Field Experiences with Handheld Diagnostic Devices to Triage Children under Five Presenting with Severe Febrile Illness in a District Hospital in DR Congo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting, Design and Period
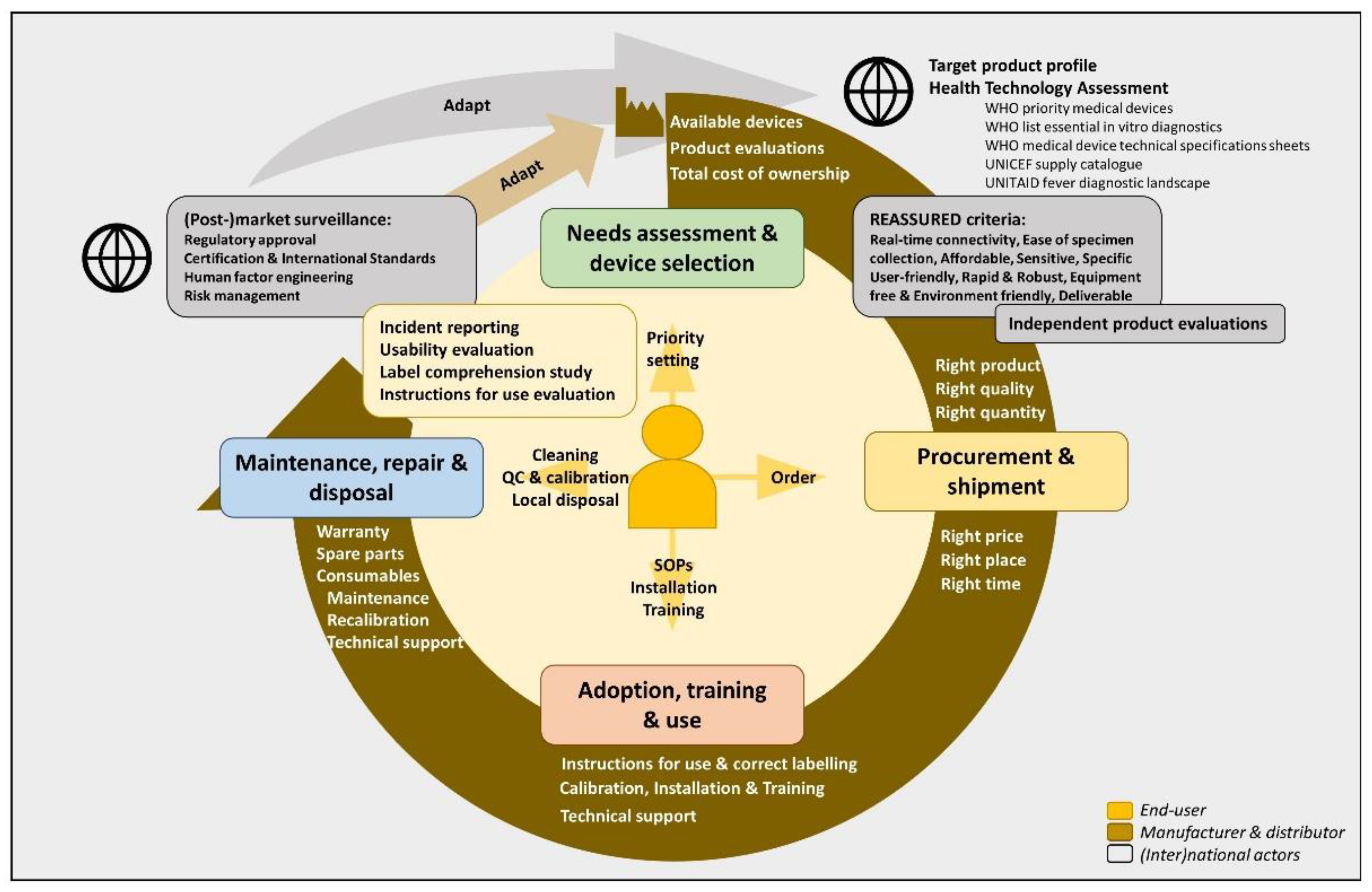
2.2. Lifecycle of Handheld Diagnostic Devices and REASSURED Criteria
2.3. Needs Assessment: Intended Use of the Handheld Diagnostic Devices and Products
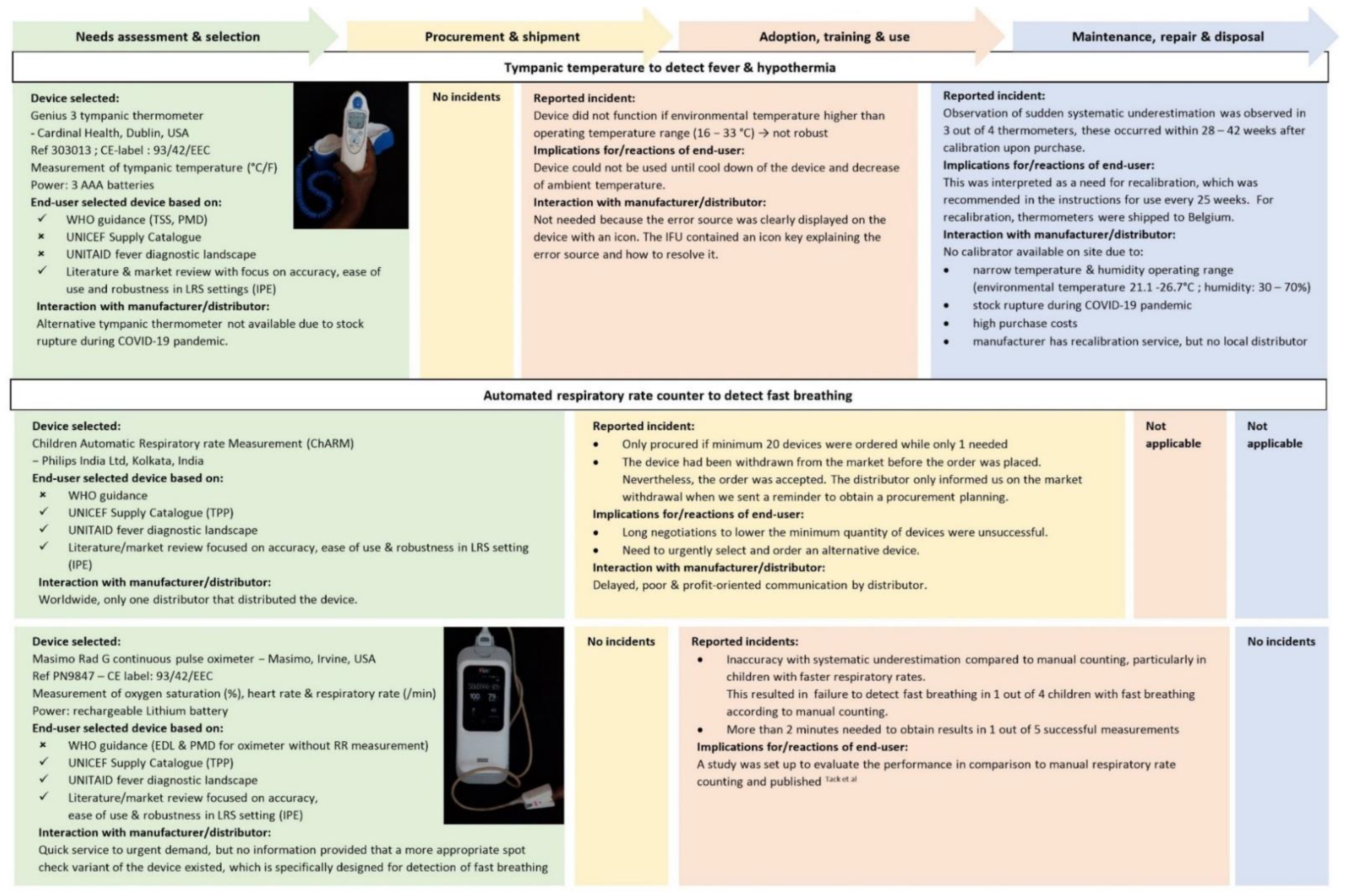
- Tympanic temperature—measured by Genius 3 tympanic thermometer (Cardinal Health, Dublin, OH, USA)
- Danger sign: fever (>37.5 °C) or hypothermia (≤35.5 °C)
- Procedure: SOP in Supplement S3. A tympanic thermometer was chosen to avoid the risks of rectal temperature and because of its superior performance compared to other non-invasive thermometers. Cut-offs were set to increase sensitivity and to harmonize with WHO cut-offs recommended for axillary temperature (see Supplement S4).
- Oxygen saturation—measured by Masimo Rad G continuous pulse oximeter (Masimo, Irvine, CA, USA)
- Danger sign: Hypoxia (<90%) [5]
- Procedure: SOP in Supplement S5
- Clinical implications: Urgent airway or breathing support needed, e.g. oxygen [5]. Hypoxia is present in severe infections such as pneumonia, sepsis, severe malaria [5,30]. It has a multifactorial pathophysiology including increased oxygen demands, pulmonary inflammation and pulmonary edema [30,31].
- Heart rate—measured by Masimo Rad G continuous pulse oximeter (Masimo, Irvine, CA, USA)
- Danger sign: Tachycardia (<12 months: >160/min; 12 months: >120/min) [5]
- Procedure: SOP in Supplement S5
- Clinical implications: Often present during fever. If a child has tachycardia, a weak pulse, a cold skin and prolonged capillary refill, the child has poor perfusion/shock requiring fluid resuscitation [5].
- Respiratory rate–measured by Masimo Rad G continuous pulse oximeter (Masimo, Irvine, CA, USA)
- Danger sign: fast breathing (<2 months: ≥60 breaths/min; >2−<12 months: ≥50 breaths/min; ≥12 months: ≥40 breaths/min) [5]
- Procedure: SOP in Supplement S5
- Clinical implications: Fast breathing can reflect pneumonia, but also be present in severe malaria or invasive bacterial infections due to metabolic acidosis [5].
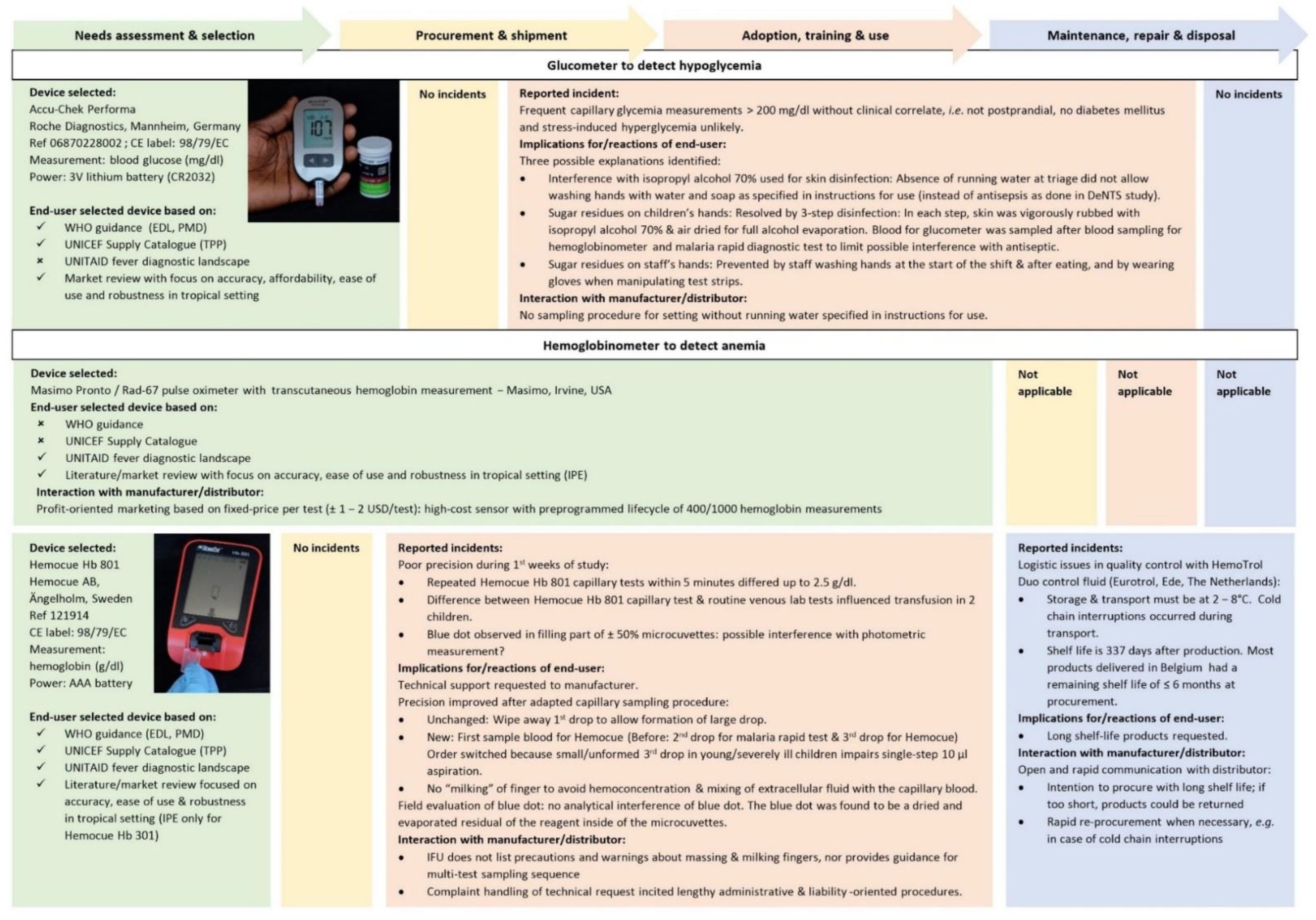
- Blood glucose–measured by Accu-Chek Performa (Roche Diagnostics, Mannheim, Germany)
- Danger sign: hypoglycemia (<45 mg/dL) [5]
- Procedure: SOP in Supplement S6
- Clinical implications: Measured on capillary blood to rapidly detect hypoglycemia, as this requires urgent treatment to avert or resolve coma or convulsions [5]. Hypoglycemia (partially) reflects the host stress response to infection with altered activity of the hypothalamic–pituitary–adrenal axis [32,33,34]. As such, hypoglycemia is a diagnostic clue pointing to life-threatening severe malaria or bacterial infections [5,30,32].
- Hemoglobin—measured by Hemocue Hb 801 (Hemocue AB, Ängelholm, Sweden)
- Danger sign: severe anemia (<5 g/dL) [5]
- Procedure: SOP in Supplement S7
- Clinical implications: Rapid and bed-side measurement on capillary blood is important for identification of children who require urgent blood transfusion [5] and for volume adaptation of venous blood samples for further diagnostic work-up to prevent iatrogenic anemia [35]. Severe anemia is a diagnostic clue pointing to severe malaria and/or invasive non-typhoidal Salmonella bloodstream infections [36,37].
2.4. Selection of Devices: General and Study-Specific Criteria
2.5. Procurement and Shipment of Handheld Diagnostic Devices
2.6. Training, Use and Maintenance of Handheld Diagnostic Devices
2.7. Evaluation of Instructions for Use
2.8. Usability Assessment and Label Comprehension Study
3. Results
3.1. Device Selection: Insufficient Guidance
3.2. Procurement: Insufficient Affordability, Delivery and Access
3.3. Training, Use and Adoption: Robustness, Timely Results, Ease of Sampling and Client-Centeredness
3.4. Instructions for Use: Insufficiently Clear and Not Adapted to Low-Resource Setting
3.5. Maintenance: Cold-Chain and Environmental Conditions Challenge Quality Control and Calibration
3.6. Usability Evaluation: Detailed Feedback and Concrete Suggestions for Device Improvement
4. Discussion
4.1. Summary of Findings
4.2. Device Selection Needs Centralized and Comprehensive Target Product Profiles
4.3. Procurement and Shipment Need for Transparent Communication and In-Country Distributors
4.4. Training and Adoption Need Prioritization and Clear and Accessible Instructions for Use
4.5. Usability and Performance Need Ease of Specimen Collection
4.6. Usability: Robustness, Size, Time-to-Result, Easy Reading, Perception by Patients and Caretakers
4.7. Usability Needs Local, Client-Centered Technical Support and Human Factor Engineering
4.8. Maintenance, Repair and Disposal: Equipment-Free and Environment-Friendly
4.9. Limitations and Strengths and Generalizability
4.10. The Broader Context of Medical Devices in Low-Resource Settings and the Way Forward
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, Regional, and National Causes of under-5 Mortality in 2000–15: An Updated Systematic Analysis with Implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [Green Version]
- Daily, J. Fever Diagnostic Technology Landscape; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Roddy, P.; Dalrymple, U.; Jensen Id, T.O.; Dittrich, S.; Rao, V.B.; Pfeffer, D.A.; Twohig, K.A.; Roberts, T.; Bernal, O.; Guillen, E. Quantifying the Incidence of Severe-Febrile-Illness Hospital Admissions in Sub-Saharan Africa. PLoS ONE 2019, 14, e022037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, K.A.; Horton, S.; Wilson, M.L.; Atun, R.; DeStigter, K.; Flanigan, J.; Sayed, S.; Adam, P.; Aguilar, B.; Andronikou, S.; et al. The Lancet Commission on Diagnostics: Transforming Access to Diagnostics. Lancet 2021, 398, S0140–S6736. [Google Scholar] [CrossRef]
- World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses, 2nd ed.; World Health Organization, Ed.; World Health Organization: Geneva, Switzerland, 2013; ISBN 978-92-4-154837-3. [Google Scholar]
- World Health Organization. Integrated Management of Childhood Illnesses, 1st ed.; World Health Organization: Geneva, Switzerland, 2014; ISBN 978-92-4-150682-3. [Google Scholar]
- World Health Organization. Global Atlas of Medical Devices; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Tack, B.; Phoba, M.-F.; van Puyvelde, S.; Kalonji, L.M.; Hardy, L.; Barbé, B.; Van der Sande, M.A.B.; Monsieurs, E.; Deborggraeve, S.; Lunguya, O.; et al. Salmonella Typhi From Blood Cultures in the Democratic Republic of the Congo: A 10-Year Surveillance. Clin. Infect. Dis. 2019, 68 (Suppl. S2), S130–S137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tack, B.; Vita, D.; Phoba, M.-F.; Mbuyi-Kalonji, L.; Hardy, L.; Barbé, B.; Jacobs, J.; Lunguya, O.; Jacobs, L. Direct association between rainfall and non-typhoidal Salmonella bloodstream infections in hospital-admitted children in the Democratic Republic of Congo. Sci. Rep. 2021, 11, 21617. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- McNally, A.; Arsenault, K.; Kumar, S.; Shukla, S.; Peterson, P.; Wang, S.; Funk, C.; Peters-Lidard, C.D.; Verdin, J.P. A land data assimilation system for sub-Saharan Africa food and water security applications. Sci. Data 2017, 4, 170012. [Google Scholar] [CrossRef] [Green Version]
- Tack, B.; Phoba, M.-F.; Barbé, B.; Kalonji, L.M.; Hardy, L.; Van Puyvelde, S.; Ingelbeen, B.; Falay, D.; Ngbonda, D.; Van Der Sande, M.A.B.; et al. Non-typhoidal Salmonella bloodstream infections in Kisantu, DR Congo: Emergence of O5-negative Salmonella Typhimurium and extensive drug resistance. PLoS Negl. Trop. Dis. 2020, 14, e0008121. [Google Scholar] [CrossRef] [Green Version]
- Ministère du Plan et Suivi de la Mise en oeuvre de la Révolution de la Modernité; Ministère de la Santé Publique; ICF International. Democratic Republic of Congo Demographic and Health Survey 2013-14: Key Findings; MPSMRM, MSP, and ICF International: Rockville, MD, USA, 2014. [Google Scholar]
- Stasse, S.; Vita, D.; Kimfuta, J.; da Silveira, V.C.; Bossyns, P.; Criel, B. Improving financial access to health care in the Kisantu district in the Democratic Republic of Congo: Acting upon complexity. Glob. Health Action 2015, 8, 25480. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Human Resources for Medical Devices, the Role of Biomedical Engineers; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Development of Medical Device Policies; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Medical Device Donations: Considerations for Solicitation and Provision; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Medical Equipment Maintenance Programme Overview; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Needs Assessment for Medical Devices; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Procurement Process Resource Guide; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Guidance for Post-Market Surveillance and Market Surveillance of Medical Devices, Including In Vitro Diagnostics; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Decommissioning Medical Devices; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Interagency List of Priority Medical Devices for Essential Interventions for Reproductive, Maternal, Newborn and Child Health; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. WHO Technical Specifications for 61 Medical Devices; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization. WHO Technical Specifications for Automated Non-Invasive Blood Pressure Measuring Devices with Cuff; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. The Selection and Use of Essential in Vitro Diagnostics: Report of the Third Meeting of the WHO Strategic Advisory Group of Experts on In Vitro Diagnostics, 2020 (Including the Third WHO Model List of Essential In Vitro Diagnostics); World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Land, K.J.; Boeras, D.I.; Chen, X.-S.; Ramsay, A.R.; Peeling, R.W. REASSURED Diagnostics to Inform Disease Control Strategies, Strengthen Health Systems and Improve Patient Outcomes. Nat. Microbiol. 2018, 4, 46–54. [Google Scholar] [CrossRef]
- Tack, B.; Vita, D.; Mbaki, T.N.; Lunguya, O.; Toelen, J.; Jacobs, J. Performance of Automated Point-of-Care Respiratory Rate Counting versus Manual Counting in Children under Five Admitted with Severe Febrile Illness to Kisantu Hospital, DR Congo. Diagnostics 2021, 11, 2078. [Google Scholar] [CrossRef] [PubMed]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension Guidelines for the Management of High Blood Pressure in Children and Adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines for the Treatment of Malaria, 3rd ed.; World Health Organization: Geneva, Switzerland, 2015; ISBN 9789241549127. [Google Scholar]
- Schaffer, K.; Taylor, C.T. The Impact of Hypoxia on Bacterial Infection. FEBS J. 2015, 282, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, S.; Maltha, J.; Deborggraeve, S.; Rattray, N.J.W.; Issa, G.; Bérenger, K.; Lompo, P.; Tahita, M.C.; Ruspasinghe, T.; McConville, M.; et al. Towards Improving Point-of-Care Diagnosis of Non-Malaria Febrile Illness: A Metabolomics Approach. PLOS Negl. Trop. Dis. 2016, 10, e0004480. [Google Scholar] [CrossRef]
- Brooks, M.H.; Barry, K.G.; Cirksena, W.J.; Malloy, J.P.; Bruton, J.; Gilliland, P.F. Pituitary-Adrenal Function in Acute Falciparum Malaria. Am. J. Trop. Med. Hyg. 1969, 18, 872–877. [Google Scholar] [CrossRef]
- Annane, D. The Role of ACTH and Corticosteroids for Sepsis and Septic Shock: An Update. Front. Endocrinol. 2016, 7, 70. [Google Scholar] [CrossRef]
- Kuijpers, L.M.F.; Maltha, J.; Guiraud, I.; Kaboré, B.; Lompo, P.; Devlieger, H.; van Geet, C.; Tinto, H.; Jacobs, J. Severe Anaemia Associated with Plasmodium Falciparum Infection in Children: Consequences for Additional Blood Sampling for Research. Malar. J. 2016, 15, 304. [Google Scholar] [CrossRef] [Green Version]
- English, M.; Berkley, J.; Mwangi, I.; Mohammed, S.; Ahmed, M.; Osier, F.; Muturi, N.; Ogutu, B.; Marsh, K.; Newton, C.R.J.C. Hypothetical Performance of Syndrome-Based Management of Acute Paediatric Admissions of Children Aged More than 60 Days in a Kenyan District Hospital. Bull. World Health Organ. 2003, 81, 166–173. [Google Scholar]
- Nadjm, B.; Amos, B.; Mtove, G.; Ostermann, J.; Chonya, S.; Wangai, H.; Kimera, J.; Msuya, W.; Mtei, F.; Dekker, D.; et al. WHO Guidelines for Antimicrobial Treatment in Children Admitted to Hospital in an Area of Intense Plasmodium Falciparum Transmission: Prospective Study. BMJ 2010, 340, c1350. [Google Scholar] [CrossRef] [Green Version]
- Baker, K.; Ward, C.; Maurel, A.; de Cola, M.A.; Smith, H.; Getachew, D.; Habte, T.; McWhorter, C.; LaBarre, P.; Karlstrom, J.; et al. Usability and Acceptability of a Multimodal Respiratory Rate and Pulse Oximeter Device in Case Management of Children with Symptoms of Pneumonia: A Cross-Sectional Study in Ethiopia. Acta Paediatr. 2021, 110, 1620–1632. [Google Scholar] [CrossRef]
- Baker, K. Acute Respiratory Infection Diagnostic Aid: Learnings and next Steps—Acute Respiratory Infection Diagnostic Aid Learnings and next Steps. Available online: https://www.malariaconsortium.org/resources/publications/1321/acute-respiratory-infection-diagnostic-aid-learnings-and-next-steps (accessed on 28 September 2021).
- Källander, K.; Ward, C.; Smith, H.; Bhattarai, R.; Ashish, K.C.; Timsina, D.; Lamichhane, B.; Maurel, A.; Ram Shrestha, P.; Baral, S.; et al. Usability and Acceptability of an Automated Respiratory Rate Counter to Assess Childhood Pneumonia in Nepal. Acta Paediatr. Int. J. Paediatr. 2020, 109, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Baker, K.; Smith, H.; Maurel, A.; Getachew, D.; Habte, T.; McWhorter, C.; LaBarre, P.; Karlstrom, J.; Black, J.; et al. Usability and Acceptability of an Automated Respiratory Rate Counter to Assess Children for Symptoms of Pneumonia: A Cross-Sectional Study in Ethiopia. Acta Paediatr. Int. J. Paediatr. 2020, 109, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Maurel, A.; Ward, C.; Getachew, D.; Habte, T.; McWhorter, C.; LaBarre, P.; Karlström, J.; Petzold, M.; Källander, K. Automated Respiratory Rate Counter to Assess Children for Symptoms of Pneumonia: Protocol for Cross-Sectional Usability and Acceptability Studies in Ethiopia and Nepal. JMIR Res. Protoc. 2020, 9, e14405. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Baker, K.; Marks, S.; Getachew, D.; Habte, T.; McWhorter, C.; Labarre, P.; Howard-Brand, J.; Miller, N.P.; Tarekegn, H.; et al. Determining the Agreement between an Automated Respiratory Rate Counter and a Reference Standard for Detecting Symptoms of Pneumonia in Children: Protocol for a Cross-Sectional Study in Ethiopia. JMIR Res. Protoc. 2020, 9, e16531. [Google Scholar] [CrossRef] [PubMed]
- Helldén, D.; Baker, K.; Habte, T.; Batisso, E.; Orsini, N.; Källander, K.; Alfvén, T. Does Chest Attachment of an Automated Respiratory Rate Monitor Influence the Actual Respiratory Rate in Children under Five? Am. J. Trop. Med. Hyg. 2020, 102, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Alwadhi, V.; Sarin, E.; Kumar, P.; Saboth, P.; Khera, A.; Gupta, S.; Kumar, H. Measuring Accuracy of Plethysmography Based Respiratory Rate Measurement Using Pulse Oximeter at a Tertiary Hospital in India. Pneumonia 2020, 12, 4. [Google Scholar] [CrossRef]
- Dale, N.M.; Parshuram, C.; Tomlinson, G.; Shepherd, S.; Mohammed Ashir, G.; Bukar, L.M.; Zlotkin, S. Performance of Automated versus Nurse-Measured Respiratory Rate Measurements in Hospitalised Malnourished Children. Acta Paediatr. Int. J. Paediatr. 2021, 110, 2249–2251. [Google Scholar] [CrossRef]
- Chue, A.L.; Moore, R.L.; Cavey, A.; Ashley, E.A.; Stepniewska, K.; Nosten, F.; McGready, R. Comparability of Tympanic and Oral Mercury Thermometers at High Ambient Temperatures. BMC Res. Notes 2012, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Oyakhirome, S.; Profanter, K.; Kremsner, P.G. Short Report: Assessment of Fever in African Children: Implication for Malaria Trials. Am. J. Trop. Med. Hyg. 2010, 82, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Abdulkadir, M.B.; Johnson, W.B.R. A Comparative Study of Rectal Tympanic and Axillary Thermometry in Febrile Children under 5 Years of Age in Nigeria. Paediatr. Int. Child Health 2013, 33, 165–169. [Google Scholar] [CrossRef]
- UNICEF. Supply Division Copenhagen Target Product Profile Acute Respiratory Infection Diagnostic Aid (ARIDA); UNICEF: Copenhagen, Denmark, 2014. [Google Scholar]
- Uyoga, S.; George, E.C.; Bates, I.; Olupot-Olupot, P.; Chimalizeni, Y.; Molyneux, E.M.; Maitland, K. Point-of-Care Haemoglobin Testing in African Hospitals: A Neglected Essential Diagnostic Test. Br. J. Haematol. 2021, 193, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, R.D.; Mei, Z.; Mapango, C.; Jefferds, M.E.D. Methods and Analyzers for Hemoglobin Measurement in Clinical Laboratories and Field Settings. Ann. N.Y. Acad. Sci. 2019, 1450, 147–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, M.; Han, Z.; Abu-Haydar, E.; Matsiko, E.; Iyakaremye, D.; Tuyisenge, L.; Magaret, A.; Lyambabaje, A. An Evaluation of Hemoglobin Measurement Tools and Their Accuracy and Reliability When Screening for Child Anemia in Rwanda: A Randomized Study. PLoS ONE 2018, 13, e0187663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, T.S.; Méndez-Gómez-Humarán, I.; Ruán, M.D.C.M.; Tapia, B.M.; Hernández, S.V.; Ávila, M.H. Validation of Masimo Pronto 7 and HemoCue 201 for Hemoglobin Determination in Children from 1 to 5 Years of Age. PLoS ONE 2017, 12, e0170990. [Google Scholar] [CrossRef] [Green Version]
- Kirby, R.; Palamountain, K. Target Product Profiles for Newborn Care in Low-Resource Settings (v1.2) Consensus Meeting Report; UNICEF: Copenhagen, Denmark, 2020. [Google Scholar]
- PATH. Diagnostics Instrument–Target Product Profile: Diagnostic Instrument: Hemoglobinometer; PATH: Seattle, WA, USA, 2018. [Google Scholar]
- UNICEF Supply Division Supply Catalogue. Available online: https://supply.unicef.org/ (accessed on 17 November 2021).
- Scolarius. Available online: https://www.scolarius.com/ (accessed on 19 November 2021).
- Food and Drug Administration. Applying Human Factors and Usability Engineering to Medical Devices; FDA: Rockville, MD, USA, 2011. [Google Scholar]
- Parker, M.; Barrett, K.; Kahn, M.; Saul, D.; Bansil, P.; Tawiah, C.; Advani, N.; Zobrist, S.; de Los Santos, T.; Gerth-Guyette, E. Potential New Tool for Anemia Screening: An Evaluation of the Performance and Usability of the TrueHb Hemometer. PLoS ONE 2020, 15, e023033. [Google Scholar] [CrossRef]
- Young, M.F.; Raines, K.; Jameel, F.; Sidi, M.; Oliveira-Streiff, S.; Nwajei, P.; McGlamry, K.; Ou, J.; Oladele, A.; Suchdev, P.S. Non-Invasive Hemoglobin Measurement Devices Require Refinement to Match Diagnostic Performance with Their High Level of Usability and Acceptability. PLoS ONE 2021, 16, e0254629. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Agyemang, C.T.; Ambler, G.; Delarosa, J.; Brunette, W.; Levari, S.; Larson, C.; Sundt, M.; Newton, S.; Borriello, G.; et al. MPneumonia, an Innovation for Diagnosing and Treating Childhood Pneumonia in Low-Resource Settings: A Feasibility, Usability and Acceptability Study in Ghana. PLoS ONE 2016, 11, e0165201. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Technical Guidance Series for WHO Prequalification—Diagnostic Assessment: Designing Instructions for Use for IVDs; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Selection of Essential in Vitro Diagnostics at Country Level Using the WHO Model List of Essential In Vitro Diagnostics to Develop and Update a National List of Essential in Vitro Diagnostics; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Seo, D.C.; Ladoni, M.; Brunk, E.; Becker, M.W.; Bix, L. Do Healthcare Professionals Comprehend Standardized Symbols Present on Medical Device Packaging?: An Important Factor in the Fight Over Label Space. Packag. Technol. Sci. 2017, 30, 61–73. [Google Scholar] [CrossRef]
- Hermans, V.; Monzote, L.; van den Sande, B.; Mukadi, P.; Sopheak, T.; Gillet, P.; Jacobs, J. Assessment of the Knowledge of Graphical Symbols Labelled on Malaria Rapid Diagnostic Tests in Four International Settings. Malar. J. 2011, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Huddy, J.R.; Ni, M.; Misra, S.; Mavroveli, S.; Barlow, J.; Hanna, G.B. Development of the Point-of-Care Key Evidence Tool (POCKET): A Checklist for Multi-Dimensional Evidence Generation in Point-of-Care Tests. Clin. Chem. Lab. Med. 2019, 57, 845–855. [Google Scholar] [CrossRef]
- Cherne, N.; Moses, R.; Piperato, S.M.; Cheung, C. Research: How Medical Device Instructions for Use Engage Users. Biomed. Instrum. Technol. 2020, 54, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Kühne, V.; Lunguya, O.; Affolabi, D.; Hardy, L.; Vandenberg, O. Implementing COVID-19 (SARS-CoV-2) Rapid Diagnostic Tests in Sub-Saharan Africa: A Review. Front. Med. 2020, 7, 684. [Google Scholar] [CrossRef] [PubMed]
- Barbé, B.; Verdonck, K.; El-Safi, S.; Khanal, B.; Teav, S.; Lilo Kalo, J.R.; Ravinetto, R.; Chappuis, F.; Boelaert, M.; Jacobs, J. Rapid Diagnostic Tests for Neglected Infectious Diseases: Case Study Highlights Need for Customer Awareness and Postmarket Surveillance. PLoS Negl. Trop. Dis. 2016, 10, e000465. [Google Scholar] [CrossRef] [Green Version]
- Fricke, T.R.; Tahhan, N.; Resnikoff, S.; Papas, E.; Burnett, A.; Ho, S.M.; Naduvilath, T.; Naidoo, K.S. Global Prevalence of Presbyopia and Vision Impairment from Uncorrected Presbyopia: Systematic Review, Meta-Analysis, and Modelling. Ophthalmology 2018, 125, 1492–1499. [Google Scholar] [CrossRef]
- Naidoo, K.; Kempen, J.H.; Gichuhi, S.; Braithwaite, T.; Casson, R.J.; Cicinelli, M.V.; Das, A.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.E.; et al. Prevalence and Causes of Vision Loss in Sub-Saharan Africa in 2015: Magnitude, Temporal Trends and Projections. Br. J. Ophthalmol. 2020, 104, 1658–1668. [Google Scholar] [CrossRef]
- Pelayo, S.; Marcilly, R.; Bellandi, T. Human Factors Engineering for Medical Devices: European Regulation and Current Issues. Int. J. Qual. Health Care 2021, 33, 31–36. [Google Scholar] [CrossRef]
- Daily, J.; Cameron, A.; Galluzo, K.; Barrett, K.; Dittrich, S.; Fernandez, L. Biomarkers for Acute Febrile Illness at the Point of Care in Low-Resource Settings; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. WHO List of Prequalified in Vitro Diagnostic Products; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. Health Technology Assessment of Medical Devices; WHO: Geneva, Switzerland, 2011. [Google Scholar]

| Tympanic Thermometer | Multimodal Oximeter | Glucometer | Hemoglobinometer | |
|---|---|---|---|---|
| Format and languages | Digital: CD-ROM, online PDF (EN, FR, NL, oth.) | Oximeter: Printed (NL), Digital: online PDF (EN) Sensor: Printed (EN, FR, oth.) | Printed and digital: online PDF (EN, FR, NL, oth.) | Printed and digital: online PDF (EN, FR, NL) |
| Readability score (in brackets) of French sampling instructions | Primary school level (60) | Based on IFU sensor only:Primary school level (66) | Primary school level (52) | Primary school level (51) |
| Symbol Key (explanation of symbols used) | Present | Present | Present | Present |
| Figures |
|
|
|
|
| Comments from end-users to adapt sampling instructions to real life situations in low-resource settings |
|
|
|
|
| Tympanic Thermometer | Multimodal Oximeter | |
|---|---|---|
| Frequency of use (numbers refer to study HCW: n = 8) | Daily: n = 8 | Daily: n = 1 2 – 4 days/month: n = 5 < 1 day/month: n = 2 |
| Training & adoption | 🗸 Rapidity facilitates patient flow 🗶 Difficult to learn correct position 🗶 Large size hampers integration in routine care 🗶 Non-function if environmental temperature is too high hampers integration in routine care | 🗶 Thorough training & practice is needed. 🗶 Difficult to know when & how to change sensitivity mode 🗶 Difficult to integrate in routine care due to slow performance & unreliable respiratory rate: “It makes the user suffer” |
| Measurement preparation | 🗸 Few steps & rapid 🗸 Ear is an easily accessible body site 🗸 Difficult access if child moves or is afraid 🗶 Sometimes difficult to take up probes from base & attach them to the thermometer 🗶 Inspection of ear canal needed | 🗸 Few steps & rapid if child is calm 🗶 Some children refuse the sensor application & it can take long to calm the child: “You sometimes have to wait until the child sleeps.” |
| Measurement | 🗸 Rapid 🗸 Easy, few steps 🗸 “Does not miss subjective fever” 🗶 Thermometer shuts down if you are too slow 🗶 Not reliable (too low) if not well positioned, if child moves, or if ear canal is wet because the child is sweety or freshly washed 🗶 Impression that the device displays the previous temperature if measurement interval between two consecutive measurements is too short, although probe was changed | 🗸 Measurement of oxygen saturation and heart rate is timely and reliable if child is calm 🗶 Measurement of oxygen saturation and heart rate take time & some- times multiple attempts needed. The plethysmography curve is irregu- lar if the child moves too much 🗶 Measurement can be so slow that a child that was initially calm gets agitated during measurement 🗶 Respiratory rate measurement is unreliable & slow |
| Result display | 🗸 Easy to read 🗸 Rapid 🗸 Memory function | 🗸 Good readability of numbers 🗸 Plethysmography curve displayed 🗶 Preliminary result displays in grey, this is not described in IFU. It can take long until definitive measurement displays in white. 🗶 If measurement is unsuccessful “- -“ is displayed. There is no error message explaining the error source. 🗶 Continuous measurement: you must be quick to read measurement be- fore sensor disconnection & there is no memory function |
| Maintenance | 🗸 Easy cleaning 🗶 Need for screwdriver to change batteries | 🗸 Easy cleaning 🗸 Rechargeable with USB-cable 🗶 Careful cleaning of electronic connection between sensor & oximeter |
| Quality control & calibration | 🗶 Need for frequent recalibration 🗶 No recalibration on site possible | 🗸 No need for recalibration 🗶 No external quality control possible |
| Hygiene & security for HCW | 🗸 No need to touch used probes (eject button) 🗶 Need to carry along a small waste bin to throw away used probes | 🗸 Feel comfortable during use |
| Security and comfort for patient & caretaker | 🗸 Parents trust the device 🗸 Considered as a play (“telephone”) by some children 🗶 Other children are afraid | 🗸 Non-invasive technique 🗸 High-tech interface is considered as high-quality care 🗶 Caretakers associate the use of an oximeter with the presence of severe illness & think that it can predict disease prognosis 🗶 The alarm sounds scare the caretakers and children: “Children are really afraid, it’s a war.” |
| Size & bedside testing | 🗸 Rapid 🗶 Too big and heavy 🗶 Size hampers integration in routine care 🗶 Probe is too large for ears of small children | 🗸 Not heavy 🗶 Sensor is too large for smallest infants |
| Suggestions | > Reduce size & weight > Reusable probes, cleaning instead of probes > Enable on site recalibration | > Spot-check mode with memory function (available on market, was not deliverable upon order) > If not improved, omit respiratory rate measurement > Integrate screen in sensor to make device smaller > Improve function in severely ill children > Provide a smaller sensor for the smallest infants > Add an indicator led light that illuminates when the device is charging |
| x | Glucometer | Hemoglobinometer |
|---|---|---|
| Frequency of use | Daily: n = 1 2 – 4 days/month: n = 5 < 1 day/month: n = 2 | Daily: n = 1 2 – 4 days/month: n = 5 < 1 day/month: n = 2 |
| Training & adoption | 🗸 For most staff: easy & quick learning 🗸 Rapidity facilitates patient flow 🗶 For less experienced nurses: initially a bit complex to remem- ber & organize all different steps | 🗸 Rapidity facilitates patient flow 🗸 Not difficult to learn which steps must be done 🗶 Correct sampling requires practice & experience 🗶 Confusion in the first weeks of use due to variable reliability of results compared to results routine lab. Improved after new sampling instructions. Trust in Hemocue Hb801 restored after on-site comparison with routine hematocrit values |
| Measurement preparation | 🗶 Cleaning of skin is difficult because fingers are often very dirty & running water is not available. It takes min. 3 disinfection steps to clean the skin. 🗶 If cleaning is not properly done, sugar residues on the skin cause falsely high glucose measurements 🗶 Slow: Waiting until disinfectant completely dries & waiting until device is ready for blood application after strip insertion 🗶 Strip must be correctly oriented upon insertion. If not: coded error message appears | 🗸 Easy to prepare measurement 🗶 If device activated too soon, it returns to sleeping mode be- fore microcuvette is filled & ready for insertion |
| Measurement | 🗸 Few steps required, rapid 🗸Blood automatically aspired in strip upon contact 🗶 Entry point for blood aspiration is small and it is counterintui- tive & impractical that it is located at the front edge, instead of on top of the strip, particularly difficult if child moves a lot 🗶 Blood glucose is last test that is sampled from the finger prick. Sometimes a 2nd finger prick is needed because meanwhile the blood stopped flowing | 🗸 Few steps required, rapid 🗸 Blood automatically aspired in strip upon contact 🗶 Difficult to fill microcuvette correctly/completely: if child moves, if blood drop is small, if drop is poorly delineated (particularly in severely anemic children). 🗶 Massing finger or strong pressure on finger can disturb result |
| Result display | 🗸 Easy to read 🗸 Rapid 🗸 Memory function 🗶 Error messages are not self-explanatory | 🗸 Easy to read 🗸 Rapid 🗸 Memory function 🗶 Error messages are not self-explanatory |
| Maintenance | 🗸 Easy cleaning 🗸 Easy to change battery 🗶 “Once, I accidently entered the set-up mode & could not use the de vice anymore until the principal investigator explained me how to leave the set-up mode.” | 🗸 Easy to change batteries 🗶 Cleaning is a bit delicate & complicated: concentration needed to clean the interior part without leaving cotton particles; wait until all parts are dry before reassembling; sometimes difficult to reinsert the microcuvette support 🗶 Once we suddenly could not turn on device anymore: AAA batteries corroded. Problem resolved by changing batteries |
| Quality control & calibration | 🗸 The quality control has always been within range. 🗶 Quality control is very complex: many steps, “sometimes you must click once & sometimes twice”, need to consult the proce- dure because failure to remember all the steps. 🗶 The 2 different control liquids are easily confused. | 🗸 Quality control is easy & has always been within range 🗶 Biosecurity risk because quality control fluid is blood 🗶 Quality control fluid is expensive and difficult to get and store due to cold chain requirements |
| Hygiene & security for HCW | 🗸 Small blood volumes increase safety. 🗸 All staff feels comfortable during the procedure. | 🗸 All staff feels comfortable during the procedure 🗶 A large drop of blood is needed: there is a risk that the blood starts flowing if the drop gets too large |
| Security and comfort for patient & caretaker | 🗸 Caretakers aware of importance of glycemia for good health: glycemia measurement highly appreciated by caretakers 🗶 Children are afraid from the finger prick and cry. Caretakers are sometimes afraid too 🗶 Caretakers worried if measurement must be repeated when glycemia possibly falsely elevated due to sugar residues on child’s hands | 🗸 Caretakers want to know if their child is anemic and if their child needs a transfusion 🗸 Caretakers are not worried about the measurement 🗸 Caretakers are very happy with the instant results 🗶 Children are afraid from the finger prick and cry Caretakers are sometimes afraid too |
| Size & bedside testing | 🗸 Small & not heavy, fits in your pocket 🗶 Single use strips 🗶 Strips not universal for all glucometers | 🗸 Small & not heavy 🗸 Easy to carry around |
| Suggestions | > Universal strips > Change to rechargeable device > Switch to non-coded, self-explanatory error messages > Simplify blood sampling: on top instead of edge strip > Appropriate disinfectant / cleaning solution | > Opt for rechargeable battery (already available on market) instead of AA battery (used in current study) > Switch to non-coded, self-explanatory error messages > Facilitate correct use by clear sampling instructions or training support |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tack, B.; Vita, D.; Mansosa, I.; Mbaki, T.N.; Wasolua, N.; Luyindula, A.; Toelen, J.; Lunguya, O.; Jacobs, J. Field Experiences with Handheld Diagnostic Devices to Triage Children under Five Presenting with Severe Febrile Illness in a District Hospital in DR Congo. Diagnostics 2022, 12, 746. https://doi.org/10.3390/diagnostics12030746
Tack B, Vita D, Mansosa I, Mbaki TN, Wasolua N, Luyindula A, Toelen J, Lunguya O, Jacobs J. Field Experiences with Handheld Diagnostic Devices to Triage Children under Five Presenting with Severe Febrile Illness in a District Hospital in DR Congo. Diagnostics. 2022; 12(3):746. https://doi.org/10.3390/diagnostics12030746
Chicago/Turabian StyleTack, Bieke, Daniel Vita, Irène Mansosa, Thomas Nsema Mbaki, Naomie Wasolua, Aimée Luyindula, Jaan Toelen, Octavie Lunguya, and Jan Jacobs. 2022. "Field Experiences with Handheld Diagnostic Devices to Triage Children under Five Presenting with Severe Febrile Illness in a District Hospital in DR Congo" Diagnostics 12, no. 3: 746. https://doi.org/10.3390/diagnostics12030746
APA StyleTack, B., Vita, D., Mansosa, I., Mbaki, T. N., Wasolua, N., Luyindula, A., Toelen, J., Lunguya, O., & Jacobs, J. (2022). Field Experiences with Handheld Diagnostic Devices to Triage Children under Five Presenting with Severe Febrile Illness in a District Hospital in DR Congo. Diagnostics, 12(3), 746. https://doi.org/10.3390/diagnostics12030746






