Overview of Lung Ultrasound in Pediatric Cardiology
Abstract
:1. Background
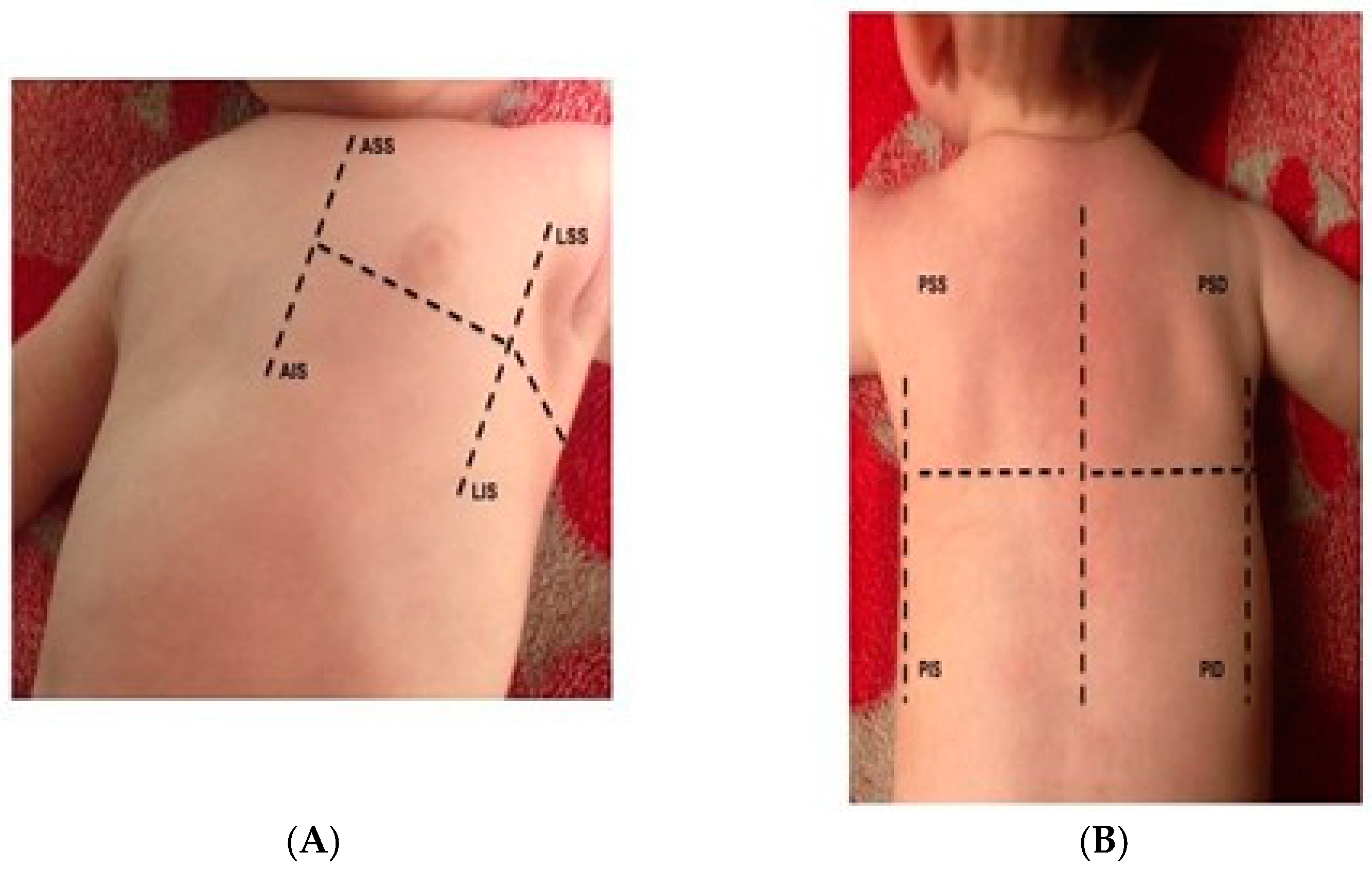
2. LUS Examination Protocols
3. When and Who Should Perform LUS
4. Common Findings
4.1. B-Lines
4.2. Classification of Lung Congestion in Children
4.3. Pleural Effusion
4.4. Atelectasis, Pneumonia, Consolidations, and Others
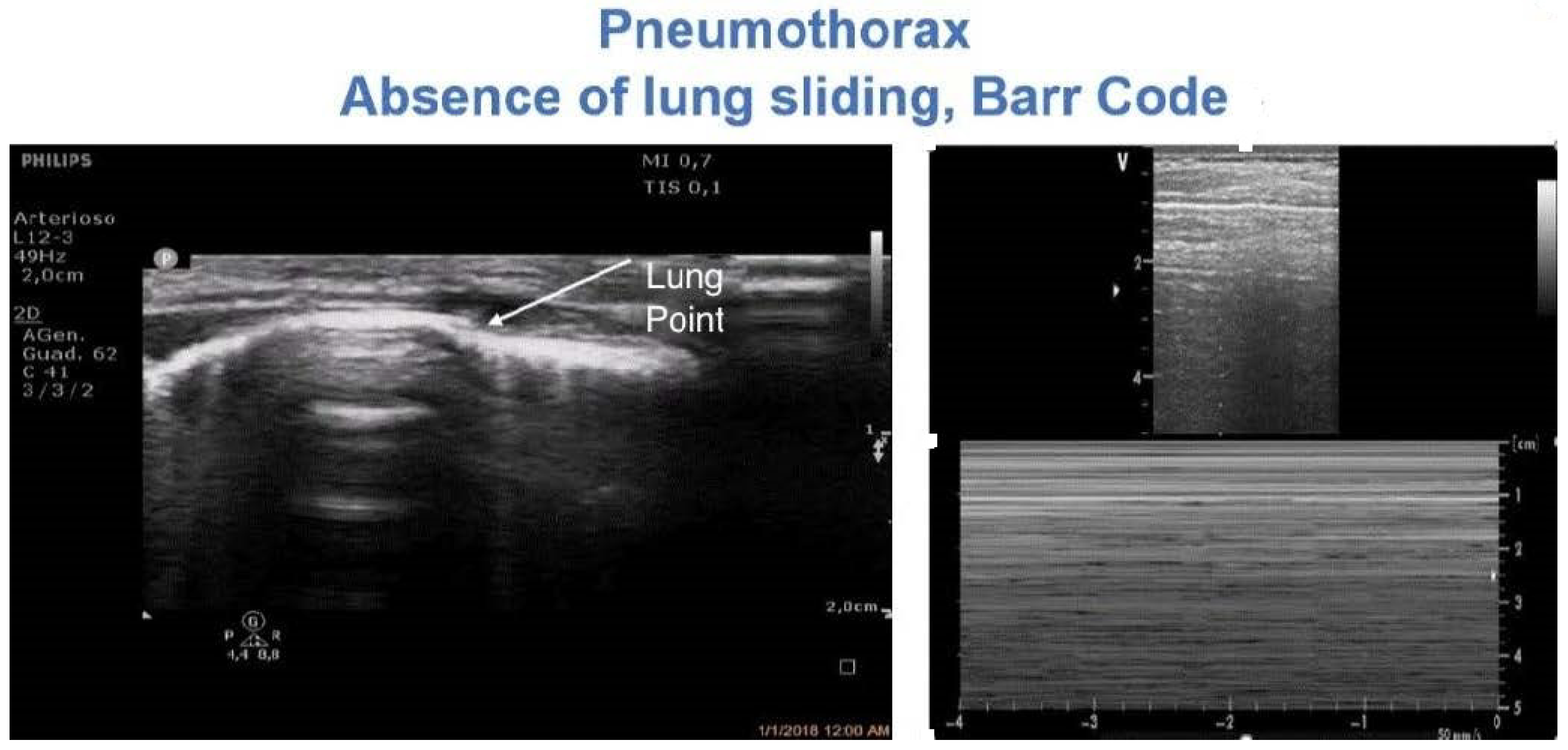
4.5. Pneumothorax
| Authors | Population | Protocol of Examination | Formula |
|---|---|---|---|
| Remérand et al. [22], France | 58 (45 M) Age 58 ± 17 years | SUPINE Transverse views positioning the transducer in each IS. The transducer was slipped between the patient’s back and mattress. The lower and upper IS where PE was detected were drawn on the patient’s skin PE length measured in paravertebral regions between the apical and caudal limits. Cross-sectional area measured at the mid-length of PE | PEV (mL) = ACT (cm2) × LCT (mm) |
| Usta [24], Germany | 135 (90 M) Age 60 (45–67) years | SITTING The transducer was moved in a cranial direction in the mid-scapular line. PE diameter: maximal distance between mid-height of the diaphragm and visceral pleura | PEV (mL) = D (mm) × 16 |
| Balik et al. [23], Czech Republic | 81 (47 M) m. ventilated patients Age 60 ± 15 years | SUPINE The transducer was moved in the cranial direction in the posterior axillary line PE diameter: maximal distance between parietal and visceral pleura at the lung base | PEV (mL) = 20 × Sep (mm) |
| Eibenberger [37], Austria | 51 (21 M) Age 28–82 years | SITTING Latero-dorsal wall of the chest PE diameter: the maximal perpendicular distance between the posterior wall of the lung and the posterior chest wall | D (mm) PEV (mL) 0 0–90 10 50–300 20 150–310 30 160–660 40 490–1670 50 650–1840 >60 950–251 |
| Vignon et al. [21], France | 97 (61 M) age 59 ± 20 years | SUPINE From the base to the apex of the chest, along the dorso-lateral part of the chest wall, as far as possible posterior between the mattress and the patient’s back without lifting the hemithorax. PE diameter: the maximal distance from the leading edge of the dependent surface of the lung to the trailing edge of the posterior chest wall, on transverse views of pleural spaces. Measurements were made at the base and at the apex of the pleural space | D > 45 mm at the RTB D > 50 mm at the LTB base predicted a PEV ≥ 800 mL sensitivity of 94% and 100 and specificity of 76 and 67%, respectively |
4.6. The Retrosternal Area: Diagnosis of Clots
4.7. Diaphragmatic Motion Anomalies
4.8. LUS Guidance of Interventional Procedures
4.9. Chest X-ray Reduction in Pediatric Cardiac Surgery
4.10. Prognostic Utility of LUS
5. Current Gaps of Knowledge
5.1. Why Is the Lung White?
5.2. Future Perspectives
6. Conclusive Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vitale, V.; Ricci, Z.; Cogo, P. Lung ultrasonography and pediatric cardiac surgery: First experience with a new tool for postoperative lung complications. Ann. Thorac. Surg. 2014, 97, e1214. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Giordano, R.; Assanta, N.; Murzi, B.; Gargani, L. Chest Ultrasound: A New, Easy, and Radiation-Free Tool to Detect Retrosternal Clot after Pediatric Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2015, 29, e59–e60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantinotti, M.; Giordano, R.; Volpicelli, G.; Kutty, S.; Murzi, B.; Assanta, N.; Gargani, L. Lung ultrasound in adult and paediatric cardiac surgery: Is it time for routine use? Interact. Cardiovasc. Thorac. Surg. 2016, 22, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Hasan, T.; Bonetti, S.; Gioachin, A.; Bulgarelli, A.; Bartolacelli, Y.; Ragni, L.; Gargiulo, G.D.; Donti, A. Lung ultrasound compared with bedside chest radiography in a paediatric cardiac intensive care unit. Acta Paediatr. 2021, 110, 1335–1340. [Google Scholar] [CrossRef]
- Conlon, T.W.; Himebauch, A.S.; Fitzgerald, J.C.; Chen, A.E.; Dean, A.J.; Panebianco, N.; Darge, K.; Cohen, M.S.; Greeley, W.J.; Berg, R.A.; et al. Implementation of a pediatric critical care focused bedside ultrasound training program in a large academic PICU. Pediatr. Crit. Care Med. 2015, 16, 219–226. [Google Scholar] [CrossRef]
- Chen, S.W.; Fu, W.; Liu, J.; Wang, Y. Routine application of lung ultrasonography in the neonatal intensive care unit. Medicine 2017, 96, e5826. [Google Scholar] [CrossRef]
- Claes, A.S.; Clapuyt, P.; Menten, R.; Michoux, N.; Dumitriu, D. Performance of chest ultrasound in pediatric pneumonia. Eur. J. Radiol. 2017, 88, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.Q.; Qiu, R.X.; Liu, J.; Zhang, L.; Ren, X.L.; Qin, S.J. Lung ultrasound completely replaced chest X-ray for diagnosing neonatal lung diseases: A 3-year clinical practice report from a neonatal intensive care unit in China. J. Matern. Fetal Neonatal Med. 2020, 9, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Ren, X.L.; Fu, W.; Liu, Y.; Xia, M.-R. Bronchoalveolar lavage for the treatment of neonatal pulmonary atelectasis under lung ultrasound monitoring. J. Matern. Fetal Neonatal Med. 2017, 30, 2362–2366. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Sher, S.; Valente, S.; Corbo, G.M. Ultrasound-guided pleural puncture in supine or recumbent lateral position—feasibility study. Multidiscip. Respir. Med. 2013, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantinotti, M.; Ait Ali, L.; Scalese, M.; Giordano, R.; Melo, M.; Remoli, E.; Franchi, E.; Clemente, A.; Moschetti, R.; Festa, P.; et al. Lung ultrasound reclassification of chest X-ray data after pediatric cardiac surgery. Paediatr. Anaesth. 2018, 28, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Giordano, R.; Scalese, M.; Marchese, P.; Franchi, E.; Viacava, C.; Molinaro, S.; Assanta, N.; Koestenberger, M.; Kutty, S.; et al. Prognostic Value of a New Lung Ultrasound Score to Predict Intensive Care Unit Stay in Pediatric Cardiac Surgery. Ann. Thorac. Surg. 2020, 109, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leech, M.; Bissett, B.; Kot, M.; Ntoumenopoulos, G. Lung ultrasound for critical care physiotherapists: A narrative review. Physiother. Res. Int. 2015, 20, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.; Mumoli, N.; Giorgi-Pierfranceschi, M.; Cresci, A.; Cei, M.; Basile, V.; Cocciolo, M.; Dentali, F. Comparison of the Accuracy of Nurse-Performed and Physician-Performed Lung Ultrasound in the Diagnosis of Cardiogenic Dyspnea. Chest 2016, 150, 470–471. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Hou, Q.; Lu, Y.; Bai, J.; Sun, L.; Huang, Y.; Zhang, M.; Zheng, J. Feasibility of lung ultrasound to assess pulmonary overflow in congenital heart disease children. Pediatr. Pulmonol. 2018, 53, 1525–1532. [Google Scholar] [CrossRef]
- Rodríguez-Fanjul, J.; Llop, A.S.; Balaguer, M.; Bautista-Rodriguez, C.; Hernando, J.M.; Jordan, I. Usefulness of Lung Ultrasound in Neonatal Congenital Heart Disease (LUSNEHDI): Lung Ultrasound to Assess Pulmonary Overflow in Neonatal Congenital Heart Disease. Pediatr. Cardiol. 2016, 37, 1482–1487. [Google Scholar] [CrossRef]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Buda, N.; Kosiak, W.; Wełnicki, M.; Skoczylas, A.; Olszewski, R.; Piotrkowski, J.; Skoczyński, S.; Radzikowska, E.; Jassem, E.; Grabczak, E.M. Recommendations for Lung Ultrasound in Internal Medicine. Diagnostics 2020, 10, 597. [Google Scholar] [CrossRef]
- Raimondi, F.; Migliaro, F.; Sodano, A.; Umbaldo, A.; Romano, A.; Vallone, G.; Capasso, L. Can neonatal ultrasound monitor fluid clearence and preditc the need of respiratory support? Crit. Care 2012, 16, R220. [Google Scholar] [CrossRef] [Green Version]
- Vignon, P.; Chastagner, C.; Berkane, V.; Chardac, E.; François, B.; Normand, S.; Bonnivard, M.; Clavel, M.; Pichon, N.; Preux, P.-M.; et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit. Care Med. 2005, 33, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Remérand, F.; Dellamonica, J.; Mao, Z.; Ferrari, F.; Bouhemad, B.; Jianxin, Y.; Arbelot, C.; Lu, Q.; Ichaï, C.; Rouby, J.J. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010, 36, 656–664. [Google Scholar] [CrossRef]
- Balik, M.; Plasil, P.; Waldauf, P.; Pazout, J.; Fric, M.; Otahal, M.; Pachl, J. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006, 32, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Usta, E.; Mustafi, M.; Ziemer, G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 204–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eibenberger, K.; Dock, W.; Ammann, M. Quantification of pleural effusions: Sonography versus radiography. Radiology 1994, 191, 681–684. [Google Scholar] [CrossRef]
- Lorenz, J.; Börner, N.; Nikolaus, H.P. Sonographic volumetry of pleural effusions. Ultraschall Med. 1988, 9, 212–215. [Google Scholar] [CrossRef]
- Yang, P.C.; Luh, K.T.; Chang, D.B.; Wu, H.D.; Yu, C.J.; Kuo, S.H. Value of sonography in determining the nature of pleural effusion: Analysis of 320 cases. AJR Am. J. Roentgenol. 1992, 159, 29–33. [Google Scholar] [CrossRef]
- Hosokawa, T.; Shibuki, S.; Tanami, Y.; Sato, Y.; Ko, Y.; Nomura, K.; Oguma, E. Extracardiac Complications in Intensive Care Units after Surgical Repair for Congenital Heart Disease: Imaging Review with a Focus on Ultrasound and Radiography. J. Pediatr. Intensive Care 2021, 10, 85–105. [Google Scholar] [CrossRef]
- Kane, J.M.; Friedman, M.; Mitchell, J.B.; Wang, D.; Huang, Z.; Backer, C.L. Association between postoperative Fever and atelectasis in pediatric patients. World J. Pediatr. Congenit. Heart Surg. 2011, 2, 359–363. [Google Scholar] [CrossRef]
- Acosta, C.M.; Maidana, G.A.; Jacovitti, D.; Belaunzarán, A.; Cereceda, S.; Rae, E.; Molina, A.; Gonorazky, S.; Bohm, S.H.; Tusman, G. Accuracy of transthoracic lung ultrasound for diagnosing anesthesia-induced atelectasis in children. Anesthesiology 2014, 120, 1370–1379. [Google Scholar] [CrossRef] [Green Version]
- Östberg, E.; Auner, U.; Enlund, M.; Zetterström, H.; Edmark, L. Minimizing atelectasis formation during general anaesthesia-oxygen washout is a non-essential supplement to PEEP. Ups. J. Med. Sci. 2017, 122, 92–98. [Google Scholar] [CrossRef]
- Monastesse, A.; Girard, F.; Massicotte, N.; Chartrand-Lefebvre, C.; Girard, M. Lung ultrasonography for the assessment of perioperative atelectasis: A pilot feasibility study. Anesth. Analg. 2017, 124, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, L.; Spahn, D.R. New concepts of atelectasis during general anaesthesia. Br. J. Anaesth. 2003, 91, 61–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Hou, Q.; Bai, J.; Zhang, J.; Sun, L.; Tan, R.; Zhang, M.; Zheng, J. Modified Lung Ultrasound Examinations in Assessment and Monitoring of Positive End-Expiratory Pressure-Induced Lung Reaeration in Young Children with Congenital Heart Disease Under General Anesthesia. Pediatr. Crit. Care Med. 2019, 20, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H.; Yu, N.; Wang, Y.H.; Gao, Y.B.; Pan, L. Lung ultrasound vs. chest radiography in the diagnosis of children pneumonia: Systematic evidence. Medicine 2020, 99, e23671. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Boero, E.; Sverzellati, N.; Cardinale, L.; Busso, M.; Boccuzzi, F.; Tullio, M.; Lamorte, A.; Stefanone, V.; Ferrari, G.; et al. Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Med. 2014, 40, 1460–1467. [Google Scholar] [CrossRef]
- Eisenberg, R.L.; Khabbaz, K.R. Are chest radiographs routinely indicated after chest tube removal following cardiac surgery? AJR Am. J. Roentgenol. 2011, 197, 122–124. [Google Scholar] [CrossRef]
- Urvoas, E.; Pariente, D.; Fausser, C.; Lipsich, J.; Taleb, R.; Devictor, D. Diaphragmatic paralysis in children: Diagnosis by TM-mode ultrasound. Pediatr. Radiol. 1994, 24, 564–568. [Google Scholar] [CrossRef]
- Joho-Arreola, A.L.; Bauersfeld, U.; Stauffer, U.G.; Baenziger, O.; Bernet, V. Incidence and treatment of diaphragmatic paralysis after cardiac surgery in children. Eur. J. Cardiothorac. Surg. 2005, 27, 53–57. [Google Scholar] [CrossRef]
- Cavanna, L.; Mordenti, P.; Bertè, R.; Palladino, M.A.; Biasini, C.; Anselmi, E.; Seghini, P.; Vecchia, S.; Civardi, G.; di Nunzio, C.; et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: Report on 445 consecutive patients with advanced cancer. World J. Surg. Oncol. 2014, 12, 139. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Tabatabaii, S.A.; Farahbakhsh, N. Role of ultrasound in confirmation of endotracheal tube in neonates: A review. J. Matern. Fetal Neonatal Med. 2019, 32, 1359–1367. [Google Scholar] [CrossRef]
- Jaeel, P.; Sheth, M.; Nguyen, J. Ultrasonography for endotracheal tube position in infants and children. Eur. J. Pediatr. 2017, 176, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Giordano, R.; Gargani, L.; Marchese, P.; Franchi, E.; Koestenberger, M.; Kutty, S.; Ait-Ali, L.; Assanta, N. Could judicious use of lung ultrasound reduce radiographic examinations in pediatric cardiac surgery patients? J. Clin. Anesth. 2020, 61, 109638. [Google Scholar] [CrossRef] [PubMed]
- Kaskinen, A.K.; Martelius, L.; Kirjavainen, T.; Rautiainen, P.; Andersson, S.; Pitkänen, O.M. Assessment of extravascular lung water by ultrasound after congenital cardiac surgery. Pediatr. Pulmonol. 2017, 52, 345–352. [Google Scholar] [CrossRef]
- Fissore, E.; Zieleskiewicz, L.; Markarian, T.; Muller, L.; Duclos, G.; Bourgoin, M.; Michelet, P.; Leone, M.; Claret, P.G.; Bobbia, X. Pneumothorax diagnosis with lung sliding quantification by speckle tracking: A prospective multicentric observational study. Am. J. Emerg. Med. 2021, 49, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Duclos, G.; Bobbia, X.; Markarian, T.; Muller, L.; Cheyssac, C.; Castillon, S.; Resseguier, N.; Boussuges, A.; Volpicelli, G.; Leone, M.; et al. Speckle tracking quantification of lung sliding for the diagnosis of pneumothorax: A multicentric observational study. Intensive Care Med. 2019, 45, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Andronikou, S.; Mentzel, H.J.; Piskunowicz, M.; Squires, J.H.; Barnewolt, C.E. Contrast-enhanced ultrasound of pediatric lungs. Pediatr. Radiol. 2021, 51, 2340–2350. [Google Scholar] [CrossRef]
- Lewińska, A.; Shahnazaryan, K. The Use of Diaphragm Ultrasonography in Pulmonary Physiotherapy of COPD Patients: A Literature Review. J. Clin. Med. 2020, 9, 3525. [Google Scholar] [CrossRef]
- Weber, M.D.; Lim, J.K.B.; Glau, C.; Conlon, T.; James, R.; Lee, J.H. A narrative review of diaphragmatic ultrasound in pediatric critical care. Pediatr. Pulmonol. 2021, 56, 2471–2483. [Google Scholar] [CrossRef]
- Ait-Ali, L.; Andreassi, M.G.; Foffa, I.; Spadoni, I.; Vano, E.; Picano, E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart 2010, 96, 269–274. [Google Scholar] [CrossRef] [Green Version]





| Authors | Classifications |
|---|---|
| Wu (34) |
|
| Cantinotti (12,13) |
|
| Raimondi, Vitale (15,20) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantinotti, M.; Marchese, P.; Giordano, R.; Franchi, E.; Assanta, N.; Jani, V.; Kutty, S.; Gargani, L. Overview of Lung Ultrasound in Pediatric Cardiology. Diagnostics 2022, 12, 763. https://doi.org/10.3390/diagnostics12030763
Cantinotti M, Marchese P, Giordano R, Franchi E, Assanta N, Jani V, Kutty S, Gargani L. Overview of Lung Ultrasound in Pediatric Cardiology. Diagnostics. 2022; 12(3):763. https://doi.org/10.3390/diagnostics12030763
Chicago/Turabian StyleCantinotti, Massimiliano, Pietro Marchese, Raffaele Giordano, Eliana Franchi, Nadia Assanta, Vivek Jani, Shelby Kutty, and Luna Gargani. 2022. "Overview of Lung Ultrasound in Pediatric Cardiology" Diagnostics 12, no. 3: 763. https://doi.org/10.3390/diagnostics12030763
APA StyleCantinotti, M., Marchese, P., Giordano, R., Franchi, E., Assanta, N., Jani, V., Kutty, S., & Gargani, L. (2022). Overview of Lung Ultrasound in Pediatric Cardiology. Diagnostics, 12(3), 763. https://doi.org/10.3390/diagnostics12030763









