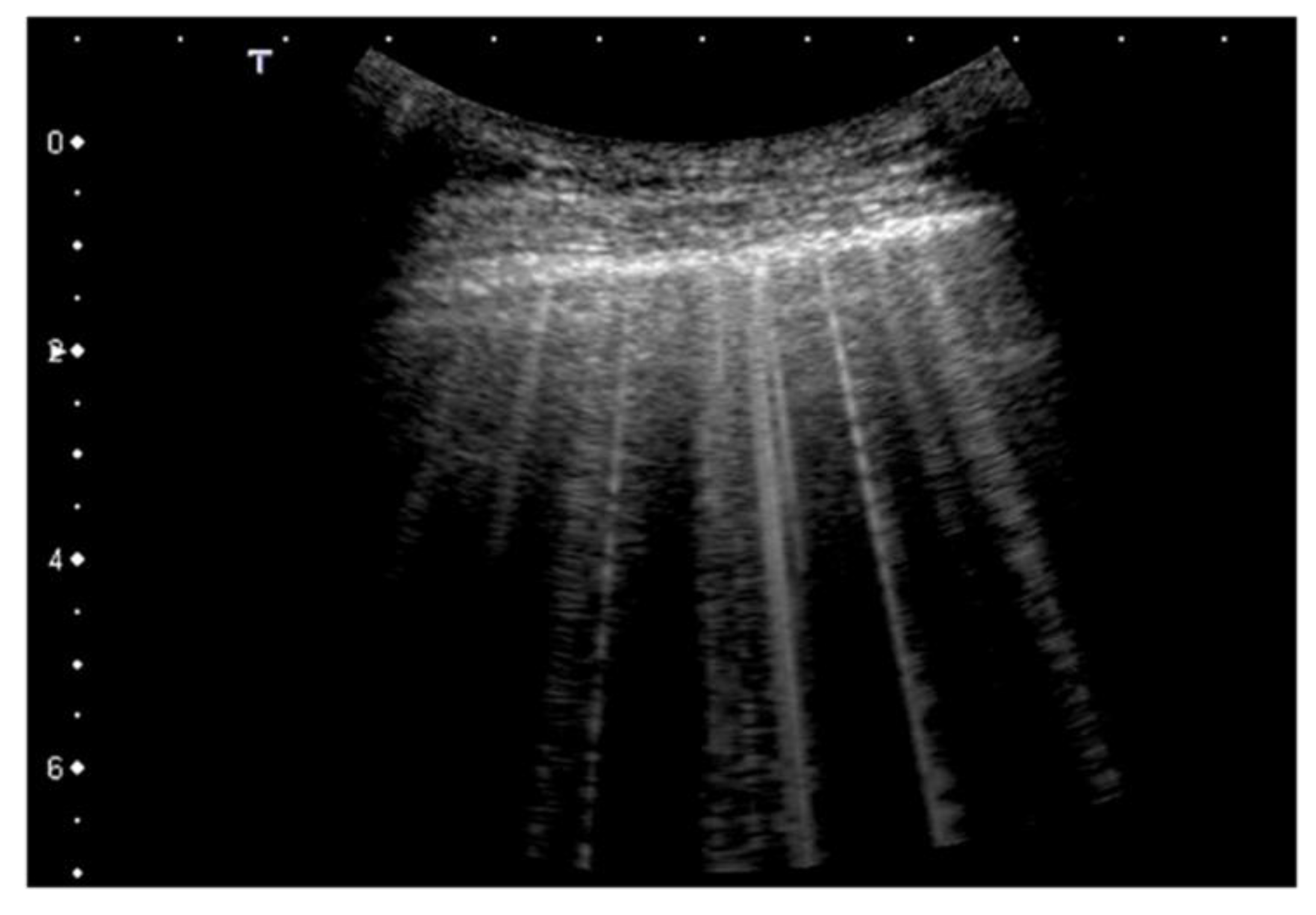
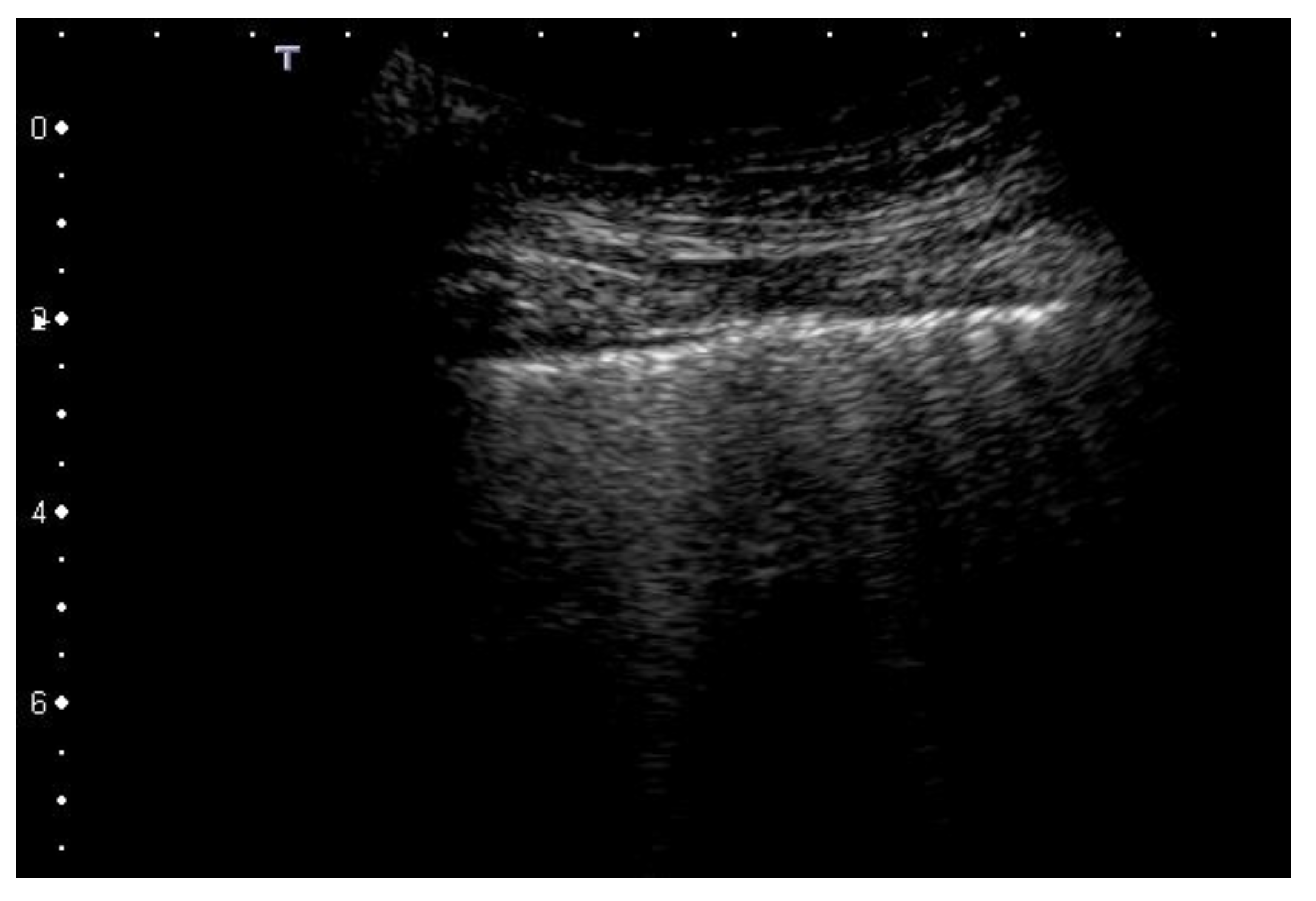
2.2. Artifacts’ Characteristics
2.3. Extension and Distribution
2.4. Relationships with Clinical Data and Integrated Multi-District Sonography
3. Clinical Basis of COVID-19 Lung Ultrasound Imaging
4. SIS in COVID-19
5. Why Does COVID-19 Consolidate the Lung?
6. COVID-19 in Pediatrics
7. Post-Acute Sequelae of COVID-19 Pneumonia
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Soldati, G.; Smargiassi, A.; Mariani, A.A.; Inchingolo, R. Novel aspects in diagnostic approach to respiratory patients: Is it the time for a new semiotics? Multidiscip. Respir. Med. 2017, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narula, J.; Chandrashekhar, Y.; Braunwald, E. Time to Add a Fifth Pillar to Bedside Physical Examination: Inspection, Palpation, Percussion, Auscultation, and Insonation. JAMA Cardiol. 2018, 1, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Demi, L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev. Respir. Med. 2019, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M.; Inchingolo, R.; Smargiassi, A.; Demi, L. On the physical basis of pulmonary sonographic interstitial syndrome. J. Ultrasound Med. 2016, 35, 2075–2086. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Demi, L.; Inchingolo, R. Artefactual lung ultrasonography: It is a matter of traps, order, and disorder. Appl. Sci. 2020, 10, 1570. [Google Scholar] [CrossRef] [Green Version]
- Demi, L.; Egan, T.; Muller, M. Lung Ultrasound Imaging, a Technical Review. Appl. Sci. 2020, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Demi, M.; Prediletto, R.; Soldati, G.; Demi, L. Physical Mechanisms Providing Clinical Information From Ultrasound Lung Images: Hypotheses and Early Confirmations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 612–623. [Google Scholar] [CrossRef]
- Mohanty, K.; Blackwel, J.; Egan, T.; Muller, M. Characterization of the Lung Parenchyma Using Ultrasound Multiple Scattering. Ultrasound Med. Biol. 2017, 43, 993–1003. [Google Scholar] [CrossRef]
- Demi, M. The impact of multiple concurrent factors on the length of the US pulmonary vertical artifacts as illustrated through the experimental and numerical analysis of simple models. J. Acoust. Soc. Am. 2021, 150, 2106–2115. [Google Scholar] [CrossRef]
- Demi, M. On the Replica of US Pulmonary Artifacts by Means of Physical Models. Diagnostics 2021, 11, 1666. [Google Scholar] [CrossRef]
- De Carvalho, L.S.; Da Silva Júnior, R.T.; Oliveira, B.V.S.; De Miranda, Y.S.; Rebouças, N.L.F.; Loureiro, M.S.; Pinheiro, S.L.R.; da Silva, R.S.; Correia, P.V.S.L.M.; Maria José Souza Silva, M.J.S.; et al. What the imaging exams show about the disease. World J. Radiol. 2021, 13, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Copetti, R.; Soldati, G.; Copetti, P. Chest sonography: A useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc. Ultrasound 2008, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldati, G.; Inchingolo, R.; Smargiassi, A.; Sher, S.; Nenna, R.; Inchingolo, C.D.; Valente, S. Ex vivo lung sonography: Morphologic-ultrasound relationship. Ultrasound Med. Biol. 2012, 38, 1169–1179. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Sher, S.; Nenna, R.; Valente, S.; Inchingolo, C.D.; Corbo, G.M. Lung ultrasonography may provide an indirect estimation of lung porosity and airspace geometry. Respiration 2014, 88, 458–468. [Google Scholar] [CrossRef]
- Ziskin, M.C.; Thickman, D.I.; Goldenberg, N.J.; Lapayowker, M.S.; Becker, J.M. The comet tail artifact. J. Ultrasound Med. 1982, 1, 1–7. [Google Scholar] [CrossRef]
- Thickman, D.I.; Ziskin, M.C.; Goldenberg, N.J.; Linder, B.E. Clinical manifestations of the comet tail artifact. J. Ultrasound Med. 1983, 2, 225–230. [Google Scholar] [CrossRef]
- Avruch, L.; Cooperberg, P.L. The ring-down artifact. J. Ultrasound Med. 1985, 4, 21–28. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Biderman, P.; Gepner, A.; Barré, O. The comet-tail artifact: An ultrasound sign of alveolar interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Mathis, G.; Blaivas, M.; Volpicelli, G.; Seibel, A.; Wastl, D.; Atkinson, N.S.S.; Cui, X.W.; Fan, M.; Yi, D. Lung B-line artifacts and their use. J. Thorac. Dis. 2016, 8, 1356–1365. [Google Scholar] [CrossRef] [Green Version]
- Picano, E.; Frassi, F.; Agricola, E.; Gligorova, S.; Gargani, L.; Mottola, G. Ultrasound lung comets: A clinically useful sign of extravascular lung water. J. Am. Soc. Echocardiogr. 2006, 19, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Reissig, A.; Kroegel, C. Transthoracic sonography of diffuse parenchymal lung disease: The role of comet tail artifacts. J. Ultrasound Med. 2003, 22, 173–180. [Google Scholar] [CrossRef]
- Soldati, G.; Giunta, V.; Sher, S.; Melosi, F.; Dini, C. “Synthetic” comets: A new look at lung sonography. Ultrasound Med. Biol. 2011, 37, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Copetti, R.; Sher, S. Sonographic interstitial syndrome: The sound of lung water. J. Ultrasound Med. 2009, 28, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; van Hoeve, W.; van Sloun, R.J.G.; Soldati, G.; Demi, M. Determination of a potential quantitative measure of the state of the lung using lung ultrasound spectroscopy. Sci. Rep. 2017, 7, 12746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldati, G.; Demi, M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J. Ultrasound 2017, 20, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Smargiassi, A.; Inchingolo, R.; Soldati, G.; Copetti, R.; Marchetti, G.; Zanforlin, A.; Giannuzzi, R.; Testa, A.; Nardini, S.; Valente, S. The role of chest ultrasonography in the management of respiratory diseases: Document II. Multidiscip. Respir. Med. 2013, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Demi, L.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Faita, F.; Soldati, G.; Task Force Group. Ultrasonography in lung pathologies: New perspectives. Multidiscip. Respir. Med. 2014, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Soldati, G.; Demi, M.; Demi, L. Ultrasound patterns of pulmonary edema. Ann. Transl. Med. 2019, 7 (Suppl. 1), S16. [Google Scholar] [CrossRef]
- Koegelenberg, C.F.; von Groote-Bidlingmaier, F.; Bolliger, C.T. Transthoracic ultrasonography for the respiratory physician. Respiration 2012, 84, 337–350. [Google Scholar] [CrossRef]
- Smargiassi, A.; Inchingolo, R.; Calandriello, C.; Lombardi, F.; Calabrese, A.; Siciliano, M.; Larici, A.R.; Demi, L.; Richeldi, L.; Soldati, G. Possible Role of Chest Ultrasonography for the Evaluation of Peripheral Fibrotic Pulmonary Changes in Patients Affected by Idiopathic Pulmonary Fibrosis-Pilot Case Series. Appl. Sci. 2020, 10, 1617. [Google Scholar] [CrossRef] [Green Version]
- Pesenti, A.; Musch, G.; Lichtenstein, D.; Mojoli, F.; Amato, M.B.P.; Cinnella, G.; Gattinoni, L.; Quintel, M. Imaging in acute respiratory distress syndrome. Intensive Care Med. 2016, 42, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Muller, N.L.; Ooi, G.C.; Kong, P.L.; Nicolaou, S. Severe Acute Respiratory Syndrome: Radiographic and CT Findings. AJR Am. J. Roentgenol. 2003, 181, 3–8. [Google Scholar] [CrossRef]
- Ajlan, A.M.; Ahyad, R.A.; Jamjoom, L.G.; Alharthy, A.; Madani, T.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection: Chest CT Findings. AJR Am. J. Roentgenol. 2014, 203, 782–787. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Koo, H.J.; Lim, S.; Choe, J.; Choi, S.H.; Sung, H.; Do, K.H. Radiographic and CT features of viral pneumonia. Radiographics 2018, 38, 719–739. [Google Scholar] [CrossRef] [Green Version]
- Ruuskanen, O.; Lathi, E.; Jennings, L.C.; Murdoch, D.R. Viral pneumonia. Lancet 2011, 377, 1264–1275. [Google Scholar] [CrossRef]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbet, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and cardiac pathology in COVID-19: The first autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Caramaschi, S.; Kapp, M.E.; Miller, S.E.; Eisemberg, R.; Johnson, J.; Epperly, G.; Maiorana, A.; Silvestri, G.; Giannico, G.A. Histopathological findings and clinicopathologic correlation in COVID-19: A systematic review. Mod. Pathol. 2021, 34, 1614–1633. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Polidori, T.; Rucci, C.; Guido, G.; Bracci, B.; De Dominicis, C.; Laghi, A. Chest Ct features of COVID-19 in Rome, Italy. Radiology 2020, 296, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Kianzad, A.; Meijboom, L.J.; Nossent, E.J.; Roos, E.; Schurink, B.; Bonta, P.I.; van den Berk, I.A.H.; Britstra, R.; Stoker, J.; Noordegraaf, A.V.; et al. COVID-19: Histopathological correlates of imaging patterns on chest computed tomography. Respirology 2021, 26, 869–877. [Google Scholar] [CrossRef]
- Hernández-Píriz, A.; Tung-Chen, Y.; Juménez-Virumbrales, D.; Ayala-Larrañaga, I.; Barba-Martín, R.; Canora-Lebrato, J.; Zapatero-Gaviria, A.; De Casasola-Sánchez, G.G. Importance of lung Ultrasound follow-up in patients who had recovered from Coronavirus Disease 2019: Results from a Prospective Study. J. Clin. Med. 2021, 10, 3196. [Google Scholar] [CrossRef]
- Mento, F.; Perrone, T.; Macioce, V.N.; Tursi, F.; Buonsenso, D.; Torri, E.; Smargiassi, A.; Inchingolo, R.; Soldati, G.; Demi, L. On the inpact of different lung ultrasound imaging protocols in the evaluation of patients affected by Coronavirus Disease 2019. How many acquisitions are needed? J. Ultrasound Med. 2021, 40, 2235–2238. [Google Scholar] [CrossRef]
- Pal, A.; Ali, A.; Young, T.R.; Oostenbrink, J.; Prabhakar, A.; Prabhakar, A.; Deacon, N.; Arnold, A.; Eltayeb, A.; Yap, C.; et al. Comprehensive literature review on the radiographic findings, imaging modalities, and the role of radiology in the COVID-19 pandemic. World J. Radiol. 2021, 13, 258–282. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J. Ultrasound Med. 2020, 39, 1459–1462. [Google Scholar] [CrossRef] [Green Version]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients with COVID-19: A Simple, Quantitative, Reproducible Method. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Perrone, T.; Soldati, G.; Padovini, L.; Fiengo, A.; Lettieri, G.; Sabatini, U.; Gori, G.; Lepore, F.; Garolfi, M.; Palumbo, I.; et al. A new Lung Ultrasound Protocol able to predict worsening in Patients affected by Severe Acute Respiratory Syndrome Coronavirus 2 pneumonia. J. Ultrasound Med. 2021, 40, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; Mento, F.; Di Sabatino, A.; Fiengo, A.; Sabatini, U.; Macioce, V.N.; Marco Robol, M.; Tursi, F.; Sofia, C.; Di Cienzo, C.; et al. Lung Ultrasound in COVID-19 and Post-COVID-19 patients, an Evidence-Based Approach. J. Ultrasound Med. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Luo, X.; Wang, L.; Estil, J.; Lv, M.; Zhu, Y.; Wang, Q.; Xiao, X.; Song, Y.; Lee, M.S.; et al. A comparison of lung Ultrasound and Computed Tomography in the diagnosis of patients with COVID-19: A systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Portale, G.; Ciolina, F.; Arcari, L.; Di Lazzaro Giraldi, G.; Danti, M.; Pietropaolo, L.; Camastra, G.; Cordischi, C.; Urbani, L.; Proietti, L.; et al. Lung Ultrasound in COVID-19: Clinical Correlates and Comparison with Chest Computed Tomography. SN Compr. Clin. Med. 2021, 3, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Buda, N.; Cylwik, J.; Mróz, K.; Rudzinska, R.; Dubik, P.; Malczewska, A.; Oraczewska, A.; Skoczyński, S.; Suska, A.; Górecki, T.; et al. Lung Ultrasound examination in patients with SARS-CoV-2Infection: Multicenter study. J. Clin. Med. 2021, 10, 3255. [Google Scholar] [CrossRef]
- Smith, M.J.; Hayward, S.A.; Innes, S.M.; Miller, A.S.C. Point-of-care lung ultrasound in patients with COVID-19—A narrative review. Anaesthesia 2020, 24, 2776. [Google Scholar] [CrossRef] [Green Version]
- Schmid, B.; Feuerstein, D.; Lang, C.N.; Fink, K.; Steger, R.; Rieder, M.; Duerschmied, D.; Busch, H.J.; Damjanovic, D. Lung ultrasound in the emergency department—A valuable tool in the management of patients presenting with respiratory symptoms during the SARS-CoV-2 pandemic. BMC Emerg. Med. 2020, 20, 96. [Google Scholar] [CrossRef]
- Nishiura, H.; Kobayashi, T.; Miyama, T.; Suzuki, A.; Jung, S.M.; Hayashi, K.; Kinoshita, R.; Yang, Y.; Yuan, B.; Akhmetzhanov, A.R.; et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int. J. Infect. Dis. 2020, 94, 154–155. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 2020, 25, 2000180. [Google Scholar] [CrossRef] [Green Version]
- Puylaert, C.A.J.; Scheijmans, J.C.G.; Borgstein, A.B.J.; Andeweg, C.S.; Bartels-Rutten, A.; Beets, G.I.; van Berge Henegouwen, M.I.; Braak, S.J.; Couvreur, R.; Daams, F.; et al. Yield of Screening for COVID-19 in Asymptomatic Patients Before Elective or Emergency Surgery Using Chest CT and RT-PCR (SCOUT). Ann. Surg. 2020, 272, 919–924. [Google Scholar] [CrossRef]
- Kumar, A.; Weng, I.; Graglia, S.; Lew, T.; Gandhi, K.; Lalani, F.; Chia, D.; Duanmu, Y.; Jensen, T.; Lobo, V.; et al. Point-of-Care Ultrasound predicts clinical outcomes in patients with COVID-19. J. Ultrasound Med. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Stecher, S.S.; Anton, S.; Fraccaroli, A.; Götschke, J. Lung ultrasound predicts clinical course but not outcome in COVID-19 ICU patients: A retrospective single-center analysis. BMC Anesthesiol. 2021, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Bulla, P.; Dicke, L.; Klein, C.; Bott, S.; Keller, R.; Malek, N.; Fröhlich, E.; Göpel, S.; Blumenstock, G.; et al. Can follow up lung ultrasound in Coronavirus Disease-19 patients indicate clinical outcome? PLoS ONE 2021, 16, e0256359. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, G.; Malfatto, G.; Perge, E.; Facchetti, L.; Pini, L.; Bosco, M.; Cernigliaro, F.; Perego, G.B.; Facchini, M.; Badano, L.P.; et al. Lung Ultrasound in patients with SARS-CoV-2 pneumonia: Correlations with chest computed tomography, respiratory impairment, and inflammatory cascade. J. Ultrasound Med. 2021. ahead of print. [Google Scholar] [CrossRef]
- Sheard, S.; Rao, P.; Devaraj, A. Imaging of acute respiratory distress syndrome. Respir. Care 2012, 57, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Magnani, E.; Mattei, L.; Paolucci, E.; Magalotti, G.; Giacalone, N.; Praticò, C.; Praticò, B.; Zani, M.C. Lung Ultrasound in Severe COVID-19 Pneumonia in the Sub-Intensive Care Unit: Beyond the Diagnostic Purpose. Respir. Med. Case Rep. 2020, 31, 101307. [Google Scholar] [CrossRef]
- Bouhemad, B.; Brisson, H.; Le-Guen, M.; Arbelot, C.; Lu, Q.; Rouby, J.J. Bedside Ultrasound assessment of Positive End-Expiratory Pressure–induced lung recruitment. Am. J. Respir. Crit. Care Med. 2011, 183, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Prü, B.M. Variants of SARS-CoV-2: Mutations, transmissibility, virulence, drug resistance, and antibody/vaccine sensitivity. Front. Biosci. 2022, 27, 65. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatment for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Shy, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspective on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef] [Green Version]
- Franks, T.J.; Chong, P.Y.; Chui, P.; Galvin, J.R.; Lourens, R.M.; Reid, A.H.; Selbs, E.; McEvoy, C.P.L.; Hayden, C.D.L.; Fukuoka, J.; et al. Lung pathology of severe acute respiratory syndrome (SARS): A study of 8 autopsy cases from Singapore. Hum. Pathol. 2003, 34, 743–748. [Google Scholar] [PubMed]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Dengju, L.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldati, G.; Giannasi, G.; Smargiassi, A.; Inchingolo, R.; Demi, L. Contrast-Enhanced Ultrasound in patients with COVID-19: Pneumonia, Acute Respiratory Distress Syndrome, or something else. J. Ultrasound Med. 2020, 39, 2483–2489. [Google Scholar] [CrossRef]
- Buonsenso, D.; Pata, D.; Chiaretti, A. COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir. Med. 2020, 8, e27. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 among children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef] [Green Version]
- Musolino, A.M.; Supino, M.C.; Buonsenso, D.; Papa, R.E.; Chiurchiù, S.; Magistrelli, A.; Barbieri, M.A.; Raponi, M.; D’Argenio, P.; Villani, A.; et al. Lung ultrasound in the diagnosis and monitoring of 30 children with coronavirus disease 2019. Pediatr. Pulmonol. 2021, 56, 1045–1052. [Google Scholar] [CrossRef]
- Denina, M.; Scolfaro, C.; Silvestro, E.; Pruccoli, G.; Mignone, F.; Zoppo, M.; Ramenghi, U.; Garazzino, S. Lung ultrasound in children with COVID-19. Pediatrics 2020, 146, e20201157. [Google Scholar]
- Caruso, D.; Guido, G.; Zerunian, M.; Polidori, T.; Lucertini, E.; Pucciarelli, F.; Polici, M.; Rucci, C.; Bracci, B.; Nicolai, M.; et al. Post-Acute sequelae of COVID-19 pneumonia: Six-month chest CT Follow-up. Radiology 2021, 301, 396–405. [Google Scholar] [CrossRef]
- Udwadia, Z.F.; Koul, P.A.; Richeldi, L. Post-COVID lung fibrosis: The tsunami that will follow the earthquake. Lung India 2021, 38 (Suppl. S1), 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ambardar, S.R.; Hightower, S.L.; Huprikar, N.A.; Chung, K.K. Post-COVID-19 pulmonary fibrosis: Novel sequelae of the current pandemic. J. Clin. Med. 2021, 10, 2452. [Google Scholar] [CrossRef] [PubMed]
| Cardiogenic | Pneumogenic |
|---|---|
| Diffuse homogenous SIS | Diffuse inhomogeneous SIS, and spared areas |
| Smooth, linear, and regular pleural line | Coarse, irregular, and cobbled pleural line |
| Bright (laser-like), and modulated artifacts | Rough attenuated vertical artifacts |
| Normal sliding sign | Reduced sliding sign |
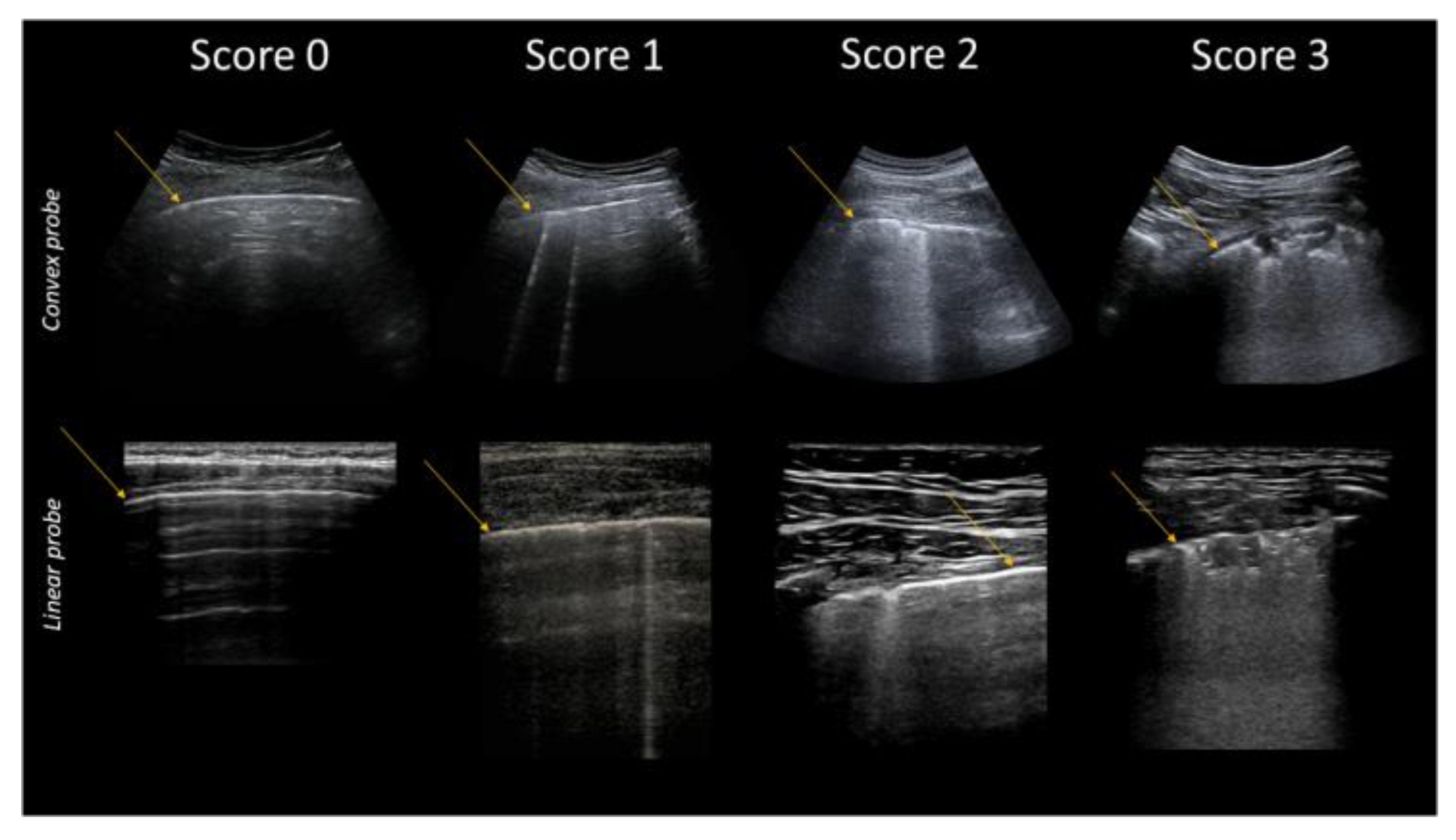
| Score | Description |
|---|---|
| 0 | Pleural line is regular. Horizontal artifacts and mirror effects are present. Normal lung. |
| 1 | Pleural line has slight alterations with sporadic vertical bright artifacts. The presence of relatively small acoustic channels due to focal interstitial thickening is speculated. |
| 2 | Pleural line has relevant alterations. Progression of subversion of peripheral air space geometry causes a predominance of vertical artifacts. Small subpleural consolidations, related to deaeration, can be present. |
| 3 | Pleural line is irregular and cobbled. Subpleural lung is denser and more disordered. White lung with or without larger consolidations may be present. Small and large consolidations are subpleural regions minimally or completely deprived of air. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldati, G.; Demi, M. What Is COVID 19 Teaching Us about Pulmonary Ultrasound? Diagnostics 2022, 12, 838. https://doi.org/10.3390/diagnostics12040838
Soldati G, Demi M. What Is COVID 19 Teaching Us about Pulmonary Ultrasound? Diagnostics. 2022; 12(4):838. https://doi.org/10.3390/diagnostics12040838
Chicago/Turabian StyleSoldati, Gino, and Marcello Demi. 2022. "What Is COVID 19 Teaching Us about Pulmonary Ultrasound?" Diagnostics 12, no. 4: 838. https://doi.org/10.3390/diagnostics12040838
APA StyleSoldati, G., & Demi, M. (2022). What Is COVID 19 Teaching Us about Pulmonary Ultrasound? Diagnostics, 12(4), 838. https://doi.org/10.3390/diagnostics12040838







