Clinical and Laboratory Characteristics of Normal Weight and Obese Individuals with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
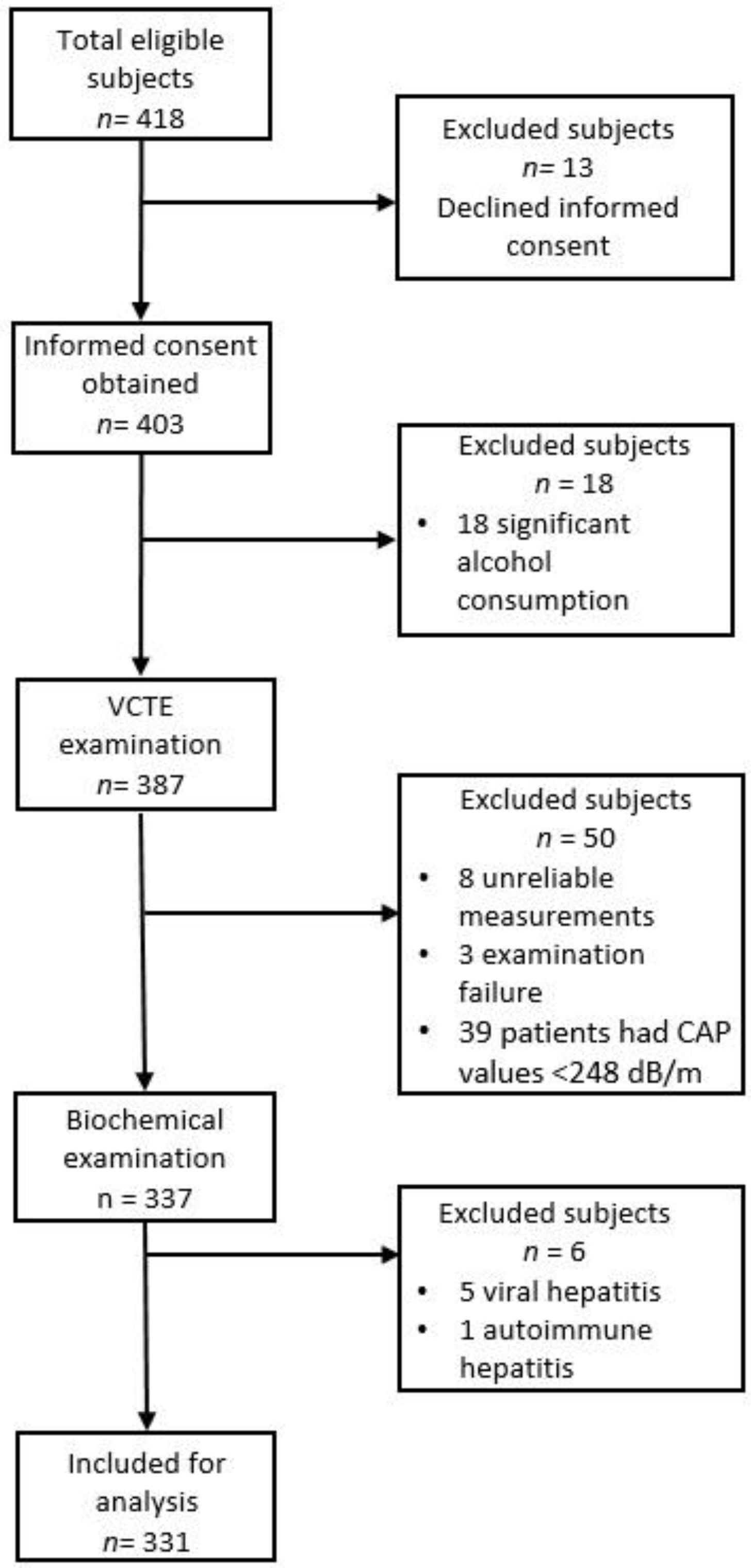
2. Materials and Methods
2.1. Participants
2.2. Liver Stiffness Measurements (LSM) and CAP Assessment
2.3. Anthropometric Measurements
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
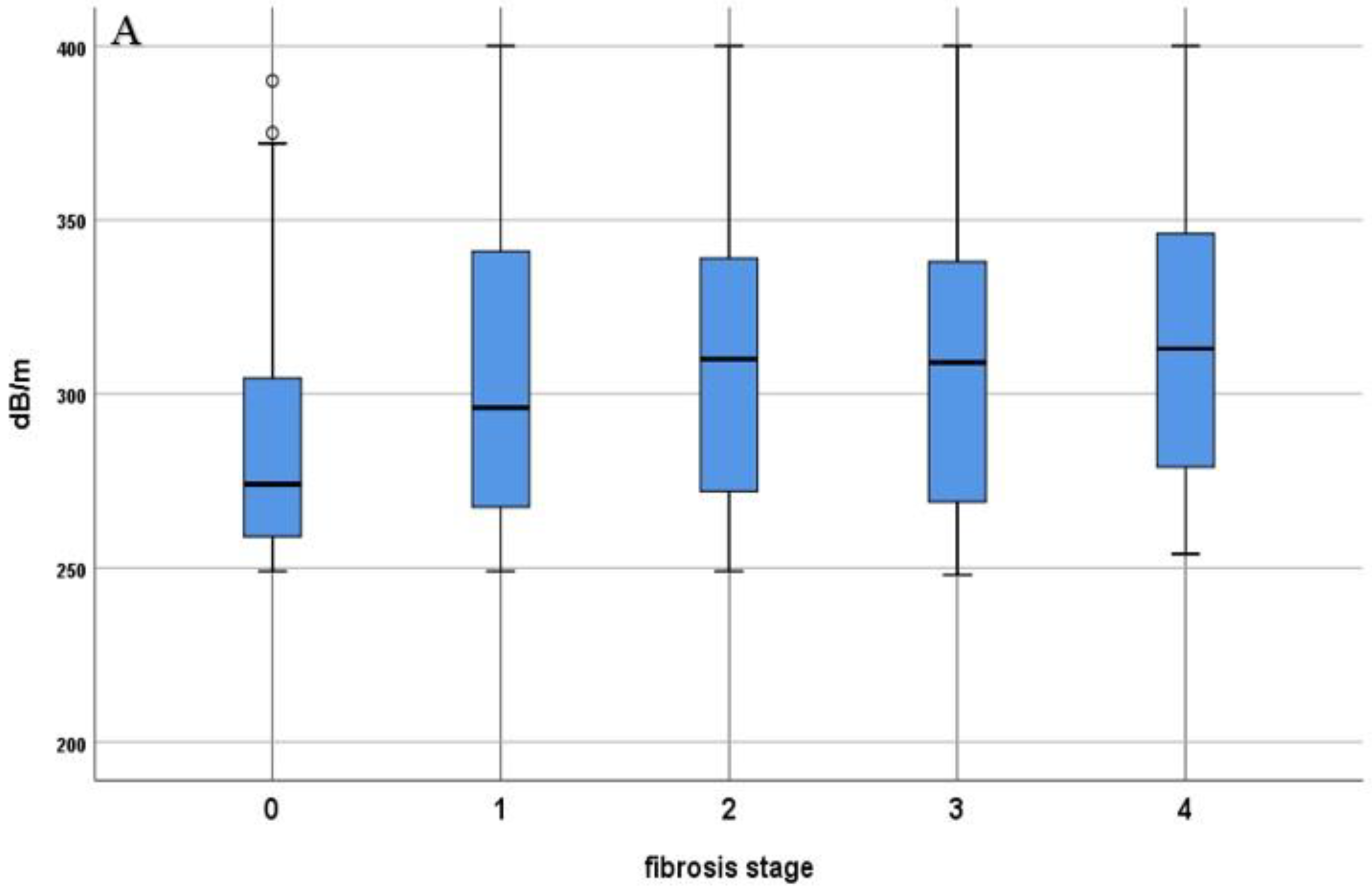
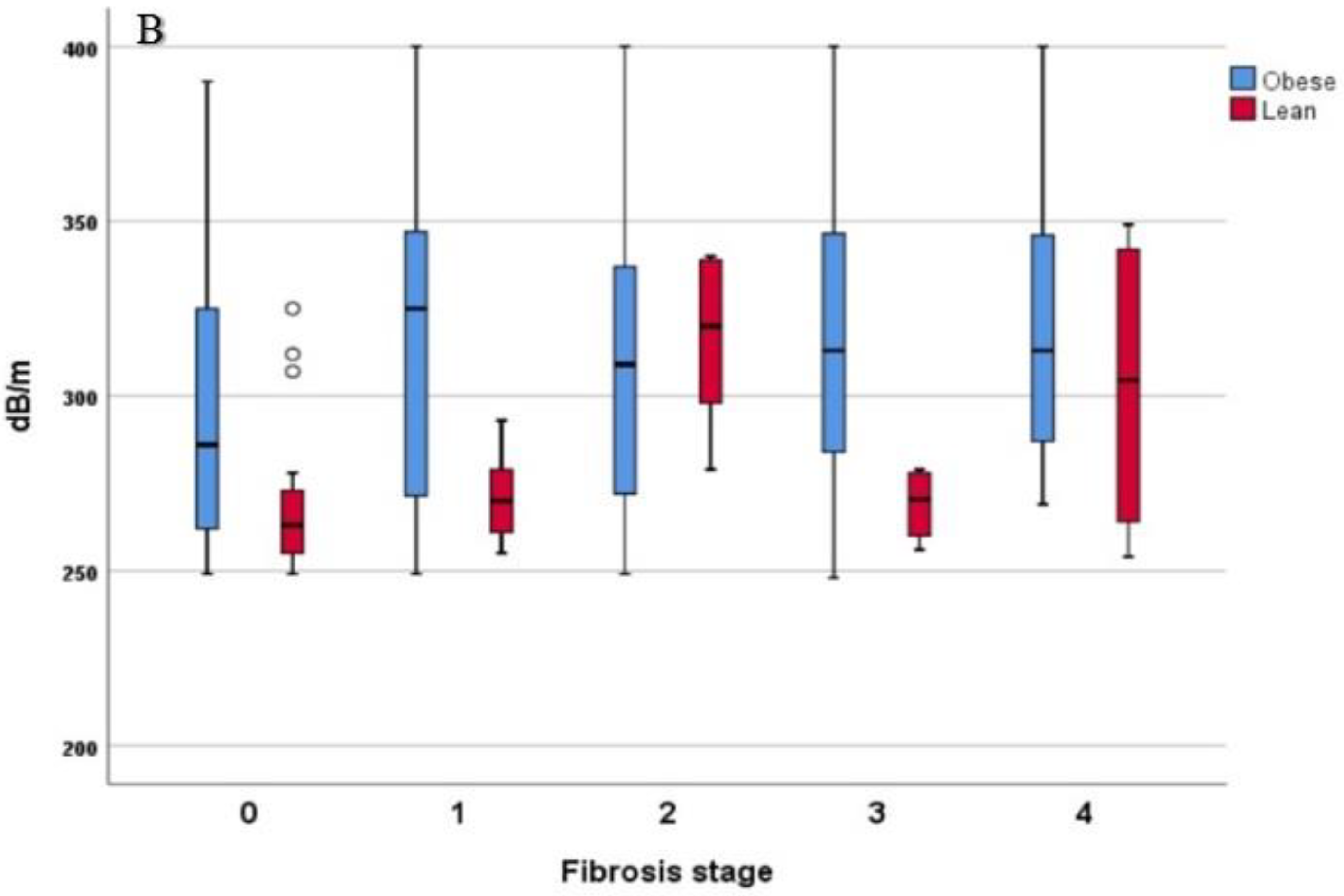
3.2. Comparison of LSM and CAP Values in Lean- and Obese-NAFLD Patients
3.3. Biochemical Profile in Lean versus Obese Subjects
3.4. Factors Associated with Advanced Fibrosis in Lean and Obese Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albhaisi, S.; Chowdhury, A.; Sanyal, A.J. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019, 1, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Ahadi, M.; Molooghi, K.; Masoudifar, N.; Namdar, A.B.; Vossoughinia, H.; Farzanehfar, M. A review of non-alcoholic fatty liver disease in non-obese and lean individuals. J. Gastroenterol. Hepatol. 2021, 36, 1497–1507. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Henry, L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021, 3, 100305. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Navarroza, A.M.C.; Wong, S.N. Comparison of clinical and metabolic profiles of lean versus non-lean nonalcoholic fatty liver disease. Indian J. Gastroenterol. 2021, 40, 380–388. [Google Scholar] [CrossRef]
- Akyuz, U.; Yesil, A.; Yilmaz, Y. Characterization of lean patients with nonalcoholic fatty liver disease: Potential role of high hemoglobin levels. Scand. J. Gastroenterol. 2015, 50, 341–346. [Google Scholar] [CrossRef]
- Bale, G.; Vishnubhotla, R.V.; Mitnala, S.; Sharma, M.; Padaki, R.N.; Pawar, S.C.; Duvvur, R.N. Whole-Exome Sequencing Identifies a Variant in Phosphatidylethanolamine N-Methyltransferase Gene to be Associated With Lean-Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 561–568. [Google Scholar] [CrossRef]
- Tilg, H.; Effenberger, M. From NAFLD to MAFLD: When pathophysiology succeeds. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 387–388. [Google Scholar] [CrossRef]
- Feldman, A.; Eder, S.K.; Felder, T.K.; Kedenko, L.; Paulweber, B.; Stadlmayr, A.; Huber-Schönauer, U.; Niederseer, D.; Stickel, F.; Auer, S.; et al. Clinical and Metabolic Characterization of Lean Caucasian Subjects with Non-alcoholic Fatty Liver. Am. J. Gastroenterol. 2017, 112, 102–110. [Google Scholar] [CrossRef]
- Wattacheril, J.; Sanyal, A.J. Lean NAFLD: An Underrecognized Outlier. Curr. Hepatol. Rep. 2016, 15, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Vos, B.; Moreno, C.; Nagy, N.; Féry, F.; Cnop, M.; Vereerstraeten, P.; Devière, J.; Adler, M. Lean non-alcoholic fatty liver disease (Lean-NAFLD): A major cause of cryptogenic liver disease. Acta Gastroenterol. Belg. 2011, 74, 389–394. [Google Scholar] [PubMed]
- Cigrovski Berkovic, M.; Bilic-Curcic, I.; Mrzljak, A.; Cigrovski, V. NAFLD and Physical Exercise: Ready, Steady, Go! Front. Nutr. 2021, 8, 734859. [Google Scholar] [CrossRef] [PubMed]
- Fracanzani, A.L.; Petta, S.; Lombardi, R.; Pisano, G.; Russello, M.; Consonni, D.; Di Marco, V.; Cammà, C.; Mensi, L.; Dongiovanni, P.; et al. Liver and Cardiovascular Damage in Patients With Lean Nonalcoholic Fatty Liver Disease, and Association With Visceral Obesity. Clin. Gastroenterol. Hepatol. 2017, 15, 1604–1611.e1. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Systematic review with meta-analysis: Risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment. Pharmacol. Ther. 2017, 46, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, R.; Bugianesi, E. NASH in Lean Individuals. Semin. Liver Dis. 2019, 39, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariya, M.; Koohpayeh, F.; Ghaemi, A.; Osati, S.; Davoodi, S.H.; Razzaz, J.M.; Javedan, G.; Ehrampoush, E.; Homayounfar, R. Assessment of the association between body composition and risk of non-alcoholic fatty liver. PLoS ONE 2021, 16, e0249223. [Google Scholar]
- Kaul, S.; Rothney, M.P.; Peters, D.M.; Wacker, W.K.; Davis, C.E.; Shapiro, M.D.; Ergun, D.L. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity 2012, 20, 1313–1318, Erratum in: Obesity 2012, 20, 1544. [Google Scholar] [CrossRef] [Green Version]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Karimi, S.; Sanginabadi, M.; Poustchi, H.; Enayati, S.; Asgarbeik, S.; Nasrollahzadeh, J.; Hekmatdoost, A. Short term effects of coffee components consumption on gut microbiota in patients with non-alcoholic fatty liver and diabetes: A pilot randomized placebo-controlled, clinical trial. EXCLI J. 2020, 19, 241–250. [Google Scholar]
- Wong, V.W.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH—Current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef]
- Piazzolla, V.A.; Mangia, A. Noninvasive Diagnosis of NAFLD and NASH. Cells 2020, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.W.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection (Updated Version). Available online: http://www.who.int/hepatitis/publications/hepatitis-cguidelines-2016/en/ (accessed on 15 December 2021).
- Wang, L.; Guo, J.; Lu, J. Risk factor compositions of nonalcoholic fatty liver disease change with body mass index in males and females. Oncotarget 2016, 7, 35632–35642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendhan, R.; Amutha, A.; Anjana, R.M.; Unnikrishnan, R.; Deepa, M.; Mohan, V. Comparison of characteristics between nonobese and overweight/obese subjects with nonalcoholic fatty liver disease in a South Indian population. Diabetes Technol. Ther. 2014, 16, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Wieland, A.; Cree-Green, M.; Nadeau, K.; Sullivan, S.; Lanaspa, M.A.; Johnson, R.J.; Jensen, T. Lean NAFLD: An underrecognized and challenging disorder in medicine. Rev. Endocr. Metab. Disord. 2021, 22, 351–366. [Google Scholar] [CrossRef]
- Feldman, A.; Wernly, B.; Strebinger, G.; Eder, S.K.; Zandanell, S.; Niederseer, D.; Strasser, M.; Haufe, H.; Sotlar, K.; Paulweber, B.; et al. Liver-related Mortality is Increased in Lean Subjects with Non- alcoholic Fatty Liver Disease Compared to Overweight and Obese Subjects. J. Gastrointest. Liver Dis. 2021, 30, 366–373. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Martínez-Montoro, J.I.; Choudhary, N.S.; Fernández-García, J.C.; Ramos-Molina, B. Non-Alcoholic Fatty Liver Disease in Lean and Non-Obese Individuals: Current and Future Challenges. Biomedicines 2021, 9, 1346. [Google Scholar] [CrossRef]
- Chahal, D.; Sharma, D.; Keshavarzi, S.; Arisar, F.A.Q.; Patel, K.; Xu, W.; Bhat, M. Distinctive clinical and genetic features of lean vs overweight fatty liver disease using the UK Biobank. Hepatol. Int. 2022, 1–12. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denkmayr, L.; Feldman, A.; Stechemesser, L.; Eder, S.K.; Zandanell, S.; Schranz, M.; Strasser, M.; Huber-Schönauer, U.; Buch, S.; Hampe, J.; et al. Lean Patients with Non-Alcoholic Fatty Liver Disease Have a Severe Histological Phenotype Similar to Obese Patients. J. Clin. Med. 2018, 7, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semmler, G.; Wernly, S.; Bachmayer, S.; Wernly, B.; Schwenoha, L.; Huber-Schönauer, U.; Stickel, F.; Niederseer, D.; Aigner, E.; Datz, C. Nonalcoholic Fatty Liver Disease in Lean Subjects: Associations with Metabolic Dysregulation and Cardiovascular Risk-A Single-Center Cross-Sectional Study. Clin. Transl. Gastroenterol. 2021, 12, e00326. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.B.; Zheng, K.I.; Rios, R.S.; Targher, G.; Byrne, C.D.; Zheng, M.H. Global epidemiology of lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2020, 35, 2041–2050. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited after a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Khayyat, Y.M. Determination of “indeterminate score” measurements in lean nonalcoholic fatty liver disease patients from western Saudi Arabia. World J. Hepatol. 2021, 13, 2150–2160. [Google Scholar] [CrossRef]
- Young, S.; Tariq, R.; Provenza, J.; Satapathy, S.K.; Faisal, K.; Choudhry, A.; Friedman, S.L.; Singal, A.K. Prevalence and Profile of Nonalcoholic Fatty Liver Disease in Lean Adults: Systematic Review and Meta-Analysis. Hepatol. Commun. 2020, 4, 953–972. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol. Commun. 2017, 2, 48–57. [Google Scholar] [CrossRef]
- Alsuhaibani, K.A.; Althunayyan, F.S.; Alsudays, A.M.; Alharbi, A.A.; Aljarallah, B.M. Nonalcoholic fatty liver disease in lean and obese patients in Saudi patients from a single center. J. Fam. Med. Prim. Care 2021, 10, 3381–3386. [Google Scholar] [CrossRef]
- Zou, B.; Yeo, Y.H.; Nguyen, V.H.; Cheung, R.; Ingelsson, E.; Nguyen, M.H. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J. Intern. Med. 2020, 288, 139–151. [Google Scholar] [CrossRef]
- Fracanzani, A.L.; Valenti, L.; Bugianesi, E.; Andreoletti, M.; Colli, A.; Vanni, E.; Bertelli, C.; Fatta, E.; Bignamini, D.; Marchesini, G.; et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology 2008, 48, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Burgert, T.S.; Taksali, S.E.; Dziura, J.; Goodman, T.R.; Yeckel, C.W.; Papademetris, X.; Constable, R.T.; Weiss, R.; Tamborlane, W.V.; Savoye, M.; et al. Alanine aminotransferase levels and fatty liver in childhood obesity: Associations with insulin resistance, adiponectin, and visceral fat. J. Clin. Endocrinol. Metab. 2006, 91, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Ulasoglu, C.; Enc, F.Y.; Kaya, E.; Yilmaz, Y. Characterization of Patients with Biopsy-Proven Non-Alcoholic Fatty Liver Disease and Normal Aminotransferase Levels. J. Gastrointest. Liver Dis. 2019, 28, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Di Marco, V.; Pipitone, R.M.; Grimaudo, S.; Buscemi, C.; Craxì, A.; Buscemi, S. Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: Genetic and metabolic risk factors in a general population. Liver Int. 2018, 38, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, H.I.; Akbar, H.O.; Fallatah, A.M. Fibroscan Compared to FIB-4, APRI, and AST/ALT Ratio for Assessment of Liver Fibrosis in Saudi Patients with Nonalcoholic Fatty Liver Disease. Hepat. Mon. 2016, 16, e38346. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Jensen, D.; Hart, J.; Mohanty, S.R. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013, 33, 1398–1405. [Google Scholar] [CrossRef]
- Natarajan, Y.; Kramer, J.R.; Yu, X.; Li, L.; Thrift, A.P.; El-Serag, H.B.; Kanwal, F. Risk of Cirrhosis and Hepatocellular Cancer in Patients with NAFLD and Normal Liver Enzymes. Hepatology 2020, 72, 1242–1252. [Google Scholar] [CrossRef]
| Overall Cohort | Lean | Obese | p-Value | |
|---|---|---|---|---|
| Patients characteristics | n= 331 | n, (%) = 73 (22.1) | n, (%) = 258 (77.9) | |
| Age (years) | 56 ± 13 | 52 ± 15 | 58 ± 11 | 0.001 |
| Height (cm) | 170 ± 9 | 172 ± 8 | 170 ± 9 | 0.076 |
| Weight (kg) | 87 ± 15 | 68 ± 8 | 93 ± 12 | <0.001 |
| Gender female | 143 (43.20%) | 36 (49.32%) | 107 (41.47%) | 0.232 |
| Gender male | 188 (56.80%) | 37 (50.68%) | 151 (58.53%) | 0.158 |
| BMI (kg/m²) | 30 ± 4 | 22 ± 1 | 32 ± 2 | <0.001 |
| LSM (kPa) | 7.40 ± 4.22 | 6.37± 3.36 | 7.69 ± 4.39 | 0.007 |
| CAP (dB/m) | 299 ± 40 | 273 ± 23 | 307 ± 41 | <0.001 |
| Platelet count (109/L) | 246 ± 74 | 238 ± 65 | 248 ± 76 | 0.271 |
| INR | 1.02 ± 0.16 | 1.01 ± 0.17 | 1.03 ± 0.16 | 0.532 |
| CRP (mg/dL) | 0.52 ± 1.50 | 0.38 ± 0.53 | 0.56 ± 1.68 | 0.154 |
| Fasting plasma glucose (mg/dL) | 110 ± 38 | 96 ± 20 | 114 ± 41 | <0.001 |
| Serum urea (mg/dL) | 38 ± 10 | 33 ± 7 | 39 ± 10 | <0.001 |
| Creatinine (mg/dL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.001 |
| ALT (IU/L) | 43 ± 26 | 31 ± 20 | 46 ± 27 | 0.003 |
| AST (IU/L) | 33 ± 19 | 28 ± 14 | 35 ± 20 | 0.003 |
| GGT (IU/L) | 44 ± 37 | 37 ± 31 | 46 ± 38 | 0.037 |
| ALP (IU/L) | 85 ± 31 | 73 ± 21 | 89 ± 33 | <0.001 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.701 |
| Total cholesterol (mg/dL) | 215 ± 39 | 207 ± 38 | 217 ± 40 | 0.047 |
| Triglycerides (mg/dL) | 157 ± 71 | 153 ± 51 | 159 ± 75 | 0.437 |
| Albumin (g/dL) | 4.6 ± 2 | 4.5 ± 0.4 | 4.7 ± 2.3 | 0.297 |
| LDL-c (mg/dL) | 128 ± 39 | 117 ± 41 | 131 ± 38 | 0.013 |
| HDL-c (mg/dL) | 45.13 ±11 | 49.41 ± 10 | 43 ± 11 | <0.001 |
| Serum uric acid (mg/dL) | 4.9 ± 1.4 | 4.2 ± 1.3 | 5.1 ± 1.4 | <0.001 |
| Fibrosis | ||||
| F0, n (%) | 122 (38.86%) | 40 (54.79%) | 82 (31.78%) | <0.001 |
| F1, n (%) | 79 (23.87%) | 16 (21.92%) | 63 (24.42%) | 0.658 |
| F2, n (%) | 63 (19.03%) | 7 (9.59%) | 56 (21.71%) | 0.020 |
| F3, n (%) | 37 (11.18%) | 6 (8.22%) | 31 (12.02%) | 0.363 |
| F4, n (%) | 30 (9.06%) | 4 (5.48%) | 26 (10.08%) | 0.227 |
| Steatosis | ||||
| S1, n (%) | 90 (27.19%) | 36 (49.32%) | 54 (20.93%) | <0.001 |
| S2, n (%) | 62 (18.73%) | 23 (31.51%) | 39 (15.12%) | 0.002 |
| S3, n (%) | 179 (54.08%) | 14 (19.18%) | 165 (63.95%) | <0.001 |
| Hypertension, n (%) | 207 (62.54%) | 30 (41.10%) | 177 (68.60%) | <0.001 |
| T2DM, n (%) | 101 (30.51%) | 14 (19.18%) | 87 (33.72%) | 0.017 |
| Parameter | Obese | Lean | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Age | 0.043 | 0.492 | 0.130 | 0.273 |
| Height | 0.140 | 0.024 | −0.107 | 0.369 |
| Weight | 0.178 | 0.004 | −0.103 | 0.388 |
| BMI | 0.100 | 0.109 | −0.034 | 0.776 |
| Platelet count (G/L) | −0.287 | <0.001 | −0.034 | 0.776 |
| INR | 0.384 | <0.001 | 0.595 | <0.001 |
| CRP (mg/dL) | 0.168 | 0.026 | 0.455 | <0.001 |
| Fasting plasma glucose (mg/dL) | 0.157 | 0.011 | −0.003 | 0.979 |
| Serum urea (mg/dL) | 0.120 | 0.054 | −0.128 | 0.281 |
| Creatinine (mg/dL) | 0.104 | 0.095 | 0.162 | 0.172 |
| ALT (IU/L) | 0.348 | <0.001 | 0.015 | 0.900 |
| AST (IU/L) | 0.370 | <0.001 | 0.273 | 0.019 |
| GGT (IU/L) | 0.426 | <0.001 | 0.109 | 0.361 |
| ALP (IU/L) | 0.238 | <0.001 | 0.055 | 0.644 |
| Total bilirubin (mg/dL) | 0.240 | <0.001 | 0.106 | 0.370 |
| Total cholesterol (mg/dL) | 0.120 | 0.054 | 0.508 | <0.001 |
| Tryglicerides (mg/dL) | 0.007 | 0.910 | 0.381 | 0.001 |
| Albumin (g/dL) | 0.082 | 0.193 | −0.294 | 0.012 |
| LDL-c (mg/dL) | 0.164 | 0.008 | 0.263 | 0.024 |
| HDL-c (mg/dL) | −0.136 | 0.028 | −0.016 | 0.896 |
| Serum uric acid (mg/dL) | 0.216 | <0.001 | 0.134 | 0.257 |
| Lean | Obese | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate | Univariate | Multivariate | ||||
| β | p | β | p | β | p | β | p | |
| Age | 0.126 | 0.287 | −0.045 | 0.473 | ||||
| Gender | 0.085 | 0.474 | −0.164 | 0.008 | −0.014 | 0.878 | ||
| Height | −0.101 | 0.393 | 0.214 | 0.001 | 0.173 | 0.150 | ||
| Weight | −0.055 | 0.643 | 0.196 | 0.002 | 0.039 | 0.690 | ||
| BMI | 0.031 | 0.796 | 0.029 | 0.640 | ||||
| Platelet count (G/L) | −0.372 | 0.001 | −0.204 | 0.042 | −0.230 | <0.001 | −0.109 | 0.031 |
| INR | 0.518 | <0.001 | 0.389 | 0.001 | 0.338 | 0.001 | 0.235 | 0.039 |
| CRP (mg/dL) | 0.256 | 0.029 | −0.025 | 0.813 | 0.008 | 0.900 | ||
| Fasting plasma glucose (mg/dL) | −0.139 | 0.239 | 0.050 | 0.424 | ||||
| Serum urea (mg/dL) | −0.023 | 0.848 | 0.148 | 0.018 | 0.123 | 0.048 | ||
| Creatinine (mg/dL) | 0.132 | 0.265 | 0.072 | 0.248 | ||||
| ALT (IU/L) | 0.106 | 0.373 | 0.283 | <0.001 | 0.182 | 0.042 | ||
| AST (IU/L) | 0.310 | 0.008 | 0.135 | 0.203 | 0.281 | <0.001 | −0.078 | 0.518 |
| GGT (IU/L) | −0.026 | 0.828 | 0.375 | <0.001 | 0.148 | 0.054 | ||
| ALP (IU/L) | 0.044 | 0.711 | 0.224 | <0.001 | 0.024 | 0.729 | ||
| Total bilirubin (mg/dL) | 0.159 | 0.180 | 0.069 | 0.273 | ||||
| Total cholesterol (mg/dL) | 0.264 | 0.024 | 0.135 | 0.030 | −0.055 | 0.419 | ||
| Triglycerides (mg/dL) | 0.268 | 0.022 | 0.256 | 0.013 | 0.026 | 0.677 | ||
| Albumin (g/dL) | −0.222 | 0.059 | −0.075 | 0.228 | ||||
| LDL-c (mg/dL) | 0.073 | 0.541 | 0.151 | 0.015 | ||||
| HDL-c (mg/dL) | 0.094 | 0.429 | −0.133 | 0.032 | 0.019 | 0.774 | ||
| Serum uric acid (mg/dL) | 0.043 | 0.719 | 0.240 | <0.001 | 0.095 | 0.183 | ||
| Hypertension | 0.153 | 0.196 | 0.199 | 0.001 | 0.133 | 0.025 | ||
| T2DM | −0.093 | 0.434 | 0.055 | 0.380 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifan, A.; Rotaru, A.; Stafie, R.; Stratina, E.; Zenovia, S.; Nastasa, R.; Huiban, L.; Cuciureanu, T.; Muzîca, C.; Chiriac, S.; et al. Clinical and Laboratory Characteristics of Normal Weight and Obese Individuals with Non-Alcoholic Fatty Liver Disease. Diagnostics 2022, 12, 801. https://doi.org/10.3390/diagnostics12040801
Trifan A, Rotaru A, Stafie R, Stratina E, Zenovia S, Nastasa R, Huiban L, Cuciureanu T, Muzîca C, Chiriac S, et al. Clinical and Laboratory Characteristics of Normal Weight and Obese Individuals with Non-Alcoholic Fatty Liver Disease. Diagnostics. 2022; 12(4):801. https://doi.org/10.3390/diagnostics12040801
Chicago/Turabian StyleTrifan, Anca, Adrian Rotaru, Remus Stafie, Ermina Stratina, Sebastian Zenovia, Robert Nastasa, Laura Huiban, Tudor Cuciureanu, Cristina Muzîca, Stefan Chiriac, and et al. 2022. "Clinical and Laboratory Characteristics of Normal Weight and Obese Individuals with Non-Alcoholic Fatty Liver Disease" Diagnostics 12, no. 4: 801. https://doi.org/10.3390/diagnostics12040801
APA StyleTrifan, A., Rotaru, A., Stafie, R., Stratina, E., Zenovia, S., Nastasa, R., Huiban, L., Cuciureanu, T., Muzîca, C., Chiriac, S., Gîrleanu, I., Sîngeap, A.-M., Sfarti, C., Cojocariu, C., & Stanciu, C. (2022). Clinical and Laboratory Characteristics of Normal Weight and Obese Individuals with Non-Alcoholic Fatty Liver Disease. Diagnostics, 12(4), 801. https://doi.org/10.3390/diagnostics12040801










