The Application of Sonovaginography for Implementing Ultrasound Assessment of Endometriosis and Other Gynaecological Diseases
Abstract
1. Introduction
2. Methods
3. Procedures
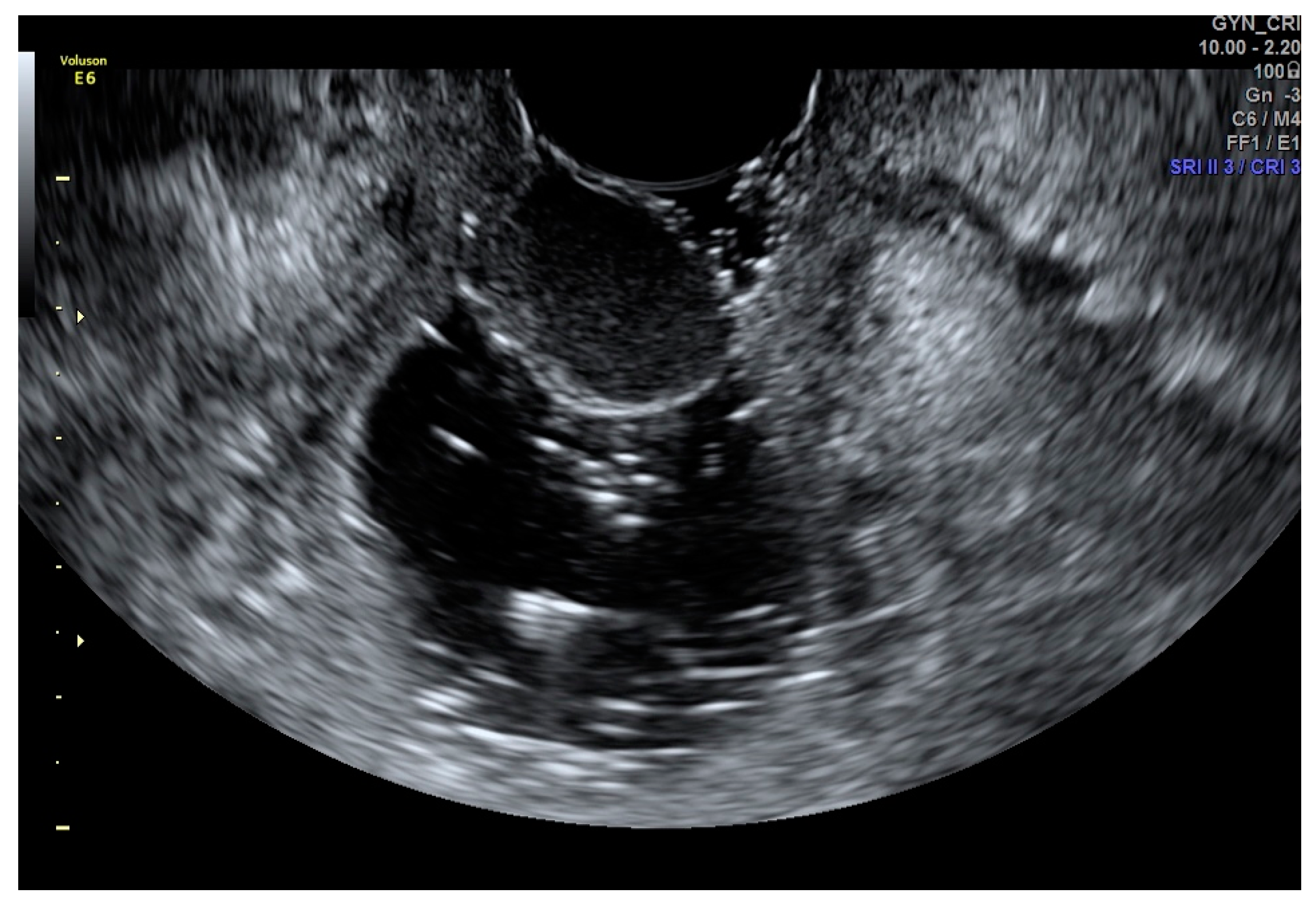
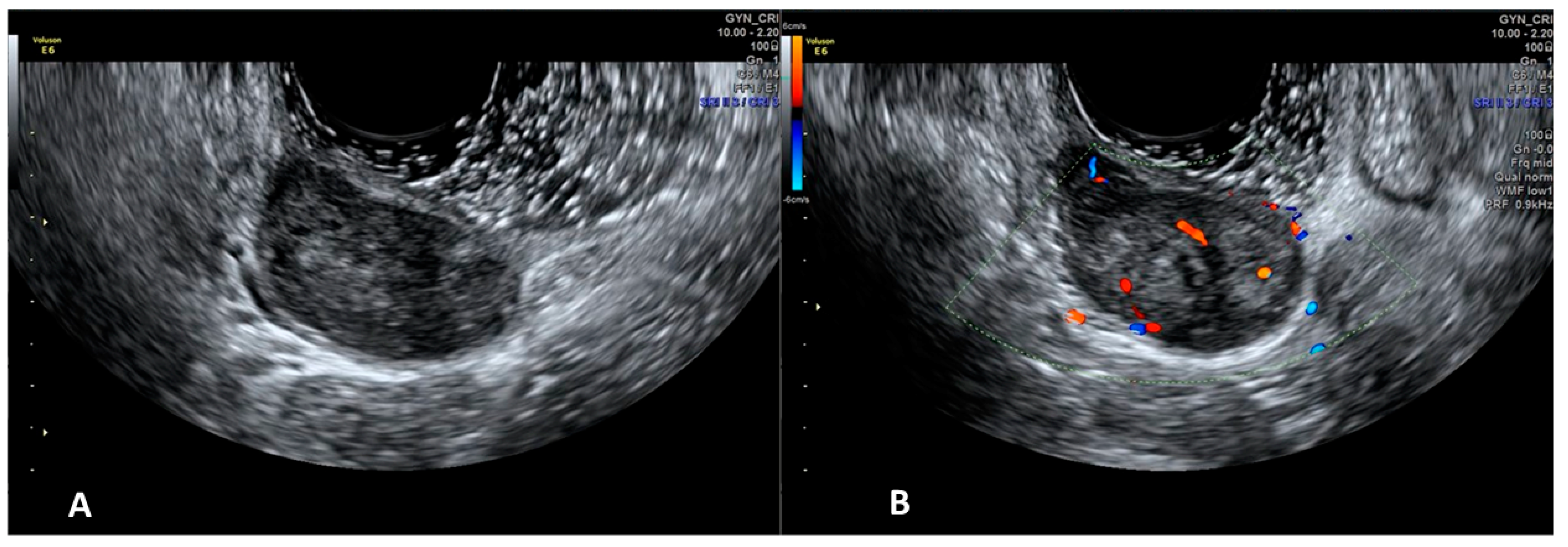
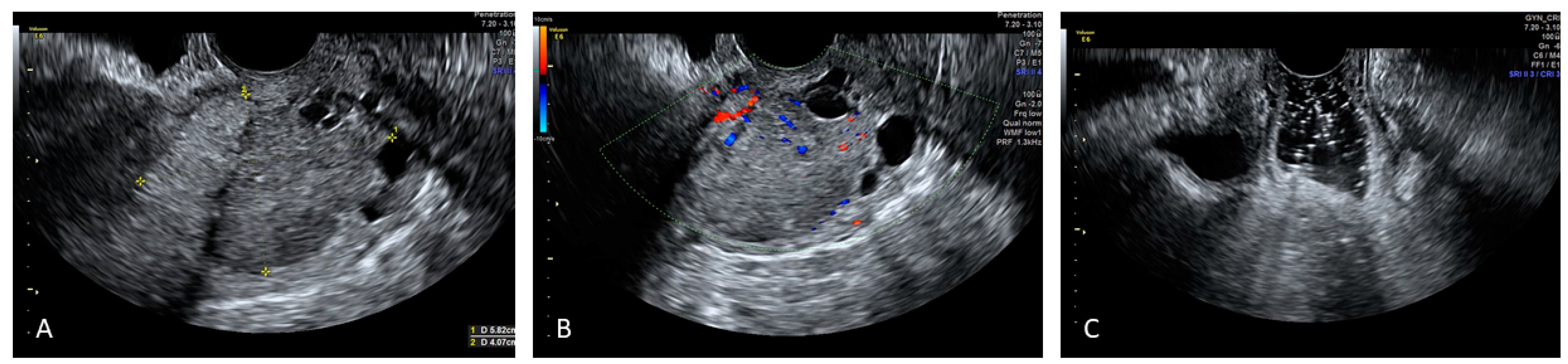
4. Applications of Sonovaginography in the Assessment of Endometriosis
4.1. Other Applications
4.1.1. Applications of Sonovaginography in the Assessment of Vaginal Diseases
4.1.2. Applications of Sonovaginography in the Assessment of Urethral Diseases
4.1.3. Applications of Sonovaginography in the Assessment of Cervical Diseases
4.1.4. Applications of Sonovaginography in the Assessment of Mullerian Anomalies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerrard, E.R., Jr.; Lloyd, L.K.; Kubricht, W.S.; Kolettis, P.N. Transvaginal ultrasound for the diagnosis of urethral diverticulum. J. Urol. 2003, 169, 1395–1397. [Google Scholar] [CrossRef]
- Bates, C.K.; Carroll, N.; Potter, J. The challenging pelvic examination. J. Gen. Intern. Med. 2011, 26, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Sibal, M. Gel Sonovaginography: A New Way of Evaluating a Variety of Local Vaginal and Cervical Disorders. J. Ultrasound Med. 2016, 35, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, M.; Cormio, G.; Giudice, G.; Legge, F.; Resta, L.; Lambo, M.; Leone, L.; Marinaccio, M.; Loizzi, V.; Arezzo, F.; et al. Whose surgery is this? Endometriosis of the round ligament. Acta Biomed. 2022, 92, e2021375. [Google Scholar]
- Bazot, M.; Malzy, P.; Cortez, A.; Roseau, G.; Amouyal, P.; Darai, E. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2007, 30, 994–1001. [Google Scholar] [CrossRef]
- Arezzo, F.; Cazzato, G.; Loizzi, V.; Ingravallo, G.; Resta, L.; Cormio, G. Peritoneal Tuberculosis Mimicking Ovarian Cancer: Gynecologic Ultrasound Evaluation with Histopathological Confirmation. Gastroenterol. Insights 2021, 12, 278–282. [Google Scholar] [CrossRef]
- Brătilă, E.; Comandaşu, D.E.; Coroleucă, C.; Cîrstoiu, M.M.; Berceanu, C.; Mehedintu, C.; Bratila, P.; Vladareanu, S. Diagnosis of endometriotic lesions by sonovaginography with ultrasound gel. Med. Ultrason. 2016, 18, 469–474. [Google Scholar] [CrossRef]
- Arezzo, F.; Franchi, D.; Loizzi, V.; Cataldo, V.; Lombardi, C.; Cazzato, G.; Cormio, G. Blue mass in the pelvis: Serous cystadenofibroma of the peritoneum. Ultrasound Obstet. Gynecol. 2021. published ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dessole, S.; Farina, M.; Rubattu, G.; Cosmi, E.; Ambrosini, G.; Nardelli, G.B. Sonovaginography is a new technique for assessing rectovaginal endometriosis. Fertil. Steril. 2003, 79, 1023–1027. [Google Scholar] [CrossRef]
- Reid, S.; Lu, C.; Hardy, N.; Casikar, I.; Reid, G.; Cario, G.; Chou, D.; Almashat, D.; Condous, G. Office gel sonovaginography for the prediction of posterior deep infiltrating endometriosis: A multicenter prospective observational study. Ultrasound Obstet. Gynecol. 2014, 44, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Saccardi, C.; Cosmi, E.; Borghero, A.; Tregnaghi, A.; Dessole, S.; Litta, P. Comparison between transvaginal sonography, saline contrast sonovaginography and magnetic resonance imaging in the diagnosis of posterior deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2012, 40, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Exacoustos, C.; Zupi, E.; Szabolcs, B.; Amadio, A.; Amoroso, C.; Vaquero, E.; Romanini, M.E.; Arduini, D. OC023: Sonographic evaluation of deep pelvic endometriosis: Endovaginal-, transrectal- and vaginosonography to assess the extension of the disease. Ultrasound Obstet. Gynecol. 2008, 32, 250. [Google Scholar] [CrossRef]
- Reid, S.; Bignardi, T.; Lu, C.; Lam, A.; Condous, G. The use of intra-operative saline sonovaginography to define the rectovaginal septum in women with suspected rectovaginal endometriosis: A pilot study. Australas. J. Ultrasound Med. 2011, 14, 4–9. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Yang, H.; Zhang, H.; Shi, T.; Ren, W. Transvaginal sonographic features of perineal masses in the female lower urogenital tract: A retrospective study of 71 patients. Ultrasound Obstet. Gynecol. 2014, 43, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.M.; Brody, J.M.; Curhan, R.P. Vaginal leiomyoma. A case report with imaging findings. J. Reprod. Med. 1996, 41, 875–877. [Google Scholar] [PubMed]
- Shadbolt, C.L.; Coakley, F.V.; Qayyum, A.; Donat, S.M. MRI of vaginal leiomyomas. J. Comput. Assist. Tomogr. 2001, 25, 355–357. [Google Scholar] [CrossRef]
- Johnson, S.C.; Yegul, N.T.; Balcacer, P. Sonovaginography: A Useful Technique in the Assessment of the Lower Genital Tract. J. Ultrasound Med. 2017, 36, 1917–1933. [Google Scholar] [CrossRef]
- Yang, J.M.; Huang, W.C. The significance of urethral hyperechogenicity in female lower urinary tract symptoms. Ultrasound Obstet. Gynecol. 2004, 24, 67–71. [Google Scholar] [CrossRef]
- O’Hara, S.; Zelesco, M.; Sun, Z. Shear Wave Elastography on the Uterine Cervix: Technical Development for the Transvaginal Approach. J. Ultrasound Med. 2019, 38, 1049–1060. [Google Scholar] [CrossRef]
- Arezzo, F.; Cormio, G.; Loizzi, V.; Cazzato, G.; Cataldo, V.; Lombardi, C.; Ingravallo, G.; Resta, L.; Cicinelli, E. HPV-Negative Cervical Cancer: A Narrative Review. Diagnostics 2021, 11, 952. [Google Scholar] [CrossRef]
- Panageas, E.; Kier, R.; McCauley, T.R.; McCarthy, S. Submucosal uterine leiomyomas: Diagnosis of prolapse into the cervix and vagina based on MR imaging. AJR Am. J. Roentgenol. 1992, 159, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Golan, A.; Zachalka, N.; Lurie, S.; Sagiv, R.; Glezerman, M. Vaginal removal of prolapsed pedunculated submucous myoma: A short, simple, and definitive procedure with minimal morbidity. Arch. Gynecol. Obstet. 2005, 271, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.C.; Di Legge, A.; De Blasis, I.; Moruzzi, M.C.; Bonatti, M.; Collarino, A.; Rufini, V.; Manfredi, R. Imaging techniques for the evaluation of cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 741–768. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Di Legge, A.; Masback, A.; Lindqvist, P.G.; Kannisto, P.; Testa, A.C. Sonographic characteristics of squamous cell cancer and adenocarcinoma of the uterine cervix. Ultrasound Obstet. Gynecol. 2010, 36, 512–516. [Google Scholar] [CrossRef]
- Epstein, E.; Testa, A.; Gaurilcikas, A.; Di Legge, A.; Ameye, L.; Atstupenaite, V.; Valentini, A.L.; Gui, B.; Wallengren, N.O.; Pudaric, S.; et al. Early-stage cervical cancer: Tumor delineation by magnetic resonance imaging and ultrasound—A European multicenter trial. Gynecol. Oncol. 2013, 128, 449–453. [Google Scholar] [CrossRef]

| Author | Year | Population | Distension Medium | Sites of Endometriosis | TVS Sensitivity | SVG Sensitivity | RMI Sensitivity | RWC-TVS Sensitivity | TVS Specificity | SVG Specificity | RMI Specificity | TVS Accuracy | SVG Accuracy | RWC-TVS Accuracy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dessole et al. [9] | 2003 | 46 | saline solution | RVS | 43.7% | 90.6% | 50% | 85.7% | ||||||
| Brătilă et al. [7] | 2016 | 193 | gel | anterior pelvic compartment | 80.1% | 81.2% | ||||||||
| posterior pelvic compartment | 73.1% | 85.3% | ||||||||||||
| urinary bladder | 65.5% | 67% | ||||||||||||
| Reid et al. [10] | 2014 | 189 | gel | rectosigmoid | 84.6% | 94.7% | ||||||||
| anterior rectal DIE | 72.2% | 92% | ||||||||||||
| PVW | 18.2% | 95% | ||||||||||||
| RVS | 18.2% | 95% | ||||||||||||
| Saccardi et al. [11] | 2012 | 54 | saline solution | vaginal fornix | 94.7% | 73.1% | 97.1% | 94.3% | ||||||
| USL | 88.9% | 66.7% | 95.6% | 95.6% | ||||||||||
| RSV | 80.6% | 83.3% | 100% | 77.8% | ||||||||||
| rectal endometriosis | 66.7% | 66.7% | 93.8% | 95.8% | ||||||||||
| Exacoustos et al. [12] | 2008 | 50 | gel | USL | 88%, | 90% | ||||||||
| distal rectal | ||||||||||||||
| sigmoid wall | ||||||||||||||
| RSV | 67% | 91% | ||||||||||||
| PVW | ||||||||||||||
| Barra et al. [13] | 2021 | 281 | gel | posterior pelvic compartment | 89.4% | 93.8% | 86.8% | 91.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arezzo, F.; Cormio, G.; La Forgia, D.; Kawosha, A.A.; Mongelli, M.; Putino, C.; Silvestris, E.; Oreste, D.; Lombardi, C.; Cazzato, G.; et al. The Application of Sonovaginography for Implementing Ultrasound Assessment of Endometriosis and Other Gynaecological Diseases. Diagnostics 2022, 12, 820. https://doi.org/10.3390/diagnostics12040820
Arezzo F, Cormio G, La Forgia D, Kawosha AA, Mongelli M, Putino C, Silvestris E, Oreste D, Lombardi C, Cazzato G, et al. The Application of Sonovaginography for Implementing Ultrasound Assessment of Endometriosis and Other Gynaecological Diseases. Diagnostics. 2022; 12(4):820. https://doi.org/10.3390/diagnostics12040820
Chicago/Turabian StyleArezzo, Francesca, Gennaro Cormio, Daniele La Forgia, Adam Abdulwakil Kawosha, Michele Mongelli, Carmela Putino, Erica Silvestris, Donato Oreste, Claudio Lombardi, Gerardo Cazzato, and et al. 2022. "The Application of Sonovaginography for Implementing Ultrasound Assessment of Endometriosis and Other Gynaecological Diseases" Diagnostics 12, no. 4: 820. https://doi.org/10.3390/diagnostics12040820
APA StyleArezzo, F., Cormio, G., La Forgia, D., Kawosha, A. A., Mongelli, M., Putino, C., Silvestris, E., Oreste, D., Lombardi, C., Cazzato, G., Cicinelli, E., & Loizzi, V. (2022). The Application of Sonovaginography for Implementing Ultrasound Assessment of Endometriosis and Other Gynaecological Diseases. Diagnostics, 12(4), 820. https://doi.org/10.3390/diagnostics12040820










