The Diagnosis of Small Gastrointestinal Subepithelial Lesions by Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy
Abstract
:1. Introduction
2. Endoscopic Features
3. EUS Imaging
4. Types of SELs
4.1. Mesenchymal Tumors
4.1.1. GISTs
4.1.2. Leiomyomas
4.1.3. Schwannomas
4.2. Neuroendocrine Neoplasm
4.3. Malignant Lymphoma
4.4. Ectopic Pancreas
4.5. Lipomas
4.6. Varices
4.7. Glomus Tumors
4.8. Metastatic Malignant Tumor
5. Indications
6. Clinical Course
7. Diagnostic Yield of EUS-FNA/B
8. Comparison with Various Biopsy Methods
9. Treatment
10. Follow-Up
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.H.; Saunders, M.D.; Rulyak, S.J.; Shaw, S.; Nietsch, H.; Kimmey, M.B. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest. Endosc. 2005, 62, 202–208. [Google Scholar] [CrossRef]
- Polkowski, M.; Butruk, E. Submucosal lesions. Gastrointest. Endosc. Clin. N. Am. 2005, 15, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Nickl, N. Endoscopic approach to gastrointestinal stromal tumors. Gastrointest. Endosc. Clin. N. Am. 2005, 15, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy 2005, 37, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Goto, O.; Raut, C.P.; Yahagi, N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016, 122, 3110–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.C.; Li, Q.L.; Yu, Y.F.; Yao, L.Q.; Xu, M.D.; Zhang, Y.Q.; Zhong, Y.S.; Chen, W.F.; Zhou, P.H. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: A meta-analysis. Surg. Endosc. 2016, 30, 2431–2441. [Google Scholar] [CrossRef]
- Attila, T.; Aydin, O. Lesion size determines diagnostic yield of EUS-FNA with onsite cytopathologic evaluation for upper gastrointestinal subepithelial lesions. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2018, 29, 436–441. [Google Scholar] [CrossRef]
- Akahoshi, K.; Oya, M.; Koga, T.; Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World J. Gastroenterol. 2018, 24, 2806–2817. [Google Scholar] [CrossRef]
- Yamada, T.; Ichikawa, H. X-ray diagnosis of elevated lesions of the stomach. Radiology 1974, 110, 79–83. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, G.H.; Park, D.Y.; Ahn, S.; Lim, W.; Lee, B.E.; Song, G.A. Endoscopic resection for duodenal subepithelial tumors: A single-center experience. Surg. Endosc. 2017, 31, 1936–1946. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, G.H.; Park, D.Y.; Shin, N.R.; Lee, B.E.; Ryu, D.Y.; Kim, D.U.; Song, G.A. Endoscopic resection of gastrointestinal lipomas: A single-center experience. Surg. Endosc. 2014, 28, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kahng, D.H.; Kim, G.H.; Park, D.Y.; Jeon, M.S.; Yi, J.W.; Choi, Y.Y.; Song, G.A. Endoscopic resection of granular cell tumors in the gastrointestinal tract: A single center experience. Surg. Endosc. 2013, 27, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Park, D.Y.; Kim, S.; Kim, D.H.; Kim, D.H.; Choi, C.W.; Heo, J.; Song, G.A. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J. Gastroenterol. 2009, 15, 3376–3381. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.M.; Kim, G.H.; Park, D.Y.; Shin, N.R.; Ahn, S.; Park, C.H.; Lee, J.S.; Lee, K.J.; Lee, B.E.; Song, G.A. Endosonographic Features of Gastric Schwannoma: A Single Center Experience. Clin. Endosc. 2016, 49, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, G.H.; Park, D.Y.; Shin, N.R.; Cheong, J.H.; Moon, J.Y.; Lee, B.E.; Song, G.A.; Seo, H.I.; Jeon, T.Y. Endosonographic findings of gastric ectopic pancreas: A single center experience. J. Gastroenterol. Hepatol. 2011, 26, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Kida, M.; Kawaguchi, Y.; Miyata, E.; Hasegawa, R.; Kaneko, T.; Yamauchi, H.; Koizumi, S.; Okuwaki, K.; Miyazawa, S.; Iwai, T.; et al. Endoscopic ultrasonography diagnosis of subepithelial lesions. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 431–443. [Google Scholar] [CrossRef]
- Sekine, M.; Imaoka, H.; Mizuno, N.; Hara, K.; Hijioka, S.; Niwa, Y.; Tajika, M.; Tanaka, T.; Ishihara, M.; Ito, S.; et al. Clinical course of gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2015, 27, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Jeon, S.W.; Lee, H.S.; Cho, C.M.; Bae, H.I.; Seo, A.N.; Kweon, O.K. Can contrast-enhanced harmonic endosonography predict malignancy risk in gastrointestinal subepithelial tumors? Endosc. Ultrasound 2016, 5, 384–389. [Google Scholar] [CrossRef] [Green Version]
- Ignee, A.; Jenssen, C.; Hocke, M.; Dong, Y.; Wang, W.P.; Cui, X.W.; Woenckhaus, M.; Iordache, S.; Saftoiu, A.; Schuessler, G.; et al. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc. Ultrasound 2017, 6, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Kitano, M.; Matsui, S.; Kamata, K.; Komaki, T.; Imai, H.; Dote, K.; Kudo, M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest. Endosc. 2011, 73, 227–237. [Google Scholar] [CrossRef]
- Pidhorecky, I.; Cheney, R.T.; Kraybill, W.G.; Gibbs, J.F. Gastrointestinal stromal tumors: Current diagnosis, biologic behavior, and management. Ann. Surg. Oncol. 2000, 7, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Sarlomo-Rikala, M.; Lasota, J. Gastrointestinal stromal tumors: Recent advances in understanding of their biology. Hum. Pathol. 1999, 30, 1213–1220. [Google Scholar] [CrossRef]
- West, R.B.; Corless, C.L.; Chen, X.; Rubin, B.P.; Subramanian, S.; Montgomery, K.; Zhu, S.; Ball, C.A.; Nielsen, T.O.; Patel, R.; et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am. J. Pathol. 2004, 165, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Kim, E.Y.; Cho, J.W.; Jeon, S.W.; Kim, J.H.; Kim, T.H.; Moon, J.S.; Kim, J.O.; The Research Group for Endoscopic Ultrasound of the Korean Society of Gastrointestinal Endoscopy. Predictive Factors for Differentiating Gastrointestinal Stromal Tumors from Leiomyomas Based on Endoscopic Ultrasonography Findings in Patients with Gastric Subepithelial Tumors: A Multicenter Retrospective Study. Clin. Endosc. 2021, 54, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Luinetti, O.; Cornaggia, M.; Capella, C.; Solcia, E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: A clinicopathologic study. Gastroenterology 1993, 104, 994–1006. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Zucca, E. Primary gastric lymphoma pathogenesis and treatment: What has changed over the past 10 years? Br. J. Haematol. 2007, 136, 521–538. [Google Scholar] [CrossRef]
- Al-Akwaa, A.M.; Siddiqui, N.; Al-Mofleh, I.A. Primary gastric lymphoma. World J. Gastroenterol. 2004, 10, 5–11. [Google Scholar] [CrossRef]
- Lee, M.J.; Chang, J.H.; Maeng, I.H.; Park, J.Y.; Im, Y.S.; Kim, T.H.; Han, S.W.; Lee, D.S. Ectopic pancreas bleeding in the jejunum revealed by capsule endoscopy. Clin. Endosc. 2012, 45, 194–197. [Google Scholar] [CrossRef]
- Matsuki, M.; Gouda, Y.; Ando, T.; Matsuoka, H.; Morita, T.; Uchida, N.; Kuriyama, S. Adenocarcinoma arising from aberrant pancreas in the stomach. J. Gastroenterol. 2005, 40, 652–656. [Google Scholar] [CrossRef]
- Cazacu, I.M.; Luzuriaga Chavez, A.A.; Nogueras Gonzalez, G.M.; Saftoiu, A.; Bhutani, M.S. Malignant Transformation of Ectopic Pancreas. Dig. Dis. Sci. 2019, 64, 655–668. [Google Scholar] [CrossRef]
- Hwang, J.H.; Rulyak, S.D.; Kimmey, M.B. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006, 130, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.Q.; Yang, J.; Zhang, F.F.; Cui, Y.; Han, A.J. Clinicopathological features of gastric glomus tumor. World J. Gastroenterol. 2010, 16, 4616–4620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Yuan, J.; Shi, H.Y. Features of gastric glomus tumor: A clinicopathologic, immunohistochemical and molecular retrospective study. Int. J. Clin. Exp. Pathol. 2014, 7, 1438–1448. [Google Scholar]
- Gheorghe, G.; Bacalbasa, N.; Ceobanu, G.; Ilie, M.; Enache, V.; Constantinescu, G.; Bungau, S.; Diaconu, C.C. Gastrointestinal Stromal Tumors-A Mini Review. J. Pers. Med. 2021, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am. J. Surg. Pathol. 2005, 29, 52–68. [Google Scholar] [CrossRef]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, iv267. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Deprez, P.H.; Jenssen, C.; Iglesias-Garcia, J.; Larghi, A.; Vanbiervliet, G.; Aithal, G.P.; Arcidiacono, P.G.; Bastos, P.; Carrara, S.; et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline-Updated January 2017. Endoscopy 2017, 49, 695–714. [Google Scholar] [CrossRef] [Green Version]
- Blackstein, M.E.; Blay, J.Y.; Corless, C.; Driman, D.K.; Riddell, R.; Soulieres, D.; Swallow, C.J.; Verma, S. Gastrointestinal stromal tumours: Consensus statement on diagnosis and treatment. Can. J. Gastroenterol. 2006, 20, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, J.; Oshima, T.; Hori, K.; Tomita, T.; Kim, Y.; Watari, J.; Oh, K.; Hirota, S.; Matsumoto, T.; Miwa, H. Small gastrointestinal stromal tumor of the stomach showing rapid growth and early metastasis to the liver. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2010, 22, 354–356. [Google Scholar] [CrossRef]
- Aso, A.; Ihara, E.; Kubo, H.; Osoegawa, T.; Oono, T.; Nakamura, K.; Ito, T.; Kakeji, Y.; Mikako, O.; Yamamoto, H.; et al. Gastric gastrointestinal stromal tumor smaller than 20 mm with liver metastasis. Clin. J. Gastroenterol. 2013, 6, 29–32. [Google Scholar] [CrossRef]
- Inoue, T.; Okumura, F.; Sano, H.; Mizushima, T.; Tsukamoto, H.; Fujita, Y.; Ibusuki, M.; Kitano, R.; Kobayashi, Y.; Ishii, N.; et al. Impact of endoscopic ultrasound-guided fine-needle biopsy on the diagnosis of subepithelial tumors: A propensity score-matching analysis. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2019, 31, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.S.; Li, Y.; Liu, W.; Yao, M.H.; Khan, N.; Hu, B. Clinical course of suspected small gastrointestinal stromal tumors in the stomach. World J. Gastrointest. Surg. 2020, 12, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O'Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002, 33, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, K.; Sumida, Y.; Matsui, N.; Oya, M.; Akinaga, R.; Kubokawa, M.; Motomura, Y.; Honda, K.; Watanabe, M.; Nagaie, T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J. Gastroenterol. 2007, 13, 2077–2082. [Google Scholar] [CrossRef]
- Mekky, M.A.; Yamao, K.; Sawaki, A.; Mizuno, N.; Hara, K.; Nafeh, M.A.; Osman, A.M.; Koshikawa, T.; Yatabe, Y.; Bhatia, V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest. Endosc. 2010, 71, 913–919. [Google Scholar] [CrossRef]
- Larghi, A.; Fuccio, L.; Chiarello, G.; Attili, F.; Vanella, G.; Paliani, G.B.; Napoleone, M.; Rindi, G.; Larocca, L.M.; Costamagna, G.; et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy 2014, 46, 39–45. [Google Scholar] [CrossRef]
- Akahoshi, K.; Oya, M.; Koga, T.; Koga, H.; Motomura, Y.; Kubokawa, M.; Gibo, J.; Nakamura, K. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J. Gastrointest. Liver Dis. JGLD 2014, 23, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Philipper, M.; Hollerbach, S.; Gabbert, H.E.; Heikaus, S.; Bocking, A.; Pomjanski, N.; Neuhaus, H.; Frieling, T.; Schumacher, B. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy 2010, 42, 300–305. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Fockens, P.; Hawes, R.H. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2012, 10, 697–703. [Google Scholar] [CrossRef]
- Tamura, T.; Yamashita, Y.; Ueda, K.; Kawaji, Y.; Itonaga, M.; Murata, S.I.; Yamamoto, K.; Yoshida, T.; Maeda, H.; Maekita, T.; et al. Rapid On-Site Evaluation by Endosonographers during Endoscopic Ultrasonography-Guided Fine-Needle Aspiration for Diagnosis of Gastrointestinal Stromal Tumors. Clin. Endosc. 2017, 50, 372–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwashita, T.; Nakai, Y.; Mukai, T.; Togawa, O.; Matsubara, S.; Hatano, Y.; Hara, A.; Tanaka, M.; Shibahara, J.; Fukayama, M.; et al. A 19-Gauge Histology Needle Versus a 19-Gauge Standard Needle in Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Solid Lesions: A Multicenter Randomized Comparison Study (GREATER Study). Dig. Dis. Sci. 2018, 63, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Cho, Y.K.; Kim, E.Y.; Kim, H.K.; Cho, J.W.; Lee, T.H.; Moon, J.S.; Korean, E.U.S.S.G. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand. J. Gastroenterol. 2014, 49, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.C.; Smith, P.P.; Faigel, D.O. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest. Endosc. 2003, 57, 68–72. [Google Scholar] [CrossRef]
- Cantor, M.J.; Davila, R.E.; Faigel, D.O. Yield of tissue sampling for subepithelial lesions evaluated by EUS: A comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest. Endosc. 2006, 64, 29–34. [Google Scholar] [CrossRef]
- Uesato, M.; Tamachi, T.; Hanari, N.; Muto, Y.; Kagaya, A.; Urahama, R.; Ogura, Y.; Suito, H.; Nakano, A.; Aikawa, M.; et al. Drill needle aspiration biopsy for submucosal tumors in an experimental study. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2017, 20, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Buscaglia, J.M.; Nagula, S.; Jayaraman, V.; Robbins, D.H.; Vadada, D.; Gross, S.A.; DiMaio, C.J.; Pais, S.; Patel, K.; Sejpal, D.V.; et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest. Endosc. 2012, 75, 1147–1152. [Google Scholar] [CrossRef]
- Iwai, T.; Kida, M.; Imaizumi, H.; Miyazawa, S.; Okuwaki, K.; Yamauchi, H.; Kaneko, T.; Hasegawa, R.; Miyata, E.; Koizumi, W. Randomized crossover trial comparing EUS-guided fine-needle aspiration with EUS-guided fine-needle biopsy for gastric subepithelial tumors. Diagn. Cytopathol. 2018, 46, 228–233. [Google Scholar] [CrossRef]
- Fujita, A.; Ryozawa, S.; Kobayashi, M.; Araki, R.; Nagata, K.; Minami, K.; Tanisaka, Y.; Kobatake, T.; Mizuide, M. Diagnostic ability of a 22G Franseen needle in endoscopic ultrasound-guided fine needle aspiration of subepithelial lesions. Mol. Clin. Oncol. 2018, 9, 527–531. [Google Scholar] [CrossRef]
- Trindade, A.J.; Benias, P.C.; Alshelleh, M.; Bazarbashi, A.N.; Tharian, B.; Inamdar, S.; Sharma, N.; Zelt, C.; Korrapati, P.; Barakat, M.; et al. Fine-needle biopsy is superior to fine-needle aspiration of suspected gastrointestinal stromal tumors: A large multicenter study. Endosc. Int. Open 2019, 7, E931–E936. [Google Scholar] [CrossRef] [Green Version]
- Minoda, Y.; Chinen, T.; Osoegawa, T.; Itaba, S.; Haraguchi, K.; Akiho, H.; Aso, A.; Sumida, Y.; Komori, K.; Ogino, H.; et al. Superiority of mucosal incision-assisted biopsy over ultrasound-guided fine needle aspiration biopsy in diagnosing small gastric subepithelial lesions: A propensity score matching analysis. BMC Gastroenterol. 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekine, M.; Miura, T.; Fujiwara, J.; Uehara, T.; Asano, T.; Matsumoto, S.; Miyatani, H.; Mashima, H. Utility of endoscopic ultrasonography-guided fine-needle biopsy (EUS-FNB) for diagnosing small subepithelial lesions (<20 mm). J. Ultrasound 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sekino, Y.; Hosono, K.; Kawana, K.; Nagase, H.; Kubota, K.; Nakajima, A. Optimal number of needle punctures in endoscopic ultrasound-guided fine-needle biopsy for gastric subepithelial lesions without rapid on-site evaluation. J. Med. Ultrason. 2021, 48, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, C.H.; Park, S.Y.; Cho, E.; Kim, H.S.; Choi, S.K. Diagnostic yields of endoscopic ultrasound-guided fine-needle tissue acquisition according to the gastric location. Medicine 2021, 100, e26477. [Google Scholar] [CrossRef] [PubMed]
- Imazu, H.; Uchiyama, Y.; Kakutani, H.; Ikeda, K.; Sumiyama, K.; Kaise, M.; Omar, S.; Ang, T.L.; Tajiri, H. A prospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol. Res. Pract. 2009, 2009, 546390. [Google Scholar] [CrossRef] [Green Version]
- Affolter, K.E.; Schmidt, R.L.; Matynia, A.P.; Adler, D.G.; Factor, R.E. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: A systematic review and meta-analysis. Dig. Dis. Sci. 2013, 58, 1026–1034. [Google Scholar] [CrossRef]
- Facciorusso, A.; Sunny, S.P.; Del Prete, V.; Antonino, M.; Muscatiello, N. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: A meta-analysis. Gastrointest. Endosc. 2020, 91, 14–22.e12. [Google Scholar] [CrossRef]
- Yamabe, A.; Irisawa, A.; Bhutani, M.S.; Shibukawa, G.; Abe, Y.; Saito, A.; Imbe, K.; Hoshi, K.; Igarashi, R. Usefulness of endoscopic ultrasound-guided fine-needle aspiration with a forward-viewing and curved linear-array echoendoscope for small gastrointestinal subepithelial lesions. Endosc. Int. Open 2015, 3, E161–E164. [Google Scholar] [CrossRef]
- Kaneko, E.; Kumagai, J.; Honda, N.; Nakamura, S.; Kino, I. Evaluation of the new giant-biopsy forceps in the diagnosis of mucosal and submucosal gastric lesions. Endoscopy 1983, 15, 322–326. [Google Scholar] [CrossRef]
- Ihara, E.; Matsuzaka, H.; Honda, K.; Hata, Y.; Sumida, Y.; Akiho, H.; Misawa, T.; Toyoshima, S.; Chijiiwa, Y.; Nakamura, K.; et al. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J. Gastrointest. Endosc. 2013, 5, 191–196. [Google Scholar] [CrossRef]
- Osoegawa, T.; Minoda, Y.; Ihara, E.; Komori, K.; Aso, A.; Goto, A.; Itaba, S.; Ogino, H.; Nakamura, K.; Harada, N.; et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: A randomized cross-over study. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2019, 31, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Takao, T.; Morita, Y.; Tanaka, S.; Toyonaga, T.; Umegaki, E.; Kodama, Y. Reasons for Diagnostic Failure in Forty-Five Consecutive Mucosal Cutting Biopsy Examinations of Gastric Subepithelial Tumors. Clin. Endosc. 2020, 53, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Hirata, Y.; Kawamura, H.; Harada, T.; Hattori, R.; Kumai, D.; Yamamoto, Y.; Kojima, Y.; Ikeuchi, H.; Hayashi, N.; et al. Efficacy of Mucosal Cutting Biopsy for the Histopathological Diagnosis of Gastric Submucosal Tumors. Case Rep. Gastroenterol. 2019, 13, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kobara, H.; Mori, H.; Nishimoto, N.; Fujihara, S.; Nishiyama, N.; Ayaki, M.; Yachida, T.; Matsunaga, T.; Chiyo, T.; Kobayashi, N.; et al. Comparison of submucosal tunneling biopsy versus EUS-guided FNA for gastric subepithelial lesions: A prospective study with crossover design. Endosc. Int. Open 2017, 5, E695–E705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Park, J.C.; Jo, J.H.; Kim, E.H.; Shin, S.K.; Lee, S.K.; Lee, Y.C. Prospective comparative study of endoscopic ultrasonography-guided fine-needle biopsy and unroofing biopsy. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2019, 51, 831–836. [Google Scholar] [CrossRef]
- Abad-Belando, R.; Varas-Lorenzo, M.J.; Pons-Vilardell, C.; Puig-Torrus, X.; Pla-Alcaraz, M.; Monleon-Getino, A.; Sanchez-Vizcaino-Mengual, E. Canalization technique to obtain deep tissue biopsy of gastrointestinal subepithelial tumors as an alternative to conventional known techniques. Endosc. Ultrasound 2018, 7, 184–190. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Kolli, S.; Dhindsa, B.S.; Devani, K.; Ramai, D.; Sayles, H.; Rangray, R.; Bhat, I.; Singh, S.; Adler, D.G. Clinical efficacy and safety of mucosal incision-assisted biopsy for the diagnosis of upper gastrointestinal subepithelial tumors: A systematic review and meta-analysis. Ann. Gastroenterol. 2020, 33, 155–161. [Google Scholar] [CrossRef]
- Nishida, T.; Hirota, S.; Yanagisawa, A.; Sugino, Y.; Minami, M.; Yamamura, Y.; Otani, Y.; Shimada, Y.; Takahashi, F.; Kubota, T.; et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int. J. Clin. Oncol. 2008, 13, 416–430. [Google Scholar] [CrossRef]
- Li, J.; Ye, Y.; Wang, J.; Zhang, B.; Qin, S.; Shi, Y.; He, Y.; Liang, X.; Liu, X.; Zhou, Y.; et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin. J. Cancer Res. 2017, 29, 281–293. [Google Scholar] [CrossRef]
- Standards of Practice, C.; Faulx, A.L.; Kothari, S.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Gurudu, S.R.; et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest. Endosc. 2017, 85, 1117–1132. [Google Scholar] [CrossRef] [Green Version]
- Coe, T.M.; Fero, K.E.; Fanta, P.T.; Mallory, R.J.; Tang, C.M.; Murphy, J.D.; Sicklick, J.K. Population-Based Epidemiology and Mortality of Small Malignant Gastrointestinal Stromal Tumors in the USA. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2016, 20, 1132–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, D.H.; Ryu, M.H.; Kim, K.M.; Yang, H.K.; Sawaki, A.; Hirota, S.; Zheng, J.; Zhang, B.; Tzen, C.Y.; Yeh, C.N.; et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res. Treat. 2016, 48, 1155–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demetri, G.D.; von Mehren, M.; Antonescu, C.R.; DeMatteo, R.P.; Ganjoo, K.N.; Maki, R.G.; Pisters, P.W.; Raut, C.P.; Riedel, R.F.; Schuetze, S.; et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J. Natl. Compr. Cancer Netw. 2010, 8 (Suppl. 2), S1–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Zhou, Y.C.; Mou, Y.P.; Xu, X.W.; Jin, W.W.; Ajoodhea, H. Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg. Endosc. 2015, 29, 355–367. [Google Scholar] [CrossRef]
- Koh, Y.X.; Chok, A.Y.; Zheng, H.L.; Tan, C.S.; Chow, P.K.; Wong, W.K.; Goh, B.K. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann. Surg. Oncol. 2013, 20, 3549–3560. [Google Scholar] [CrossRef]
- Iordanou, C.; Theodoridis, C.A.; Lykoudis, P.M.; Dimitroulis, D.; Machairas, N.; Spartalis, E.; Kouki, P.; Pikoulis, E.; Nikiteas, N. Current evidence on laparoscopic vs. open resection for gastric stromal tumours. Oncol. Lett. 2021, 22, 734. [Google Scholar] [CrossRef]
- Inoue, H.; Ikeda, H.; Hosoya, T.; Onimaru, M.; Yoshida, A.; Eleftheriadis, N.; Maselli, R.; Kudo, S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 2012, 44, 225–230. [Google Scholar] [CrossRef]
- Ye, L.P.; Zhang, Y.; Mao, X.L.; Zhu, L.H.; Zhou, X.; Chen, J.Y. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg. Endosc. 2014, 28, 524–530. [Google Scholar] [CrossRef]
- Bialek, A.; Wiechowska-Kozlowska, A.; Pertkiewicz, J.; Polkowski, M.; Milkiewicz, P.; Karpinska, K.; Lawniczak, M.; Starzynska, T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest. Endosc. 2012, 75, 276–286. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Lien, J.M.; Tsai, M.H.; Chiu, C.T.; Chen, T.C.; Yang, K.C.; Ng, S.C. Modified endoscopic submucosal dissection with enucleation for treatment of gastric subepithelial tumors originating from the muscularis propria layer. BMC Gastroenterol. 2012, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Minami, H.; Kobayashi, Y.; Sato, Y.; Kaga, M.; Suzuki, M.; Satodate, H.; Odaka, N.; Itoh, H.; Kudo, S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010, 42, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Tan, S.; Huang, S.; Ren, Y.; Li, H.; Peng, Y.; Fu, X.; Tang, X. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors with more than 1-year’ follow-up: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2019, 54, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Nunobe, S.; Ohashi, M.; Hiki, N. Laparoscopic endoscopic cooperative surgery (LECS) for the upper gastrointestinal tract. Transl. Gastroenterol. Hepatol. 2017, 2, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiki, N.; Nunobe, S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann. Gastroenterol. Surg. 2019, 3, 239–246. [Google Scholar] [CrossRef]
- Hsiao, S.W.; Chen, M.W.; Yang, C.W.; Lin, K.H.; Chen, Y.Y.; Kor, C.T.; Huang, S.P.; Yen, H.H. A Nomogram for Predicting Laparoscopic and Endoscopic Cooperative Surgery during the Endoscopic Resection of Subepithelial Tumors of the Upper Gastrointestinal Tract. Diagnostics 2021, 11, 2160. [Google Scholar] [CrossRef]
- Agaimy, A.; Wunsch, P.H.; Hofstaedter, F.; Blaszyk, H.; Rummele, P.; Gaumann, A.; Dietmaier, W.; Hartmann, A. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am. J. Surg. Pathol. 2007, 31, 113–120. [Google Scholar] [CrossRef]
- Kawanowa, K.; Sakuma, Y.; Sakurai, S.; Hishima, T.; Iwasaki, Y.; Saito, K.; Hosoya, Y.; Nakajima, T.; Funata, N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum. Pathol. 2006, 37, 1527–1535. [Google Scholar] [CrossRef]
- Landi, B.; Blay, J.Y.; Bonvalot, S.; Brasseur, M.; Coindre, J.M.; Emile, J.F.; Hautefeuille, V.; Honore, C.; Lartigau, E.; Mantion, G.; et al. Gastrointestinal stromal tumours (GISTs): French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2019, 51, 1223–1231. [Google Scholar] [CrossRef]
- Cho, J.W.; Korean ESD Study Group. Current Guidelines in the Management of Upper Gastrointestinal Subepithelial Tumors. Clin. Endosc. 2016, 49, 235–240. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; Xue, Q.; Wang, J.; Shen, Z.; Jiang, K.; Shen, K.; Liang, B.; Yang, X.; Xie, Q.; et al. The cut-off value of tumor size and appropriate timing of follow-up for management of minimal EUS-suspected gastric gastrointestinal stromal tumors. BMC Gastroenterol. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joensuu, H. Gastrointestinal stromal tumors: Risk assessment and adjuvant therapy. Hematol. Oncol. Clin. N. Am. 2013, 27, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.S.; Gonen, M.; Gutierrez, A.; Broto, J.M.; Garcia-del-Muro, X.; Smyrk, T.C.; Maki, R.G.; Singer, S.; Brennan, M.F.; Antonescu, C.R.; et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 2009, 10, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Blay, J.Y.; Kang, Y.K.; Nishida, T.; von Mehren, M. Gastrointestinal stromal tumours. Nat. Rev. Dis. Primers 2021, 7, 22. [Google Scholar] [CrossRef]
- Joensuu, H.; Rutkowski, P.; Nishida, T.; Steigen, S.E.; Brabec, P.; Plank, L.; Nilsson, B.; Braconi, C.; Bordoni, A.; Magnusson, M.K.; et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 634–642. [Google Scholar] [CrossRef]
- Joensuu, H.; Vehtari, A.; Riihimaki, J.; Nishida, T.; Steigen, S.E.; Brabec, P.; Plank, L.; Nilsson, B.; Cirilli, C.; Braconi, C.; et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012, 13, 265–274. [Google Scholar] [CrossRef]
- Nishida, T.; Holmebakk, T.; Raut, C.P.; Rutkowski, P. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann. Surg. Oncol. 2019, 26, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Holmebakk, T.; Bjerkehagen, B.; Boye, K.; Bruland, O.; Stoldt, S.; Sundby Hall, K. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br. J. Surg. 2016, 103, 684–691. [Google Scholar] [CrossRef]
- Casali, P.G.; Le Cesne, A.; Poveda Velasco, A.; Kotasek, D.; Rutkowski, P.; Hohenberger, P.; Fumagalli, E.; Judson, I.R.; Italiano, A.; Gelderblom, H.; et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4276–4283. [Google Scholar] [CrossRef] [Green Version]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Reichardt, A.; Hermes, B.; Schutte, J.; Cameron, S.; Hohenberger, P.; Jost, P.J.; Al-Batran, S.E.; et al. Survival Outcomes Associated With 3 Years vs 1 Year of Adjuvant Imatinib for Patients With High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial After 10-Year Follow-up. JAMA Oncol. 2020, 6, 1241–1246. [Google Scholar] [CrossRef]
- Benjamin, R.S.; Casali, P.G. Adjuvant Imatinib for GI Stromal Tumors: When and For How Long? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Hedenstrom, P.; Andersson, C.; Sjovall, H.; Enlund, F.; Nilsson, O.; Nilsson, B.; Sadik, R. Pretreatment Tumor DNA Sequencing of KIT and PDGFRA in Endosonography-Guided Biopsies Optimizes the Preoperative Management of Gastrointestinal Stromal Tumors. Mol. Diagn. Ther. 2020, 24, 201–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
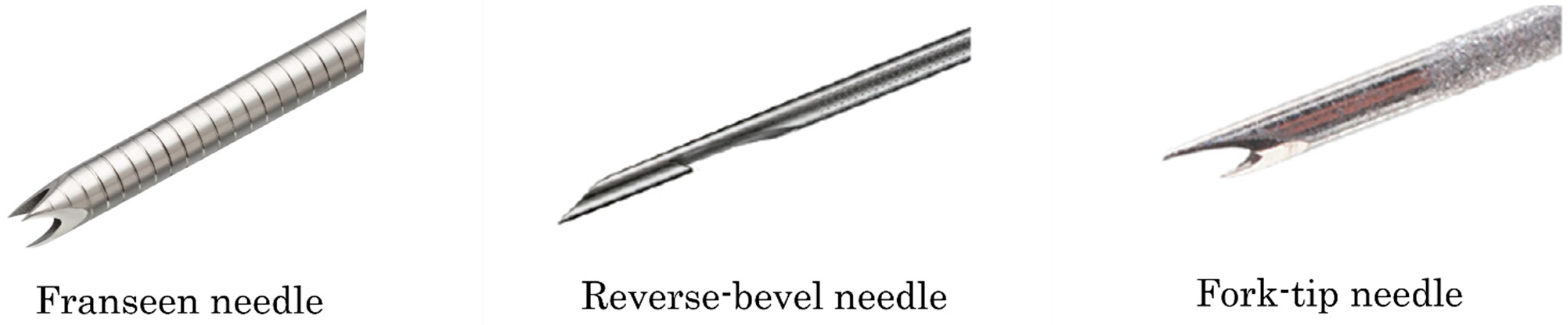
| Author | Year | FNA/FNB | Gauge of Needle | FNB Needle Used | Number of FNA | Number of <20 mm | Number of ≥20 mm | Diagnostic Rate of FNA | Diagnostic Rate of FNA <20 mm | Diagnostic Rate of FNA ≥20 mm | Number of FNB | Number of <20 mm | Number of ≥20 mm | Diagnostic Rate of FNB | Diagnostic Rate of FNB <20 mm | Diagnostic Rate of FNB ≥20 mm | Adverse Event (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akahoshi et al. [42] | 2007 | FNA | 22 | 51 | 21 | 30 | 0.82 | 0.71 | 0.90 | 0 | |||||||
| Kobara et al. [54] | 2017 | FNA | 19/22/25 | 23 | 17 | 6 | 0.35 | 0.35 | 0.33 | 0 | |||||||
| Attila et al. [7] | 2018 | FNA | 22 | 22 | 10 | 12 | 0.73 | 0.50 | 0.92 | 4.5 | |||||||
| Adachi et al. [55] | 2019 | FNA | 22 | 31 | 3 | 28 | 0.81 | 0.67 | 0.82 | 0 | |||||||
| Osoegawa et al. [56] | 2019 | FNA | 20/22/25 | 24 | 13 | 11 | 0.71 | 0.54 | 0.91 | 0 | |||||||
| Park et al. [57] | 2019 | FNB | 20/22 | Reverse-bevel | 28 | 15 | 13 | 0.64 | 0.47 | 0.85 | 0 | ||||||
| Inoue et al. [38] | 2019 | FNB | 19/20/22/25 | Reverse-bevel, Franseen | 57 | 30 | 27 | 0.82 | 0.67 | 0.97 | 3.5 | ||||||
| Iwai et al. [51] | 2018 | FNA/FNB | 19/22 | Reverse-bevel | 12 | 3 | 9 | 0.74 | 0.83 | 0.71 | 11 | 3 | 8 | 0.91 | 0.83 | 0.94 | 0 |
| Fujita et al. [52] | 2018 | FNA/FNB | 22 | Franseen | 44 | 15 | 29 | 0.75 | 0.60 | 0.83 | 17 | 5 | 12 | 0.94 | 1 | 0.92 | 0 |
| Trindade et al. [53] | 2019 | FNA/FNB | 19/22/25 | Fork-tip | 46 | 23 | 23 | 0.37 | 0.39 | 0.35 | 101 | 39 | 62 | 0.89 | 0.82 | 0.94 | 0 |
| Minoda et al. [58] | 2020 | FNA/FNB | 19/20/22/25 | Reverse-bevel, Franseen | 69 | 38 | 31 | 0.74 | 0.68 | 0.80 | 37 | 18 | 19 | 0.89 | 0.78 | 1 | 0 |
| Sekine et al. [59] | 2021 | FNA/FNB | 19/20/22/25 | Reverse-bevel, Franseen | 31 | 11 | 20 | 0.74 | 0.73 | 0.75 | 31 | 13 | 18 | 0.87 | 1 | 0.78 | 3.2 (FNB) |
| Author | Year | Procedure | Number of <20 mm | Number of ≥20 mm | Diagnostic Rate of <20 mm | Diagnostic Rate of ≥20 mm | Adverse Event (%) |
|---|---|---|---|---|---|---|---|
| Ihara et al. [68] | 2013 | MIAB | 15 | 12 | 0.80 | 0.92 | 0 |
| Osoegawa et al. [56] | 2019 | MIAB | 11 | 12 | 0.91 | 0.92 | 0 |
| Minoda et al. [58] | 2020 | MIAB | 45 | 26 | 0.93 | 0.92 | 0 |
| Adachi et al. [55] | 2019 | MCB | 7 | 9 | 0.86 | 0.89 | 0 |
| Nakano et al. [69] | 2019 | MCB | 18 | 27 | 0.72 | 0.81 | 4.4 |
| Kobara et al. [54] | 2017 | STB | 29 | 14 | 1 | 1 | 0 |
| Park et al. [57] | 2019 | unroofing biopsy | 15 | 13 | 0.80 | 0.77 | 0 |
| Abad-Belando et al. [70] | 2018 | deep biopsy | 16 | 16 | 0.94 | 1 | 9.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekine, M.; Asano, T.; Mashima, H. The Diagnosis of Small Gastrointestinal Subepithelial Lesions by Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy. Diagnostics 2022, 12, 810. https://doi.org/10.3390/diagnostics12040810
Sekine M, Asano T, Mashima H. The Diagnosis of Small Gastrointestinal Subepithelial Lesions by Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy. Diagnostics. 2022; 12(4):810. https://doi.org/10.3390/diagnostics12040810
Chicago/Turabian StyleSekine, Masanari, Takeharu Asano, and Hirosato Mashima. 2022. "The Diagnosis of Small Gastrointestinal Subepithelial Lesions by Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy" Diagnostics 12, no. 4: 810. https://doi.org/10.3390/diagnostics12040810
APA StyleSekine, M., Asano, T., & Mashima, H. (2022). The Diagnosis of Small Gastrointestinal Subepithelial Lesions by Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy. Diagnostics, 12(4), 810. https://doi.org/10.3390/diagnostics12040810






