Clinical Applications of Fetal MRI in the Brain
Abstract
:1. Introduction
2. Imaging Modalities
2.1. Fetal Ultrasound
2.2. Fetal MRI
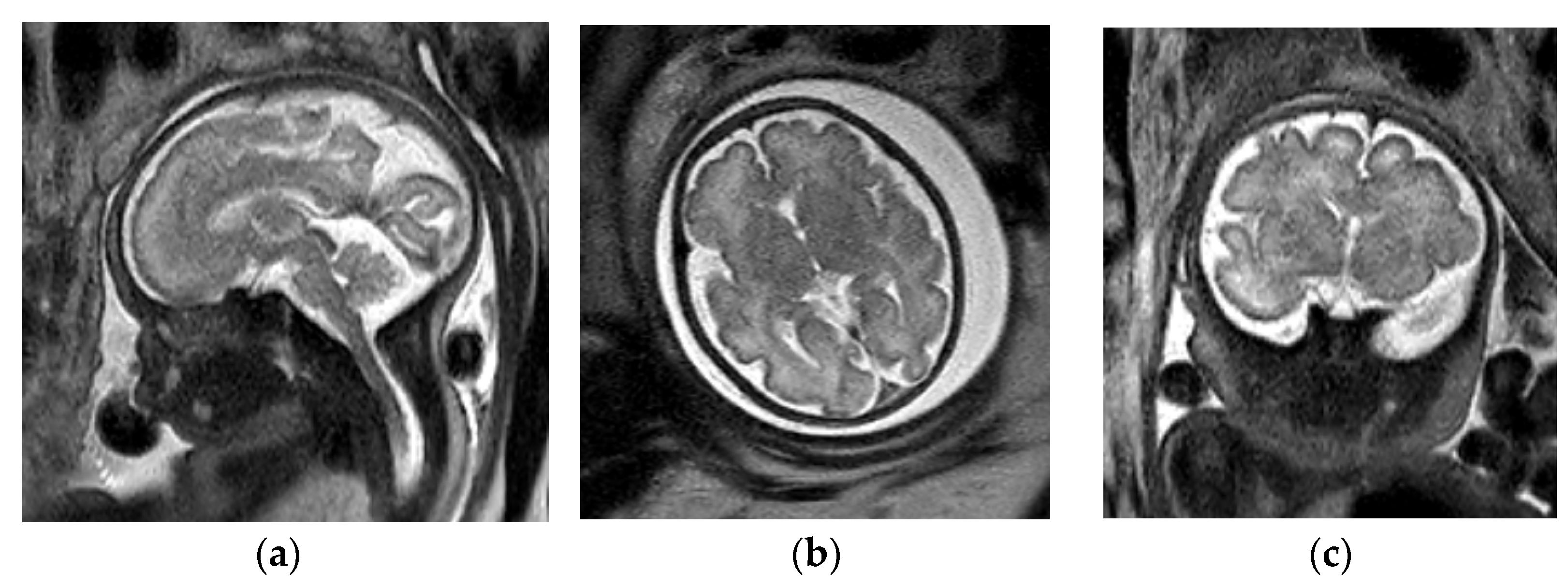
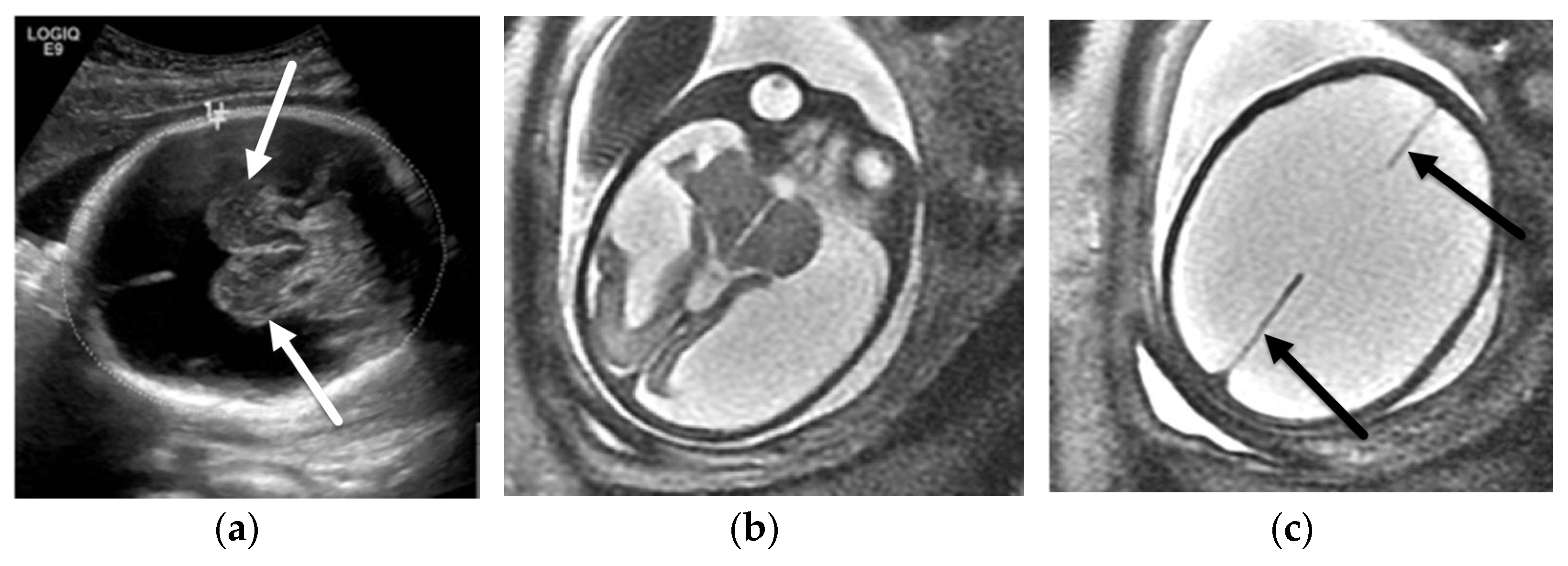
3. Ventriculomegaly
3.1. Obstructive Hydrocephalus
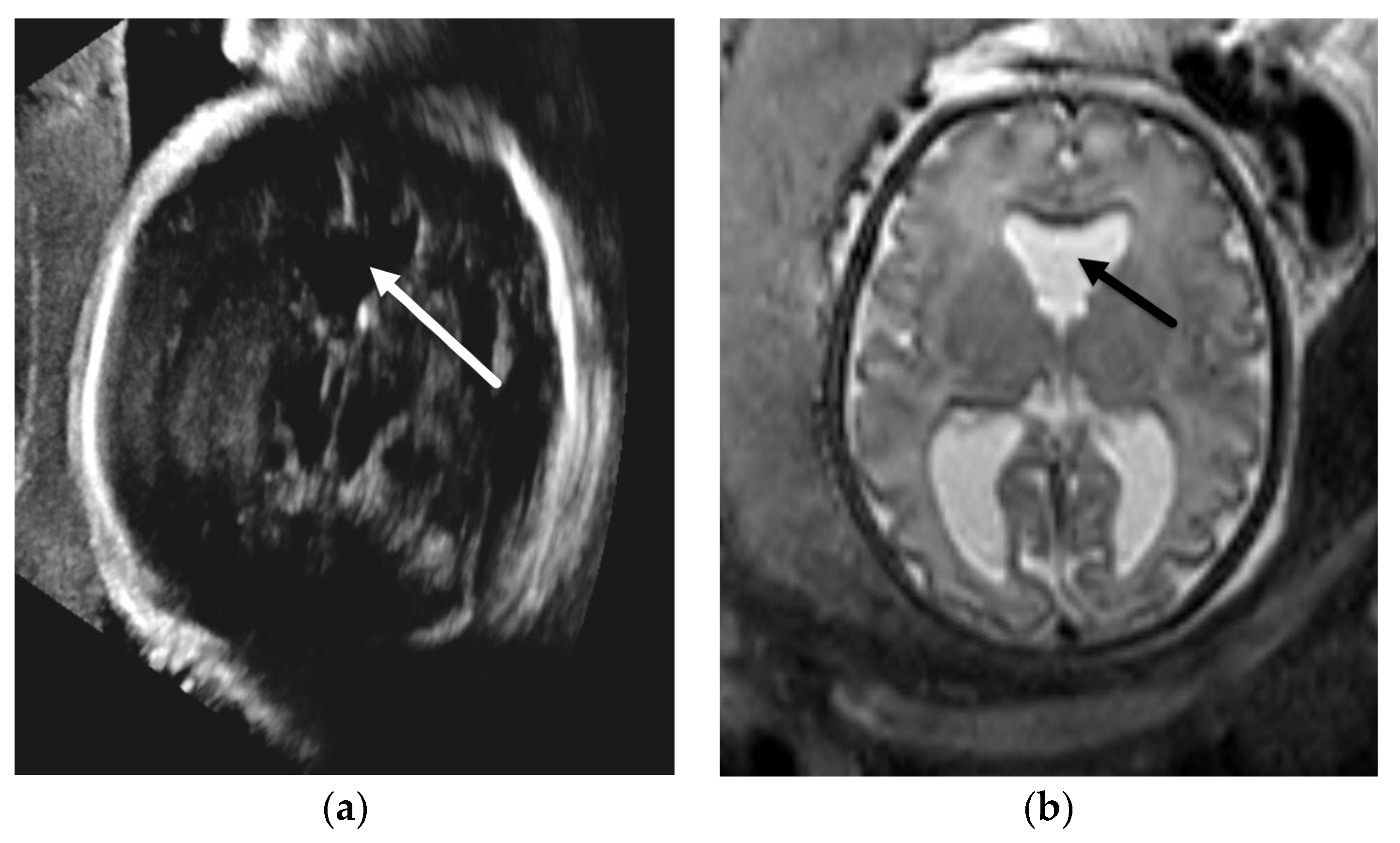
3.1.1. Aqueductal Stenosis
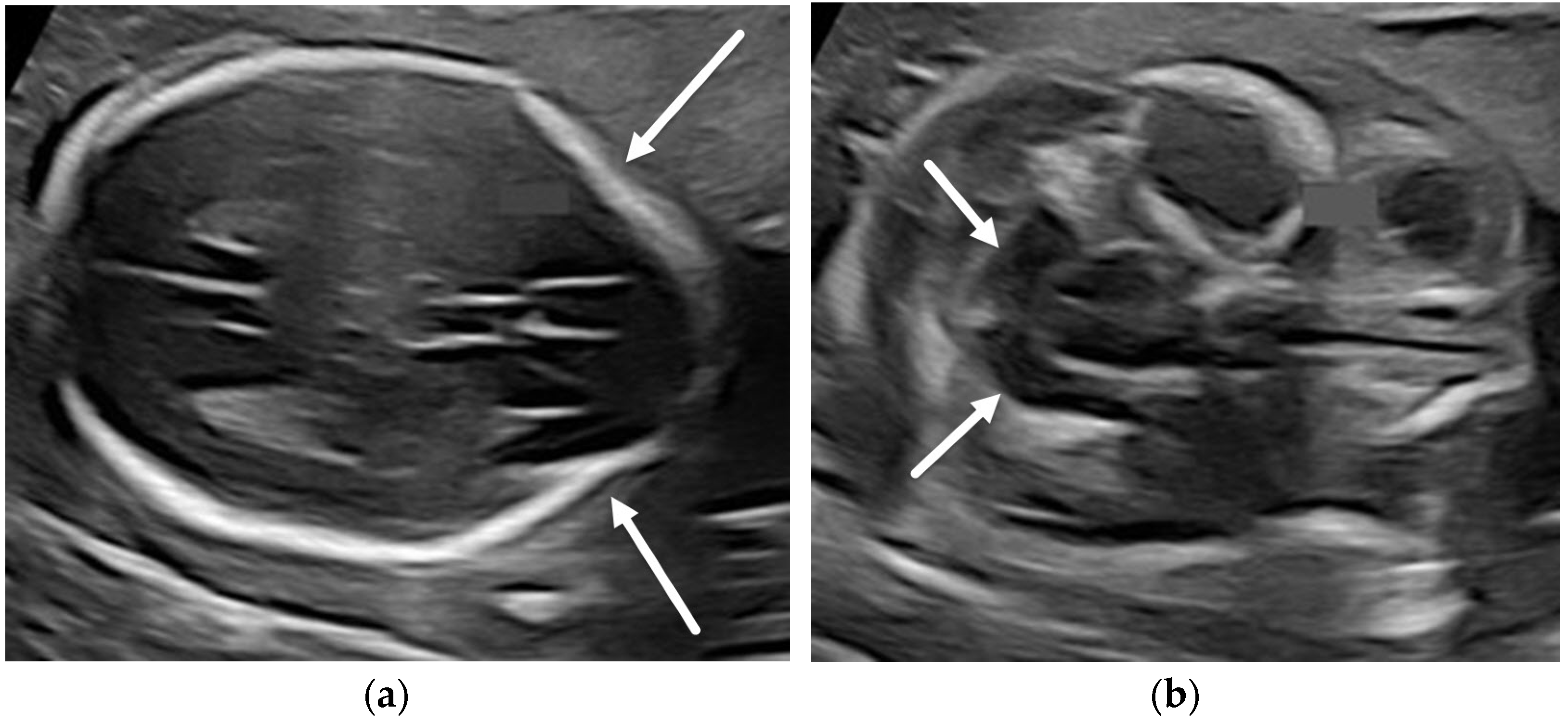
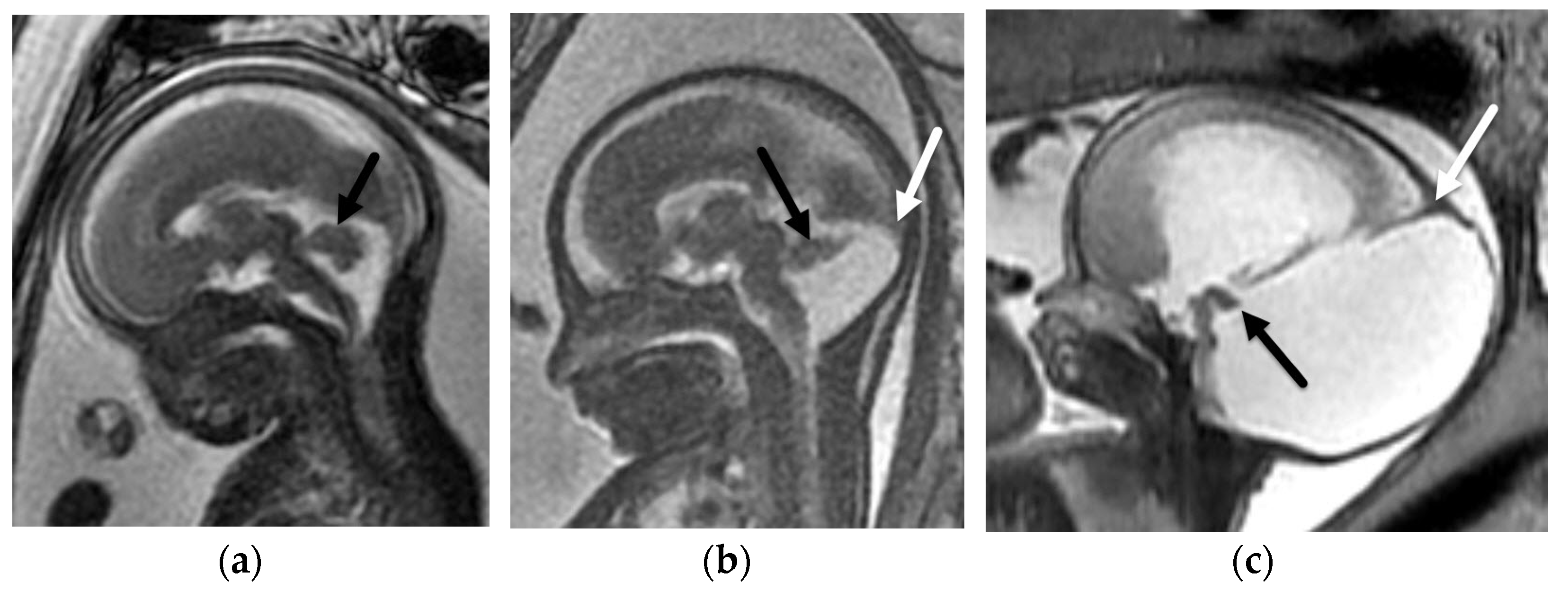
3.1.2. Chiari II Malformation
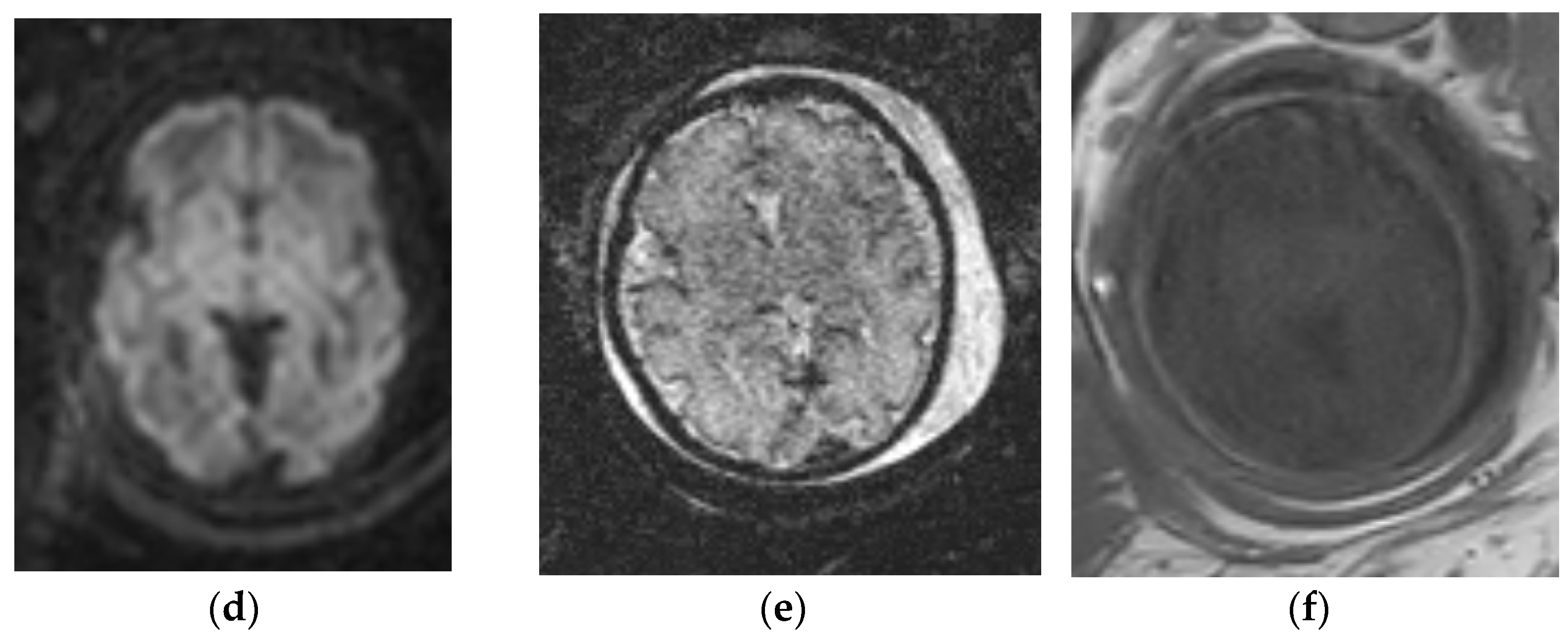
3.2. Decreased Cerebral Volume
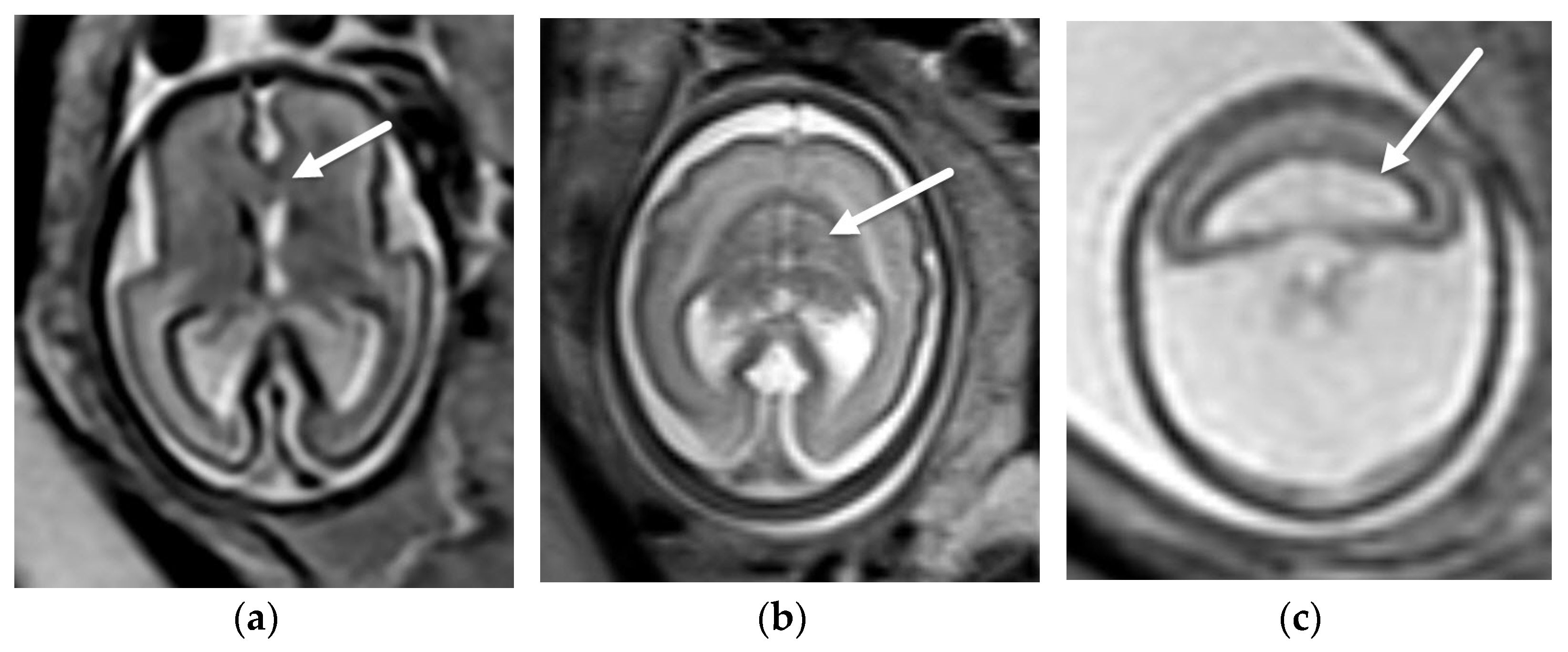
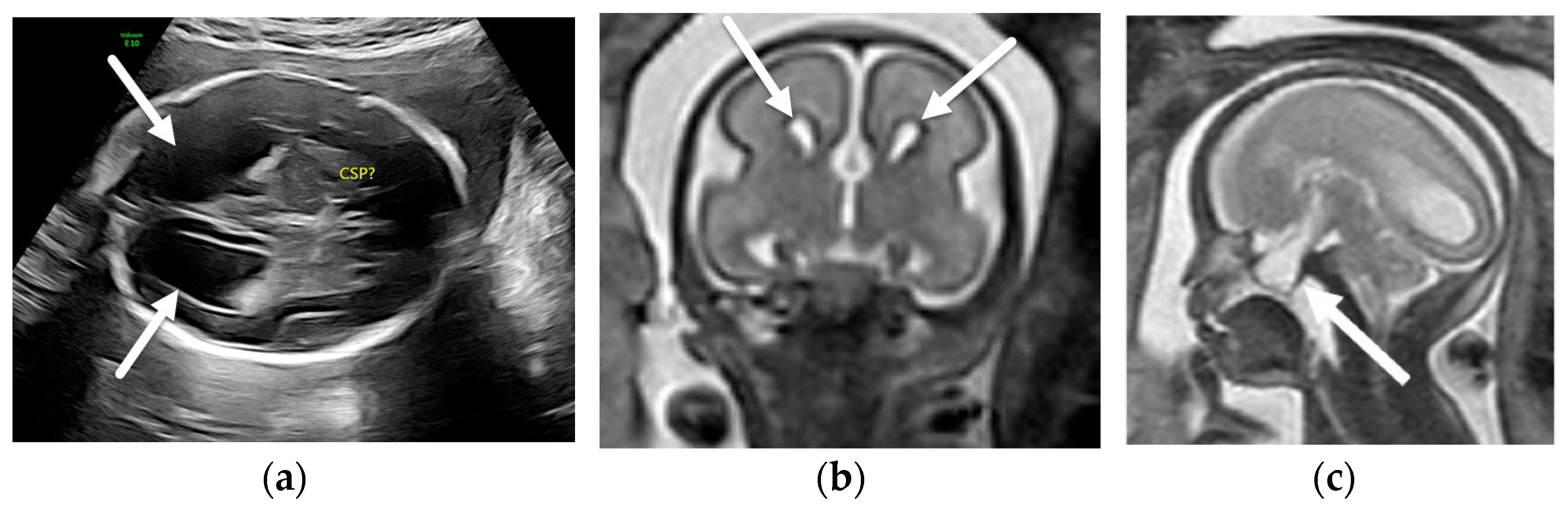
4. Absent Cavum Septi Pellucidi
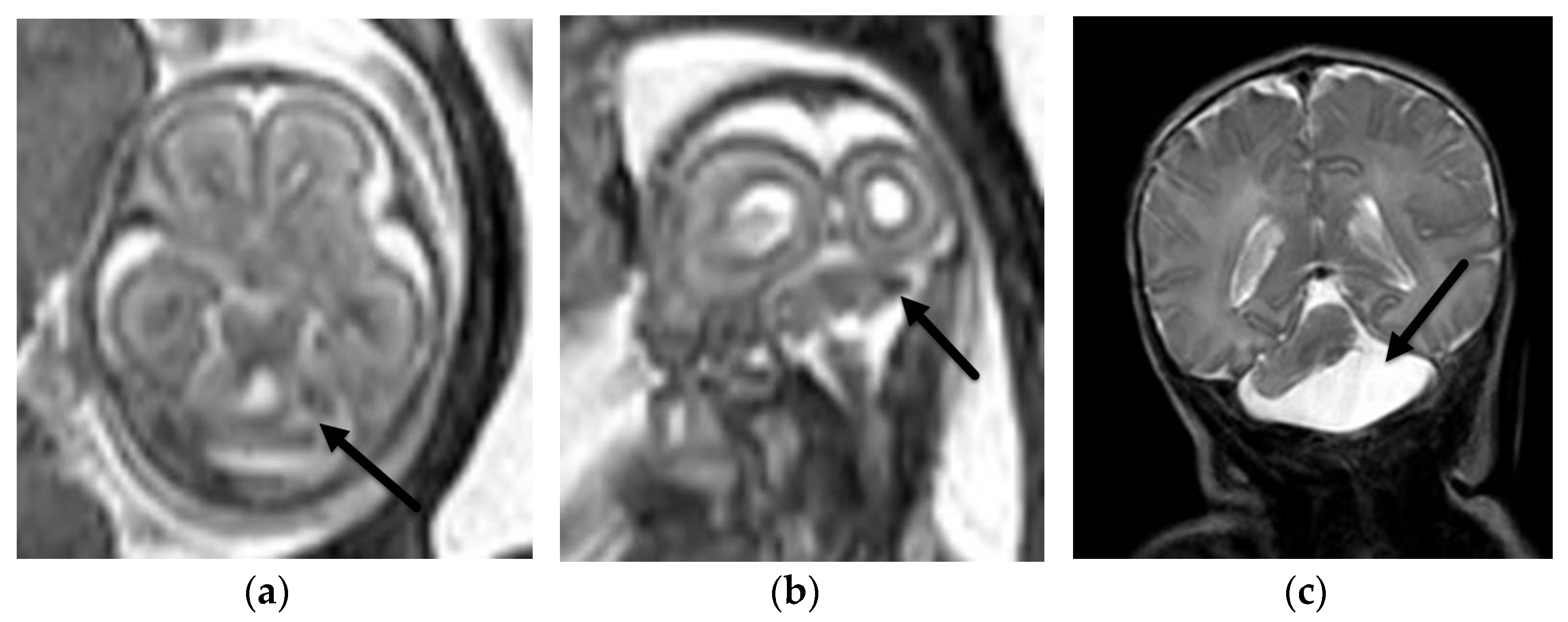
4.1. Holoprosencephaly Spectrum
4.2. Corpus Callosum Anomalies
4.3. Hypoplastic Optic Nerves vs. Isolated Septal Deficiency
5. Posterior Fossa Malformations
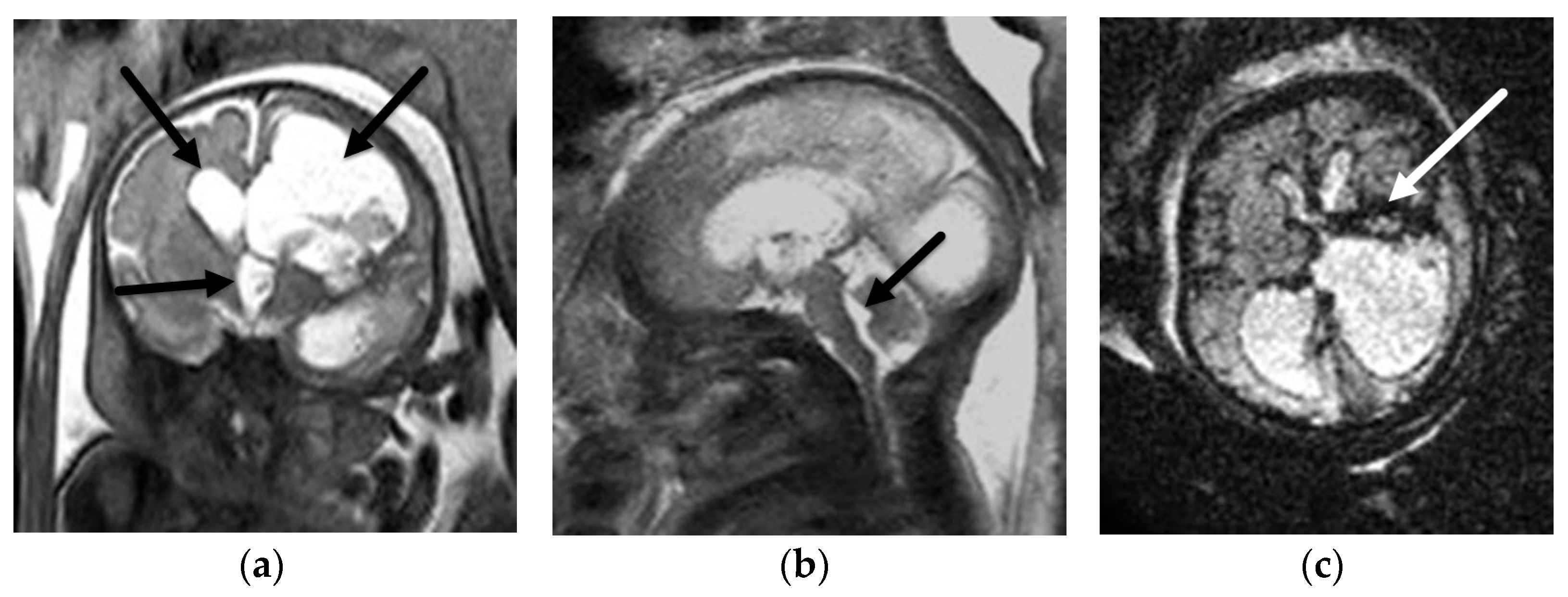
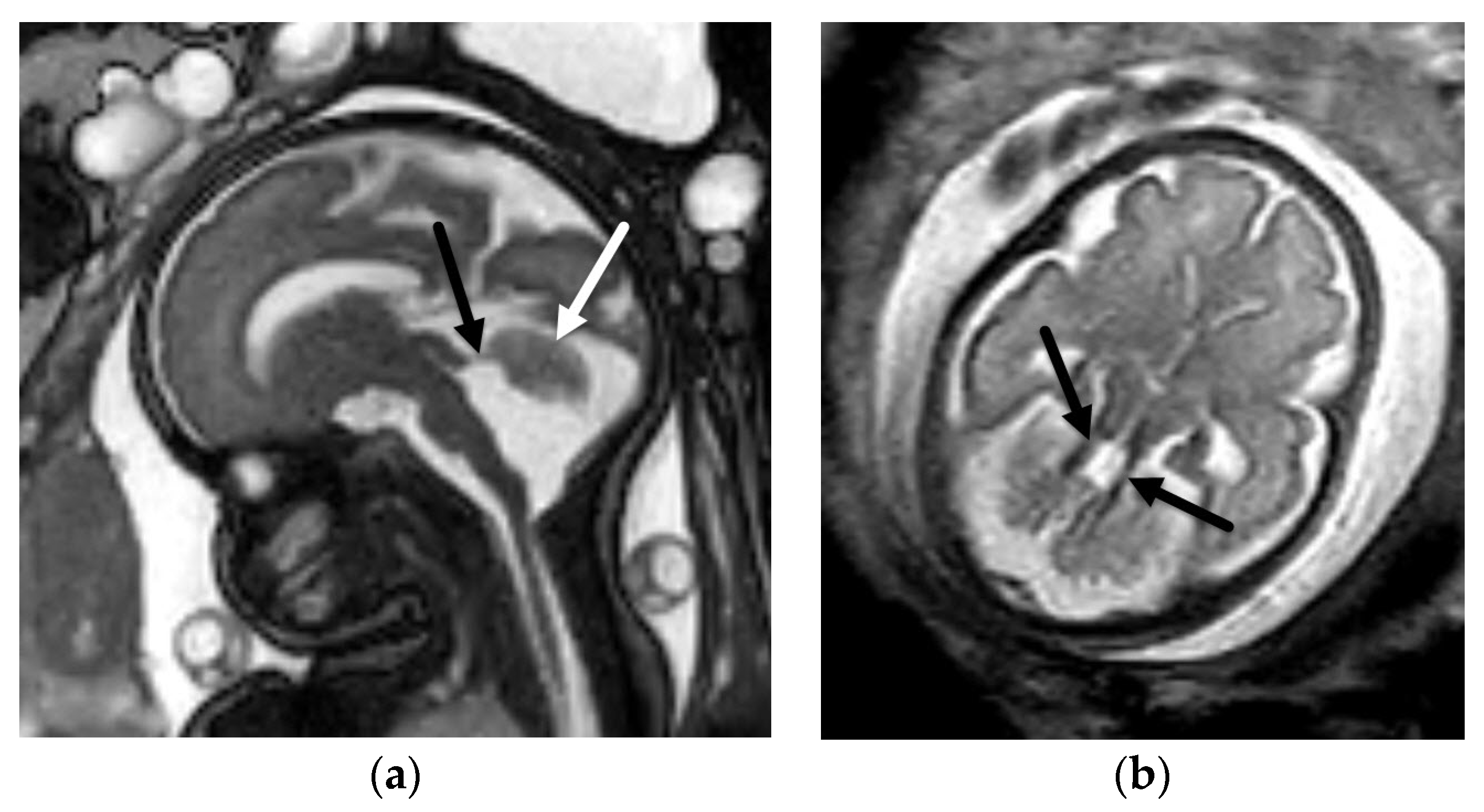
5.1. Dandy-Walker Continuum
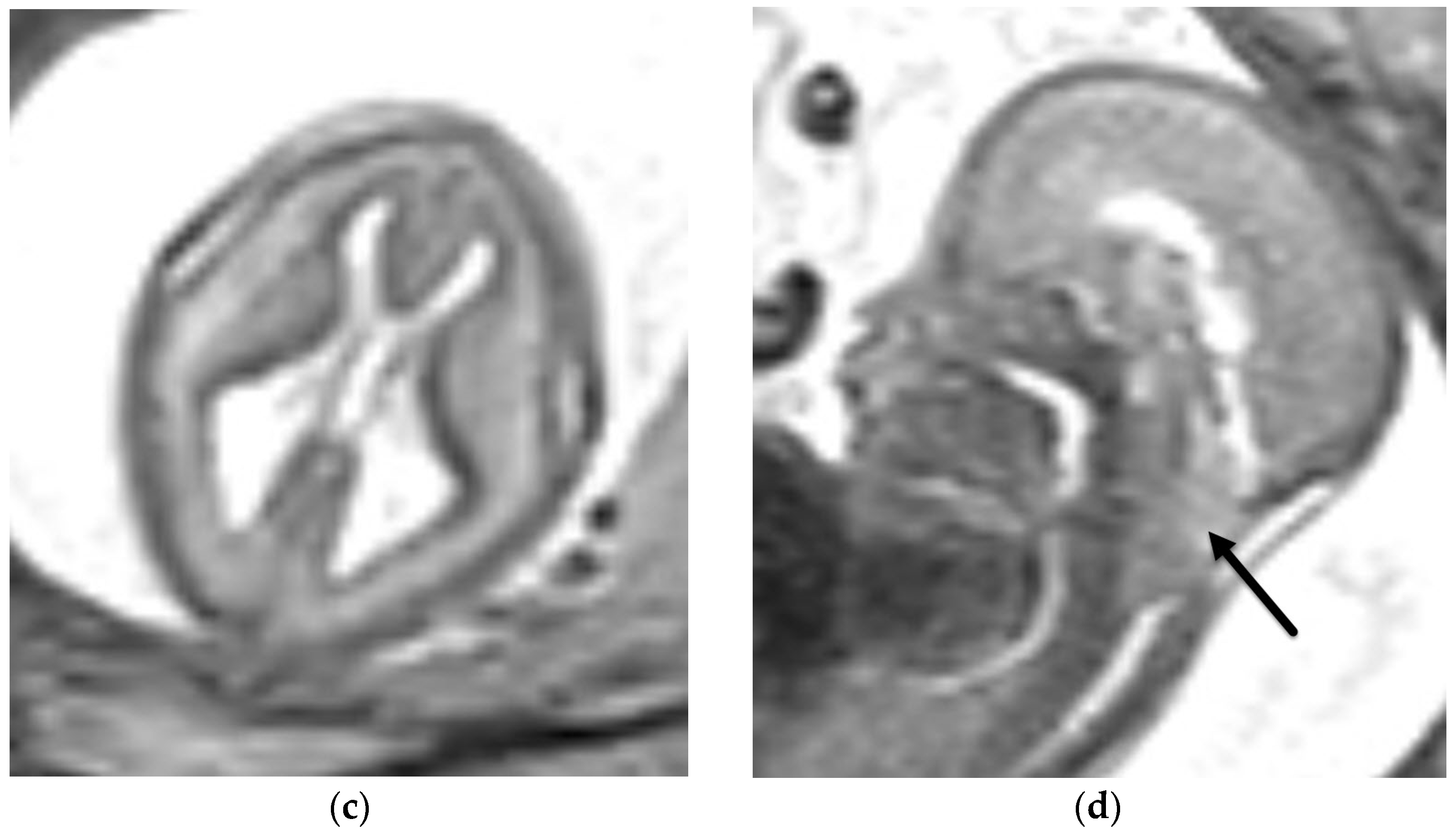
5.2. Other Abnormalities of the Cerebellar Hemispheres
5.3. Brainstem Anomalies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pfeifer, C.M.; Willard, S.D.; Cornejo, P. MRI depiction of fetal brain abnormalities. Acta Radiol. Open 2019, 8, 2058460119894987. [Google Scholar] [CrossRef]
- Pellerito, J.; Bromely, B.; Allison, S.; Chauhan, A.; Destounis, S.; Dickman, E.; Kline-Fath, B.; Mastrobattista, J.; Neumyer, M.; Rundek, T.; et al. AIUM-ACR-ACOG-SMFM-SRU Practice Parameter for the Performance of Standard Diagnostic Obstetric Ultrasound Examinations. J. Ultrasound Med. 2018, 37, E13–E24. [Google Scholar]
- Buscarini, E.; Lutz, H.; Mirk, P. Manual of Diagnostic Ultrasound, 2nd ed.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Filly, R.A.; Cardoza, J.D.; Goldstein, R.B.; Barkovich, A.J. Detection of fetal central nervous system anomalies: A practical level of effort for a routine sonogram. Radiology 1989, 172, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Nonaka, M.; Bamba, Y.; Teramoto, C.; Ban, C.; Pooh, R.K. Seminars in Fetal & Neonatal Medicine Diagnosis, treatment, and long-term outcomes of fetal hydrocephalus. Semin. Fetal Neonatal Med. 2012, 17, 330–335. [Google Scholar] [PubMed]
- Platt, L.D.; Barth, R.A.; Pugash, D. Current controversies in prenatal diagnosis 3: Fetal MRI should be performed in all prenatally detected fetuses with a major structural abnormality. Prenat. Diagn. 2018, 38, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Levine, D.; Barnes, P.D.; Robertson, R.R.; Wong, G.; Mehta, T.S. Fast MR imaging of fetal central nervous system abnormalities. Radiology 2003, 229, 51–61. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Bradburn, M.; Campbell, M.J.; Cooper, C.L.; Graham, R.; Jarvis, D.; Kilby, M.D.; Mason, G.; Mooney, C.; Robson, S.C.; et al. Use of MRI in the diagnosis of fetal brain abnormalities in utero (MERIDIAN): A multicentre, prospective cohort study. Lancet 2017, 389, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.L.; Byrne, J.J.; Clark, H.R.; Twickler, D.M.; Dashe, J.S. Use of Fetal Magnetic Resonance Imaging After Sonographic Identification of Major Structural Anomalies. J. Ultrasound Med. 2020, 39, 2053–2058. [Google Scholar] [CrossRef]
- American College of Radiology. ACR–SPR Practice Parameter for the Safe and Optimal Performance of Fetal Magnetic Resonance Imaging (MRI); American College of Radiology: Reston, VA, USA, 2020. [Google Scholar]
- Prayer, D.; Malinger, G.; Brugger, P.C.; Cassady, C.; De Catte, L.; De Keersmaecker, B.; Fernandes, G.L.; Glanc, P.; Gonçalves, L.F.; Gruber, G.M.; et al. ISUOG Practice Guidelines: Performance of fetal magnetic resonance imaging. Ultrasound Obstet. Gynecol. 2017, 49, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Epstein, K.N.; Kline-Fath, B.M.; Zhang, B.; Venkatesan, C.; Habli, M.; Dowd, D.; Nagaraj, U.D. Prenatal Evaluation of Intracranial Hemorrhage on Fetal MRI: A Retrospective Review. Am. J. Neuroradiol. 2021, 42, 2222–2228. [Google Scholar] [CrossRef]
- Nagaraj, U.D.; Calvo-Garcia, M.A.; Merrow, A.C.; Zhang, B.; Tkach, J.A.; Kline-Fath, B.M. Utilization of 3-T fetal magnetic resonance imaging in clinical practice: A single-institution experience. Pediatr. Radiol. 2021, 51, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.B.; La Pidus, A.S.; Filly, R.A.; Cardoza, J. Mild lateral cerebral ventricular dilatation in utero: Clinical significance and prognosis. Radiology 1990, 176, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Achiron, R.; Schimmel, M.; Achiron, A.; Mashiach, S. Fetal mild idiopathic lateral ventriculomegaly: Is there a correlation with fetal trisomy? Ultrasound Obstet. Gynecol. 1993, 3, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Bernard, J.P.; Ville, Y. Reference ranges for fetal ventricular width: A non-normal approach. Ultrasound Obstet. Gynecol. 2007, 30, 61–66. [Google Scholar] [CrossRef]
- Nagaraj, U.D.; Kline-Fath, B.M. Imaging diagnosis of ventriculomegaly: Fetal, neonatal, and pediatric. Child’s Nerv Syst. 2020, 36, 1669–1679. [Google Scholar] [CrossRef]
- Gaglioti, P.; Oberto, M.; Todros, T. The significance of fetal ventriculomegaly: Etiology, short- and long-term outcomes. Prenat. Diagn. 2009, 29, 381–388. [Google Scholar] [CrossRef]
- Falip, C.; Blanc, N.; Maes, E.; Zaccaria, I.; Oury, J.F.; Sebag, G.; Garel, C. Postnatal clinical and imaging follow-up of infants with prenatal isolated mild ventriculomegaly: A series of 101 cases. Pediatr. Radiol. 2007, 37, 981–989. [Google Scholar] [CrossRef]
- Garel, C.; Luton, D.; Oury, J.F.; Gressens, P. Ventricular dilatations. Child’s Nerv. Syst. 2003, 19, 517–523. [Google Scholar] [CrossRef]
- Addario, P.V.D.; Pinto, V.; Pintucci, A.; Vincenzo, P.; Pinto, V.; Pintucci, A. Sonographic diagnosis of fetal cerebral ventriculomegaly: An update Sonographic diagnosis of fetal cerebral ventriculomegaly: An update. J. Matern. Neonatal Med. 2007, 20, 7–14. [Google Scholar] [CrossRef]
- Cinalli, G.; Spennato, P.; Nastro, A.; Aliberti, F.; Trischitta, V.; Ruggiero, C.; Mirone, G.; Cianciulli, E. Hydrocephalus in aqueductal stenosis. Child’s Nerv. Syst. 2011, 27, 1621–1642. [Google Scholar] [CrossRef]
- Zhang, J.; Williams, M.A.; Rigamonti, D. Genetics of human hydrocephalus. J. Neurol. 2006, 253, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jissendi-Tchofo, P.; Kara, S.; Barkovich, A.J. Midbrain-hindbrain involvement in lissencephalies. Neurology 2009, 72, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Heaphy-Henault, K.; Guimaraes, C.; Mehollin-Ray, A.; Cassady, C.I.; Zhang, W.; Desai, N.K.; Paldino, M.J. Congenital aqueductal stenosis: Findings at fetal MRI that accurately predict a postnatal diagnosis. Am. J. Neuroradiol. 2018, 39, 942–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline-Fath, B.M.; Arroyo, M.S.; Calvo-Garcia, M.A.; Horn, P.S.; Thomas, C. Prenatal aqueduct stenosis: Association with rhombencephalosynapsis and neonatal outcome. Prenat. Diagn. 2018, 38, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K.L. Chiari Type II malformation: Past, present, and future. Neurosurg. Focus 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Van den Hof, M.C.; Nicolaides, K.H.; Campbell, J.; Campbell, S. Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. Am. J. Obstet. Gynecol. 1990, 162, 322–327. [Google Scholar] [CrossRef]
- Blaas, H.; Eik-Nes, S. Sonoembryology and early prenatal diagnosis of neural anamalies. Prenat. Diagn. 2009, 29, 312–325. [Google Scholar] [CrossRef]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W., III; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Committee on Obstetric Practice Society for Maternal-Fetal Medicine. Maternal-Fetal Surgery for Myelomeningocele. Obstet. Gynecol. 2017, 130, 164–167. [Google Scholar] [CrossRef]
- Sutton, L.N.; Adzick, N.S.; Bilaniuk, L.T.; Johnson, M.P.; Crombleholme, T.M.; Flake, A.W. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. J. Am. Med. Assoc. 1999, 282, 1826–1831. [Google Scholar] [CrossRef] [Green Version]
- Nagaraj, U.D.; Bierbrauer, K.S.; Zhang, B.; Peiro, J.L.; Kline-Fath, B.M. Hindbrain Herniation in Chiari II Malformation on Fetal and Postnatal MRI. AJNR Am. J. Neuroradiol. 2017, 38, 1031–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, N.S.; Monteagudo, A.; Kuller, J.A.; Craigo, S.; Norton, M.E. Mild fetal ventriculomegaly: Diagnosis, evaluation, and management. Am. J. Obstet. Gynecol. 2018, 219, B2–B9. [Google Scholar] [CrossRef] [PubMed]
- Madakshira, M.G.; Gupta, K.; Uthamalingam, P.; Kapatia, G.; Saini, S.S. Multicystic encephalomalacia: An autopsy report of 4 cases. Autops. Case Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.A.; Finney, C.R.; Griffiths, P.D. Normative volume measurements of the fetal intra-cranial compartments using 3D volume in utero MR imaging. Eur Radiol. 2019, 29, 3488–3495. [Google Scholar] [CrossRef] [Green Version]
- Bethune, M.; Alibrahim, E.; Davies, B.; Yong, E. A pictorial guide for the second trimester ultrasound. Australas. J. Ultrasound Med. 2013, 16, 98–113. [Google Scholar] [CrossRef] [Green Version]
- Nagaraj, U.D.; Calvo-Garcia, M.A.; Kline-Fath, B.M. Abnormalities Associated With the Cavum Septi Pellucidi on Fetal MRI: What Radiologists Need to Know. AJR Am. J. Roentgenol. 2018, 210, 989–997. [Google Scholar] [CrossRef]
- Winter, T.C.; Kennedy, A.M.; Woodward, P.J. Holoprosencephaly: A survey of the entity, with embryology and fetal imaging. Radiographics 2015, 35, 275–290. [Google Scholar] [CrossRef]
- DeMyer, W.; Zeman, W.; Palmer, C. The face predicts the brain: Diagnostic significance of median facial anomalies for holoprosencephaly (arhinencephaly). Pediatrics 1964, 34, 256–263. [Google Scholar] [CrossRef]
- Barkovich, A.; Raybaud, C.A. Pediatric Neuroimaging, 6th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2019. [Google Scholar]
- Riddle, A.; Nagaraj, U.; Hopkin, R.J.; Kline-Fath, B.; Venkatesan, C. Fetal Magnetic Resonance Imaging (MRI) in Holoprosencephaly and Associations With Clinical Outcome: Implications for Fetal Counseling. J. Child Neurol. 2021, 36, 357–364. [Google Scholar] [CrossRef]
- Palmer, E.E.; Mowat, D. Agenesis of the corpus callosum: A clinical approach to diagnosis. Am. J. Med. Genet. Part C Semin. Med. Genet. 2014, 166, 184–197. [Google Scholar] [CrossRef]
- Paladini, D.; Pastore, G.; Cavallaro, A.; Massaro, M.; Nappi, C. Agenesis of the fetal corpus callosum: Sonographic signs change with advancing gestational age. Ultrasound Obstet. Gynecol. 2013, 42, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.H.; Bartha, A.I.; Norton, M.E.; Barkovich, A.J.; Sherr, E.H.; Glenn, O.A. Agenesis of the corpus callosum: An MR imaging analysis of associated abnormalities in the fetus. Am. J. Neuroradiol. 2009, 30, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaraj, U.D.; Moudgal, R.; Hopkin, R.J.; Venkatesan, C.; Kline-Fath, B.M. Prenatal evaluation of the Sakoda complex. Pediatr. Radiol. 2019, 49, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- De Morsier, G. Studies on malformation of cranio-encephalic sutures. III. Agenesis of the septum lucidum with malformation of the optic tract. Schweiz. Arch. Neurol. Neurochir. Psychiatr. 1956, 77, 267–292. [Google Scholar]
- Acers, T.E. Optic nerve hypoplasia: Septo-optic-pituitary dysplasia syndrome. Trans. Am. Ophthalmol. Soc. 1981, 79, 425–457. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1312194&tool=pmcentrez&rendertype=abstract (accessed on 9 March 2022).
- Ryabets-Lienhard, A.; Stewart, C.; Borchert, M.; Geffner, M.E. The Optic Nerve Hypoplasia Spectrum: Review of the Literature and Clinical Guidelines. Adv. Pediatr. 2016, 63, 127–146. [Google Scholar] [CrossRef]
- Cemeroglu, A.P.; Coulas, T.; Kleis, L. Spectrum of clinical presentations and endocrinological findings of patients with septo-optic dysplasia: A retrospective study. J. Pediatr. Endocrinol. Metab. 2015, 28, 1057–1063. [Google Scholar] [CrossRef]
- Vawter-Lee, M.M.; Wasserman, H.; Thomas, C.W.; Nichols, B.; Nagaraj, U.D.; Schapiro, M.; Venkatesan, C. Outcome of Isolated Absent Septum Pellucidum Diagnosed by Fetal Magnetic Resonance Imaging (MRI) Scan. J. Child Neurol. 2018, 33, 693–699. [Google Scholar] [CrossRef]
- Venkatesan, C.; Kline-Fath, B.; Horn, P.S.; Poisson, K.E.; Hopkin, R.; Nagaraj, U.D. Short- and Long-Term Outcomes of Prenatally Diagnosed Dandy-Walker Malformation, Vermian Hypoplasia, and Blake Pouch Cyst. J. Child Neurol. 2021, 36, 1111–1119. [Google Scholar] [CrossRef]
- Kollias, S.S.; Ball, W.S.; Prenger, E.C. Cystic malformations of the posterior fossa: Differential diagnosis clarified through embryologic analysis. Radiographics 1993, 13, 1211–1231. [Google Scholar] [CrossRef]
- Chapman, T.; Menashe, S.J.; Zare, M.; Alessio, A.M.; Ishak, G.E. Establishment of normative values for the fetal posterior fossa by magnetic resonance imaging. Prenat. Diagn. 2018, 38, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, U.D.; Kline-Fath, B.M.; Horn, P.S.; Venkatesan, C. Evaluation of Posterior Fossa Biometric Measurements on Fetal MRI in the Evaluation of Dandy-Walker Continuum. AJNR Am. J. Neuroradiol. 2021, 42, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.J.; Ederies, M.A. Seminars in Fetal & Neonatal Medicine Diagnostic imaging of posterior fossa anomalies in the fetus. Semin. Fetal Neonatal Med. 2016, 21, 312–320. [Google Scholar] [PubMed]
- Nagaraj, U.D.; Kline-Fath, B.M.; Calvo-Garcia, M.A.; Vadivelu, S.; Venkatesan, C. Fetal and postnatal MRI findings of Blake pouch remnant causing obstructive hydrocephalus. Radiol. Case Rep. 2020, 15, 2535–2539. [Google Scholar] [CrossRef]
- Boseman, T.; Orman, G.; Boltshauser, E.; Tekes, A.; Huisman, T.A.G.M.; Poretti, A. Congenital abnormalities of the posterior fossa. Radiographics 2015, 35, 200–220. [Google Scholar] [CrossRef]
- Hayashi, M.; Poretti, A.; Gorra, M.; Farzin, A.; Graham, E.M.; Huisman, T.A.G.M.; Northington, F.J. Prenatal Cerebellar Hemorrhage: Fetal and Postnatal Neuroimaging Findings and Postnatal Outcome. Pediatr Neurol. 2015, 52, 529–534. [Google Scholar] [CrossRef]
- Haines, K.M.; Wang, W.; Pierson, C.R. Cerebellar hemorrhagic injury in premature infants occurs during a vulnerable developmental period and is associated with wider neuropathology. Acta Neuropathol. Commun. 2013, 1, 69. [Google Scholar] [CrossRef] [Green Version]
- Poretti, A.; Huisman, T.A.G.M.; Scheer, I.; Boltshauser, E. Joubert syndrome and related disorders: Spectrum of neuroimaging findings in 75 patients. Am. J. Neuroradiol. 2011, 32, 1459–1463. [Google Scholar] [CrossRef] [Green Version]
- Parisi, M.A. The molecular genetics of Joubert syndrome and related ciliopathies: The challenges of genetic and phenotypic heterogeneity. Transl. Sci. Rare Dis. 2019, 4, 25–49. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, C.V.A.; Dahmoush, H.M. Imaging phenotype correlation with molecular and molecular pathway defects in malformations of cortical development. Pediatr. Radiol. 2020, 50, 1974–1987. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraj, U.D.; Kline-Fath, B.M. Clinical Applications of Fetal MRI in the Brain. Diagnostics 2022, 12, 764. https://doi.org/10.3390/diagnostics12030764
Nagaraj UD, Kline-Fath BM. Clinical Applications of Fetal MRI in the Brain. Diagnostics. 2022; 12(3):764. https://doi.org/10.3390/diagnostics12030764
Chicago/Turabian StyleNagaraj, Usha D., and Beth M. Kline-Fath. 2022. "Clinical Applications of Fetal MRI in the Brain" Diagnostics 12, no. 3: 764. https://doi.org/10.3390/diagnostics12030764
APA StyleNagaraj, U. D., & Kline-Fath, B. M. (2022). Clinical Applications of Fetal MRI in the Brain. Diagnostics, 12(3), 764. https://doi.org/10.3390/diagnostics12030764





