Imaging Evaluation of Intervertebral Disc Degeneration and Painful Discs—Advances and Challenges in Quantitative MRI
Abstract
1. Introduction
2. Mechanism of IVD Degeneration
2.1. Structure and Characteristics of IVD
2.2. Mechanism of Degeneration
3. Clinical Features and MRI Diagnosis of Discogenic LBP
3.1. T2-Weighted Imaging (T2WI)
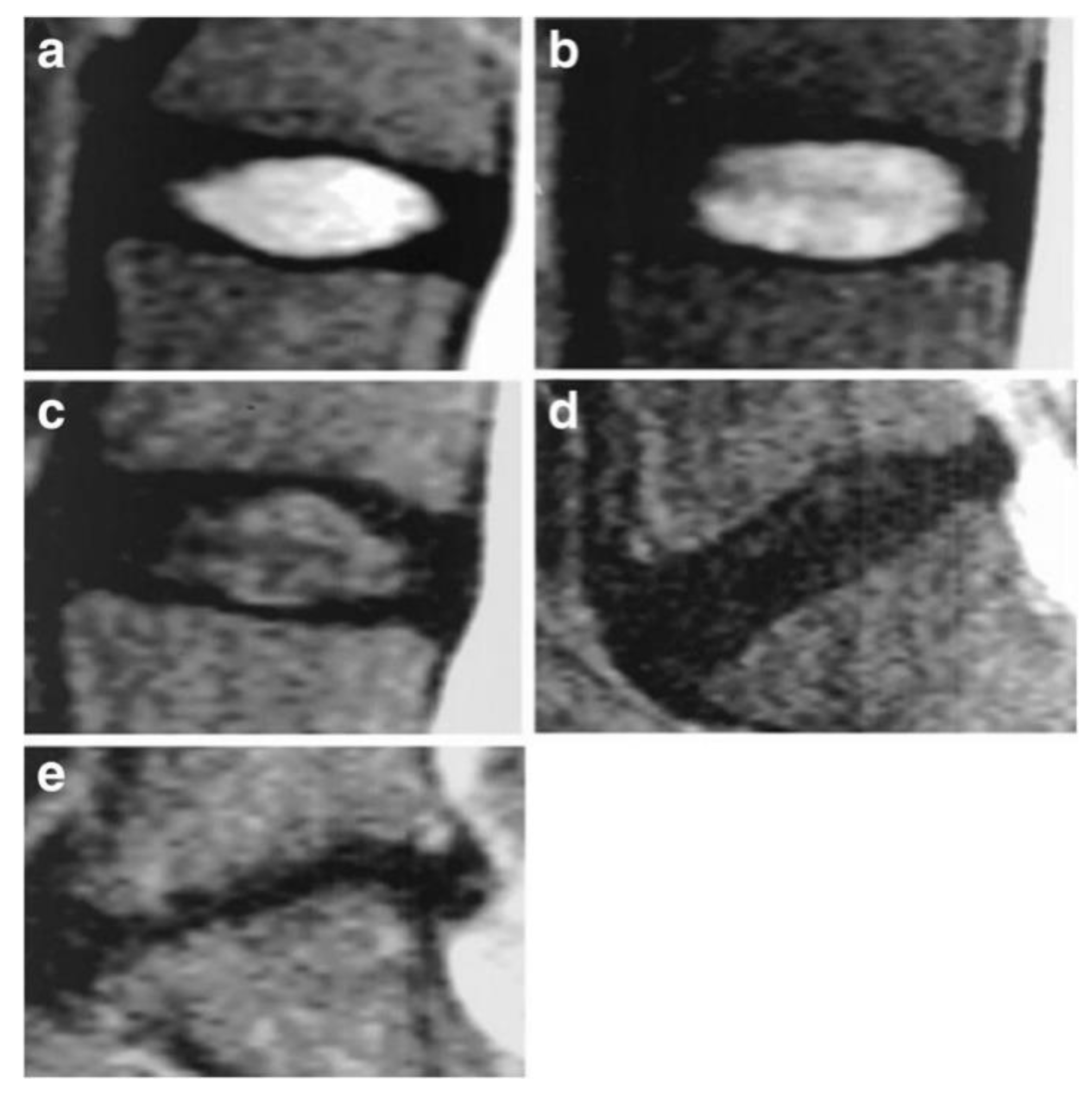
3.2. Pfirrmann Classification
3.3. Modic Changes
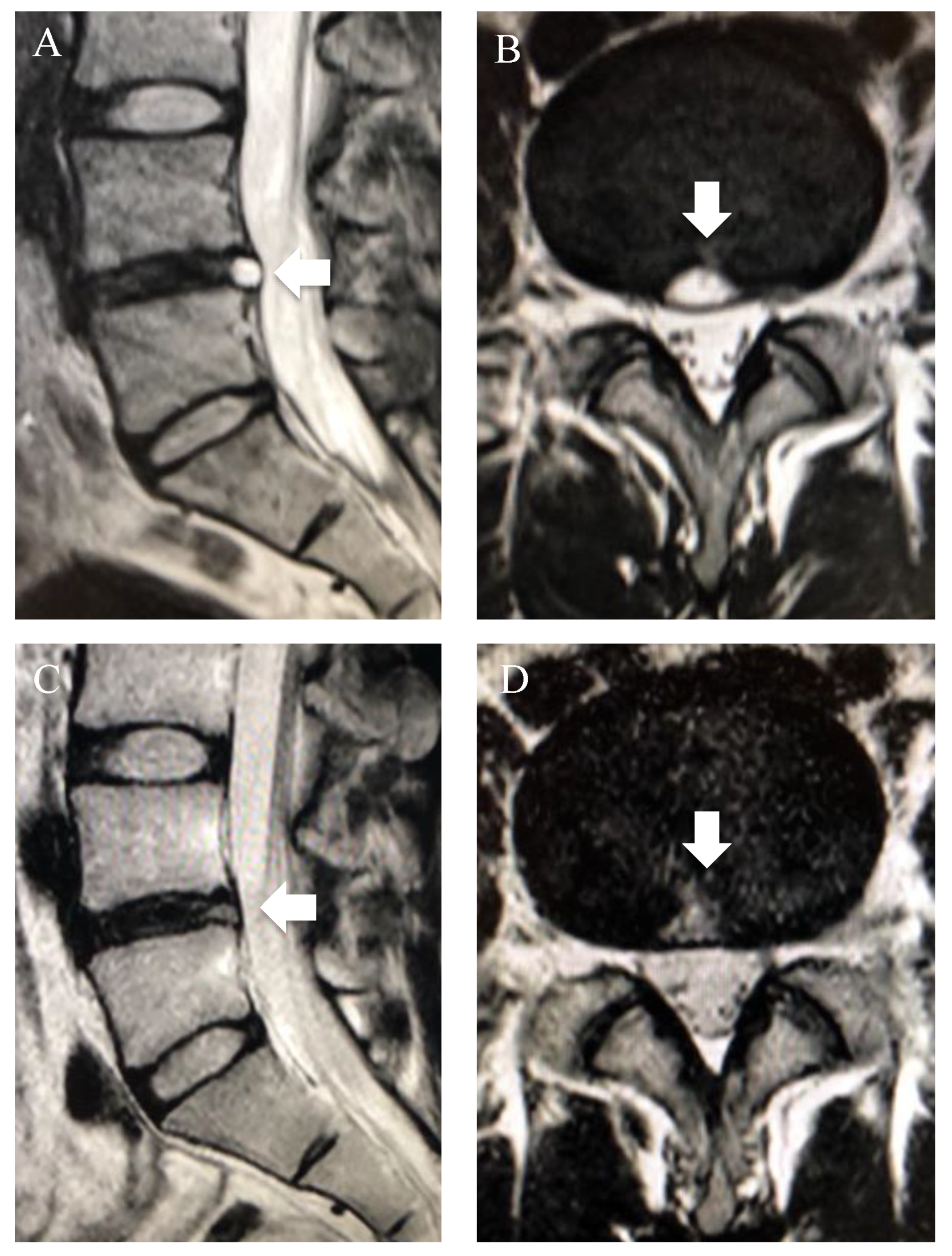
3.4. High-Intensity Zone (HIZ)
4. Quantitative MRI in IVD Degeneration
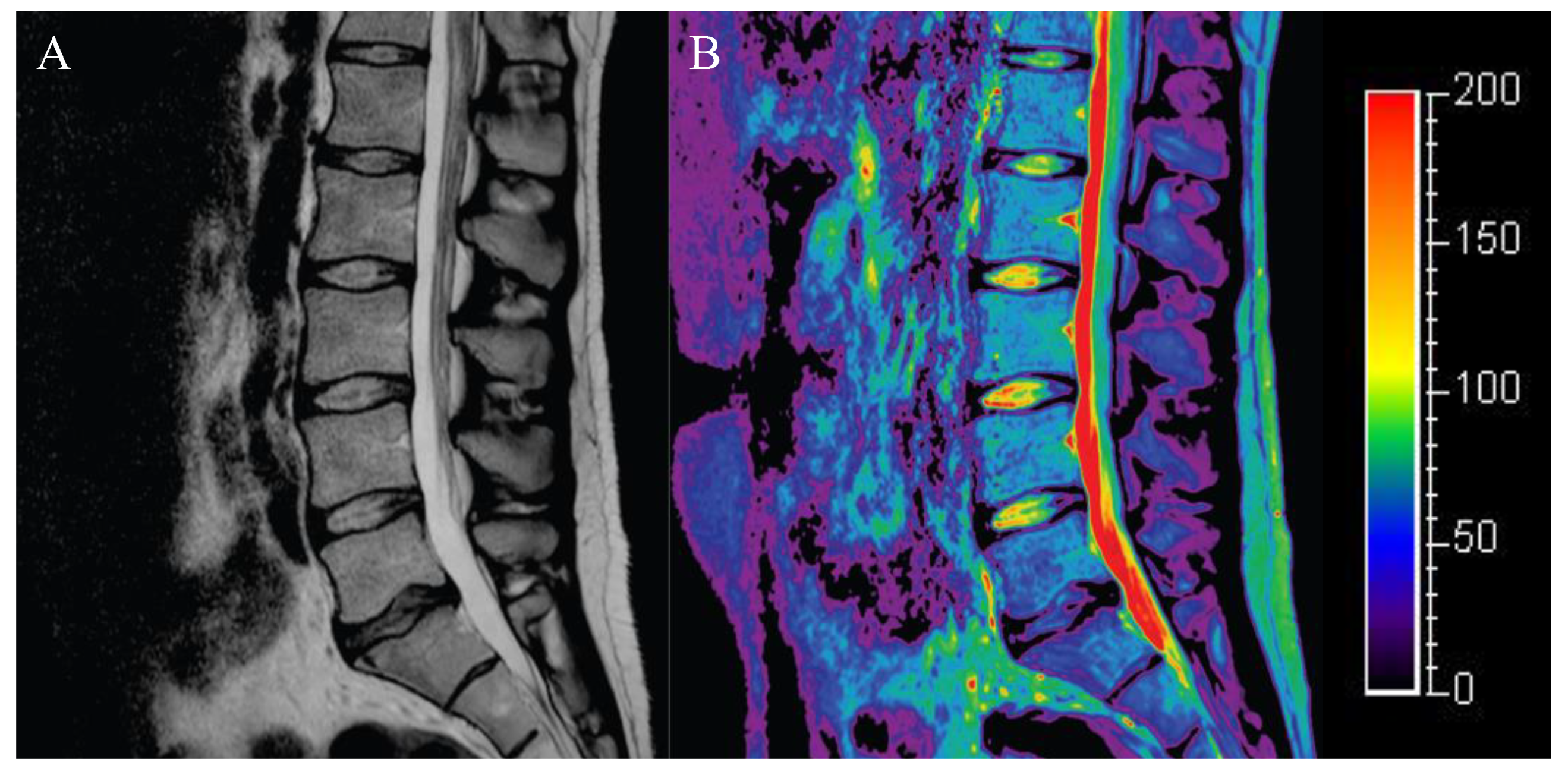
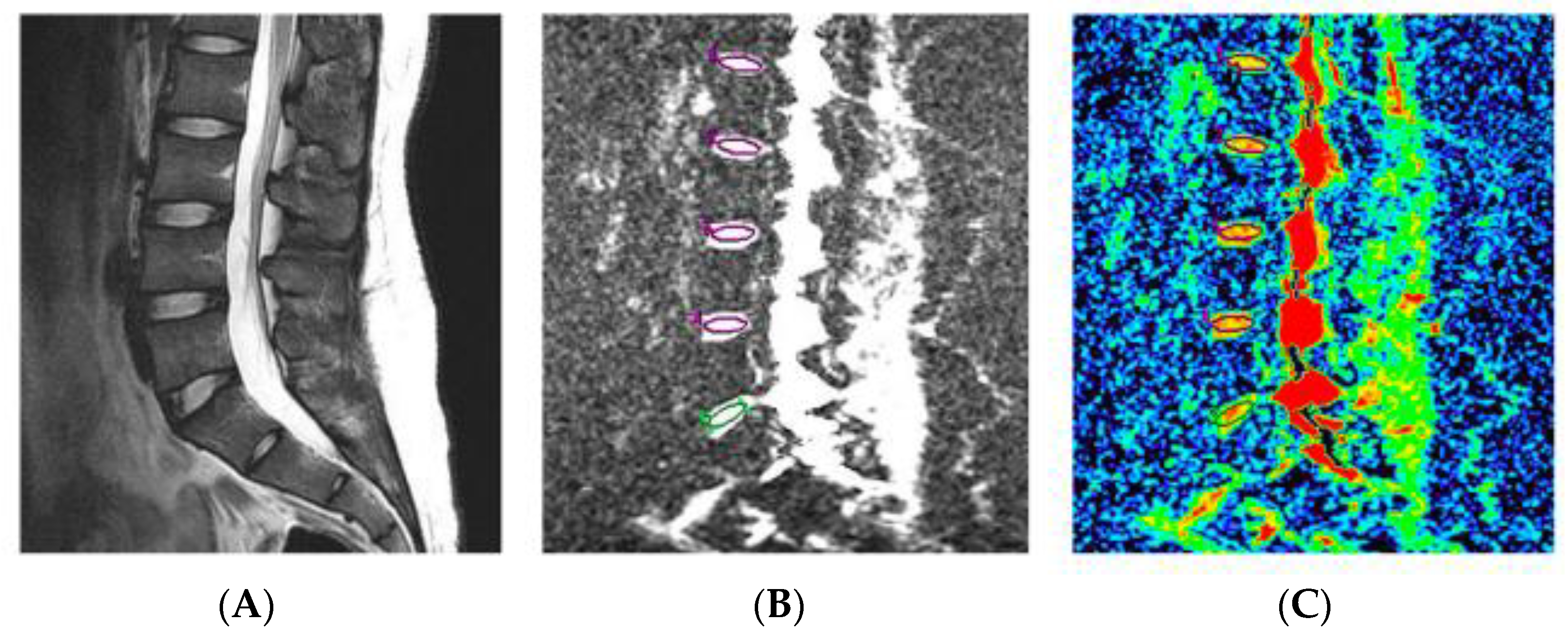
4.1. T2 Mapping
4.2. Diffusion-Weighted MRI with ADC Mapping
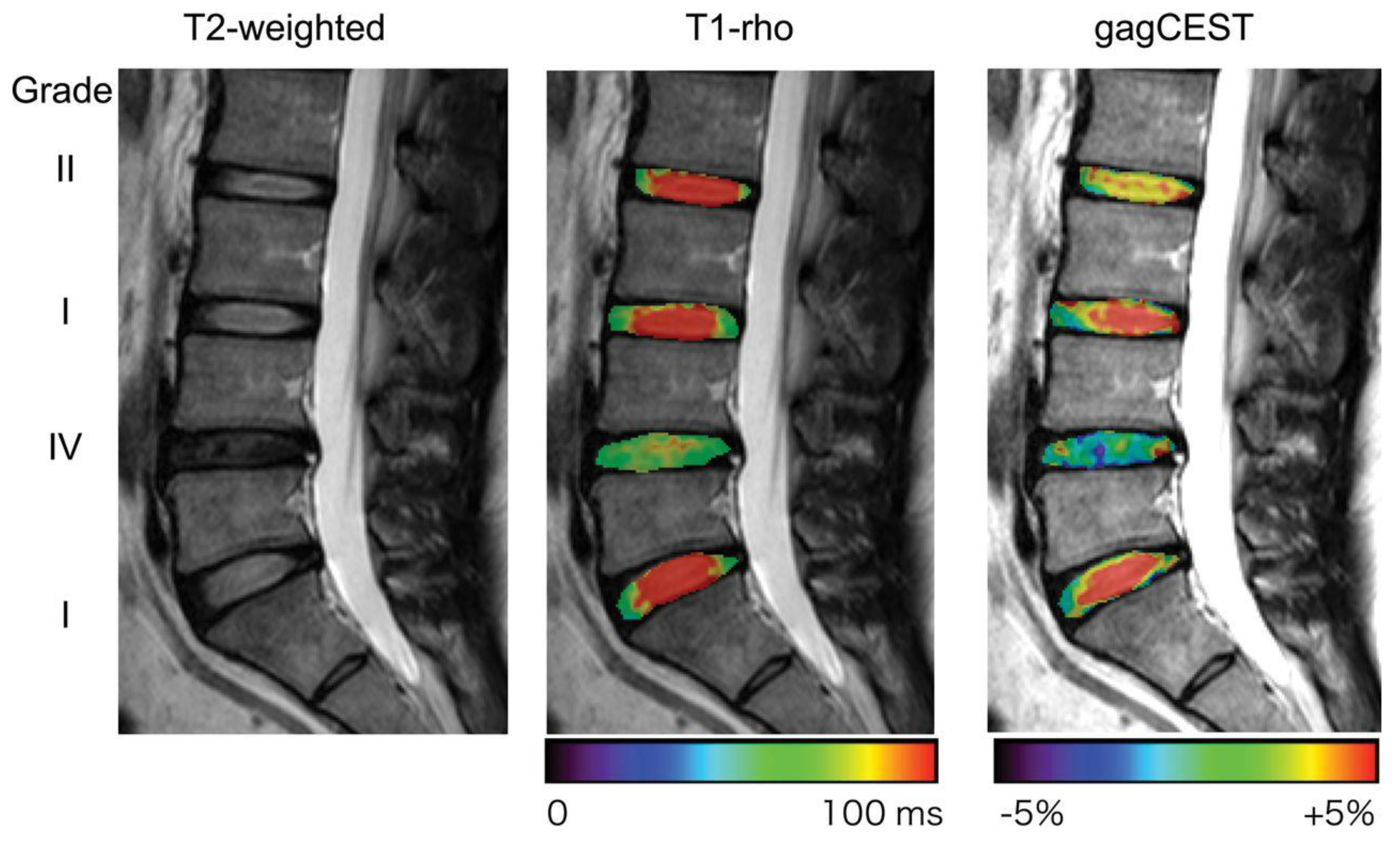
4.3. T1ρ Mapping
4.4. Sodium MRI
4.5. Q-Space Imaging (QSI)
4.6. Chemical Exchange Saturation Transfer (CEST)
4.7. MR Spectroscopy (MRS)
4.8. Ultrahigh-Field MRI
5. Identification of Painful Discs
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Katz, J.N. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. 2), 21–24. [Google Scholar] [CrossRef]
- Manchikanti, L.; Singh, V.; Pampati, V.; Damron, K.S.; Barnhill, R.C.; Beyer, C.; Cash, K.A. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician 2001, 4, 308–316. [Google Scholar] [CrossRef]
- Livshits, G.; Popham, M.; Malkin, I.; Sambrook, P.N.; Macgregor, A.J.; Spector, T.; Williams, F.M. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann. Rheum. Dis. 2011, 70, 1740–1745. [Google Scholar] [CrossRef]
- DePalma, M.J.; Ketchum, J.M.; Saullo, T. What Is the Source of Chronic Low Back Pain and Does Age Play a Role? Pain Med. 2011, 12, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, A.C.; Aprill, C.N.; Derby, R.; Fortin, J.; Kine, G.; Bogduk, N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine 1995, 20, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. 2003, 5, 120–130. [Google Scholar] [CrossRef]
- Katz, J.N.; Harris, M.B. Clinical practice. Lumbar spinal stenosis. N. Engl. J. Med. 2008, 358, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.C.; Brant-Zawadzki, M.N.; Obuchowski, N.; Modic, M.T.; Malkasian, D.; Ross, J.S. Magnetic resonance imaging of the lumbar spine in people without back pain. N. Engl. J. Med. 1994, 331, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.D.; Davis, D.O.; Dina, T.S.; Patronas, N.J.; Wiesel, S.W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J. Bone Jt. Surg. Am. 1990, 72, 403–408. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.F.; Wang, Y.X.J.; Antonio, G.E.; Choi, K.C.; Yu, A.; Ahuja, A.T.; Leung, P.C. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine 2007, 32, E708–E712. [Google Scholar] [CrossRef]
- Smith, L.J.; Silverman, L.; Sakai, D.; Le Maitre, C.L.; Mauck, R.L.; Malhotra, N.R.; Lotz, J.C.; Buckley, C.T. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine 2018, 1, e1036. [Google Scholar] [CrossRef] [PubMed]
- Schol, J.; Sakai, D. Cell therapy for intervertebral disc herniation and degenerative disc disease: Clinical trials. Int. Orthop. 2019, 43, 1011–1025. [Google Scholar] [CrossRef]
- Takeoka, Y.; Yurube, T.; Nishida, K. Gene Therapy Approach for Intervertebral Disc Degeneration: An Update. Neurospine 2020, 17, 3–14. [Google Scholar] [CrossRef]
- Krupkova, O.; Cambria, E.; Besse, L.; Besse, A.; Bowles, R.; Wuertz-Kozak, K. The potential of CRISPR/Cas9 genome editing for the study and treatment of intervertebral disc pathologies. JOR Spine 2018, 1, e1003. [Google Scholar] [CrossRef]
- Gullbrand, S.E.; Smith, L.J.; Smith, H.E.; Mauck, R.L. Promise, progress, and problems in whole disc tissue engineering. JOR Spine 2018, 1, e1015. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.T.; Hoyland, J.A.; Fujii, K.; Pandit, A.; Iatridis, J.C.; Grad, S. Critical aspects and challenges for intervertebral disc repair and regeneration-Harnessing advances in tissue engineering. JOR Spine 2018, 1, e1029. [Google Scholar] [CrossRef]
- Marchand, F.; Ahmed, A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990, 15, 402–410. [Google Scholar] [CrossRef]
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battie, M.C.; Seguin, C.A. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020, 3, e1123. [Google Scholar] [CrossRef]
- Boos, N.; Weissbach, S.; Rohrbach, H.; Weiler, C.; Spratt, K.F.; Nerlich, A.G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 2002, 27, 2631–2644. [Google Scholar] [CrossRef]
- Cheung, K.M.; Karppinen, J.; Chan, D.; Ho, D.W.; Song, Y.Q.; Sham, P.; Cheah, K.S.; Leong, J.C.; Luk, K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef]
- Lyons, G.; Eisenstein, S.M.; Sweet, M.B. Biochemical changes in intervertebral disc degeneration. Biochim. Biophys. Acta 1981, 673, 443–453. [Google Scholar] [CrossRef]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef]
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 1996, 98, 996–1003. [Google Scholar] [CrossRef]
- Roberts, S.; Caterson, B.; Menage, J.; Evans, E.H.; Jaffray, D.C.; Eisenstein, S.M. Matrix metalloproteinases and aggrecanase: Their role in disorders of the human intervertebral disc. Spine 2000, 25, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. Accelerated cellular senescence in degenerate intervertebral discs: A possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res. 2007, 9, R45. [Google Scholar] [CrossRef] [PubMed]
- Nachemson, A.L. Disc pressure measurements. Spine 1981, 6, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Kinoshita, T.; Yamashita, M.; Inoue, G.; Yamauchi, K.; Koshi, T.; Suzuki, M.; Orita, S.; Eguchi, Y.; Nakamura, S.; et al. Results of surgery for discogenic low back pain: A randomized study using discography versus discoblock for diagnosis. Spine 2009, 34, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.C.; Chenot, J.F.; van Tulder, M.; Koes, B.W. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.P.B.; Tognarelli, J.M.; Crossey, M.M.E.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J.W. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Gengwu, L.; Chao, C.; Yifan, L.; Shang, S.; Ruixi, H.; Yunhan, J.; Xiaodong, Z.; Zhikun, L. Correlations between the sagittal plane parameters of the spine and pelvis and lumbar disc degeneration. J. Orthop. Surg. Res. 2018, 13, 137. [Google Scholar] [CrossRef]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Urquhart, D.M.; Wang, Y.; Wluka, A.E.; O’Sullivan, R.; Jones, G.; Cicuttini, F.M. Modic changes in the lumbar spine and their association with body composition, fat distribution and intervertebral disc height—A 3.0 T-MRI study. BMC Musculoskelet. Disord. 2016, 17, 92. [Google Scholar] [CrossRef]
- Toyone, T.; Takahashi, K.; Kitahara, H.; Yamagata, M.; Murakami, M.; Moriya, H. Vertebral bone-marrow changes in degenerative lumbar disc disease. An MRI study of 74 patients with low back pain. J. Bone Jt. Surg. Br. 1994, 76, 757–764. [Google Scholar] [CrossRef]
- Ohtori, S.; Inoue, G.; Ito, T.; Koshi, T.; Ozawa, T.; Doya, H.; Saito, T.; Moriya, H.; Takahashi, K. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine 2006, 31, 1026–1031. [Google Scholar] [CrossRef]
- Albert, H.B.; Manniche, C.; Sorensen, J.S.; Deleuran, B.W. Antibiotic treatment in patients with low-back pain associated with Modic changes Type 1 (bone oedema): A pilot study. Br. J. Sports Med. 2008, 42, 969–973. [Google Scholar] [CrossRef]
- Splendiani, A.; Bruno, F.; Marsecano, C.; Arrigoni, F.; Di Cesare, E.; Barile, A.; Masciocchi, C. Modic I changes size increase from supine to standing MRI correlates with increase in pain intensity in standing position: Uncovering the “biomechanical stress” and “active discopathy” theories in low back pain. Eur. Spine J. 2019, 28, 983–992. [Google Scholar] [CrossRef]
- Jensen, T.S.; Karppinen, J.; Sorensen, J.S.; Niinimäki, J.; Leboeuf-Yde, C. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with non-specific low back pain. Eur. Spine J. 2008, 17, 1407–1422. [Google Scholar] [CrossRef] [PubMed]
- Aprill, C.; Bogduk, N. High-intensity zone: A diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br. J. Radiol. 1992, 65, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, S.; Sakai, D. Imaging evaluation of intervertebral disc degeneration. J. Jpn. Soc. Clin. Sports Med. 2022, 3, 234–240. [Google Scholar]
- Fang, C.; Zhang, W.; Chen, L.; Li, H. The correlation between the high-intensity zone on a T2-weighted MRI and positive outcomes of discography: A meta-analysis. J. Orthop. Surg. Res. 2017, 12, 26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jha, S.C.; Higashino, K.; Sakai, T.; Takata, Y.; Abe, M.; Yamashita, K.; Morimoto, M.; Fukuta, S.; Nagamachi, A.; Sairyo, K. Clinical Significance of High-intensity Zone for Discogenic Low Back Pain: A Review. J. Med. Investig. 2016, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Teraguchi, M.; Yim, R.; Cheung, J.P.-Y.; Samartzis, D. The association of high-intensity zones on MRI and low back pain: A systematic review. Scoliosis Spinal Disord. 2018, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, L.; Mosley, G.E.; Laudier, D.; Gallate, Z.S.; Gansau, J.; Hoy, R.C.; Poeran, J.; Iatridis, J.C. Spatial mapping of collagen content and structure in human intervertebral disk degeneration. JOR Spine 2020, 3, e1129. [Google Scholar] [CrossRef]
- Marinelli, N.L.; Haughton, V.M.; Anderson, P.A. T2 relaxation times correlated with stage of lumbar intervertebral disk degeneration and patient age. Am. J. Neuroradiol. 2010, 31, 1278–1282. [Google Scholar] [CrossRef]
- Stelzeneder, D.; Welsch, G.H.; Kovács, B.K.; Goed, S.; Paternostro-Sluga, T.; Vlychou, M.; Friedrich, K.; Mamisch, T.C.; Trattnig, S. Quantitative T2 evaluation at 3.0 T compared to morphological grading of the lumbar intervertebral disc: A standardized evaluation approach in patients with low back pain. Eur. J. Radiol. 2012, 81, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Benneker, L.M.; Boesch, C.; Watanabe, T.; Obata, T.; Anderson, S.E. Classification of intervertebral disk degeneration with axial T2 mapping. AJR Am. J. Roentgenol. 2007, 189, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Trattnig, S.; Stelzeneder, D.; Goed, S.; Reissegger, M.; Mamisch, T.C.; Paternostro-Sluga, T.; Weber, M.; Szomolanyi, P.; Welsch, G.H. Lumbar intervertebral disc abnormalities: Comparison of quantitative T2 mapping with conventional MR at 3.0 T. Eur. Radiol. 2010, 20, 2715–2722. [Google Scholar] [CrossRef]
- Ogon, I.; Takebayashi, T.; Takashima, H.; Tanimoto, K.; Ida, K.; Yoshimoto, M.; Fujiwara, H.; Kubo, T.; Yamashita, T. Analysis of chronic low back pain with magnetic resonance imaging T2 mapping of lumbar intervertebral disc. J. Orthop. Sci. 2015, 20, 295–301. [Google Scholar] [CrossRef]
- Bruno, F.; Palumbo, P.; Tommasino, E.; Bianchi, G.; Varrassi, M.; Arrigoni, F.; Barile, A.; Di Cesare, E.; Masciocchi, C.; Splendiani, A. Evaluation of intervertebral disc using T2 mapping sequences in patients undergoing O(2)-O(3) chemiodiscolysis: An instrumental study with clinical correlation. Neuroradiology 2020, 62, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Detiger, S.E.L.; Holewijn, R.M.; Hoogendoorn, R.J.W.; van Royen, B.J.; Helder, M.N.; Berger, F.H.; Kuijer, J.P.A.; Smit, T.H. MRI T2* mapping correlates with biochemistry and histology in intervertebral disc degeneration in a large animal model. Eur. Spine J. 2015, 24, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Detiger, S.E.L.; Karfeld-Sulzer, L.S.; Smit, T.H.; Yayon, A.; Weber, F.E.; Helder, M.N. BMP-2 and BMP-2/7 Heterodimers Conjugated to a Fibrin/Hyaluronic Acid Hydrogel in a Large Animal Model of Mild Intervertebral Disc Degeneration. BioRes. Open Access 2015, 4, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Wu, X.T.; Xie, X.H.; Wang, F.; Hong, X.; Zhuang, S.Y.; Zhu, L.; Rui, Y.F.; Shi, R. Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: A study in rabbits. Int. Orthop. 2015, 39, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Obata, S.; Akeda, K.; Imanishi, T.; Masuda, K.; Bae, W.; Morimoto, R.; Asanuma, Y.; Kasai, Y.; Uchida, A.; Sudo, A. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: A preclinical study. Arthritis Res. Ther. 2012, 14, R241. [Google Scholar] [CrossRef]
- Moritani, T.; Kim, J.; Capizzano, A.A.; Kirby, P.; Kademian, J.; Sato, Y. Pyogenic and non-pyogenic spinal infections: Emphasis on diffusion-weighted imaging for the detection of abscesses and pus collections. Br. J. Radiol. 2014, 87, 20140011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, X.; Wang, Y.; Zhao, J.; Zhang, X.; Gao, Y.; Li, S. Assessment of apparent diffusion coefficient in lumbar intervertebral disc degeneration. Eur. Spine J. 2014, 23, 1830–1836. [Google Scholar] [CrossRef]
- Antoniou, J.; Demers, C.N.; Beaudoin, G.; Goswami, T.; Mwale, F.; Aebi, M.; Alini, M. Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Magn. Reson. Imaging 2004, 22, 963–972. [Google Scholar] [CrossRef]
- Wu, N.; Liu, H.; Chen, J.; Zhao, L.; Zuo, W.; Ming, Y.; Liu, S.; Liu, J.; Su, X.; Gao, B.; et al. Comparison of apparent diffusion coefficient and T2 relaxation time variation patterns in assessment of age and disc level related intervertebral disc changes. PLoS ONE 2013, 8, e69052. [Google Scholar] [CrossRef] [PubMed]
- Mochida, J.; Sakai, D.; Nakamura, Y.; Watanabe, T.; Yamamoto, Y.; Kato, S. Intervertebral disc repair with activated nucleus pulposus cell transplantation: A three-year, prospective clinical study of its safety. Eur. Cells Mater. 2015, 29, 202–212, discussion 212. [Google Scholar] [CrossRef] [PubMed]
- Shalash, W.; Ahrens, S.R.; Bardonova, L.A.; Byvaltsev, V.A.; Giers, M.B. Patient-specific apparent diffusion maps used to model nutrient availability in degenerated intervertebral discs. JOR Spine 2021, 4, e1179. [Google Scholar] [CrossRef] [PubMed]
- Akella, S.V.; Regatte, R.R.; Gougoutas, A.J.; Borthakur, A.; Shapiro, E.M.; Kneeland, J.B.; Leigh, J.S.; Reddy, R. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn. Reson. Med. 2001, 46, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, W.; Auerbach, J.D.; Wheaton, A.J.; Kurji, A.; Borthakur, A.; Reddy, R.; Elliott, D.M. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine 2006, 31, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Togao, O.; Hiwatashi, A.; Wada, T.; Yamashita, K.; Kikuchi, K.; Tokunaga, C.; Keupp, J.; Yoneyama, M.; Honda, H. A Qualitative and Quantitative Correlation Study of Lumbar Intervertebral Disc Degeneration Using Glycosaminoglycan Chemical Exchange Saturation Transfer, Pfirrmann Grade, and T1-ρ. Am. J. Neuroradiol. 2018, 39, 1369–1375. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr. Course Lect. 1998, 47, 487–504. [Google Scholar] [PubMed]
- Takayama, Y.; Hatakenaka, M.; Tsushima, H.; Okazaki, K.; Yoshiura, T.; Yonezawa, M.; Nishikawa, K.; Iwamoto, Y.; Honda, H. T1ρ is superior to T2 mapping for the evaluation of articular cartilage denaturalization with osteoarthritis: Radiological-pathological correlation after total knee arthroplasty. Eur. J. Radiol. 2013, 82, e192–e198. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, A.; Maurer, P.M.; Fenty, M.; Wang, C.; Berger, R.; Yoder, J.; Balderston, R.A.; Elliott, D.M. T1ρ magnetic resonance imaging and discography pressure as novel biomarkers for disc degeneration and low back pain. Spine 2011, 36, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Fenty, M.; Crescenzi, R.; Fry, B.; Squillante, D.; Turk, D.; Maurer, P.M.; Borthakur, A. Novel imaging of the intervertebral disk and pain. Glob. Spine J. 2013, 3, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, G.; Zuo, J.; Li, X.; Kornak, J.; Link, T.M.; Majumdar, S. In vivo 3.0-tesla magnetic resonance T1ρ and T2 relaxation mapping in subjects with intervertebral disc degeneration and clinical symptoms. Magn. Reson. Med. 2010, 63, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Zobel, B.B.; Vadalà, G.; Del Vescovo, R.; Battisti, S.; Martina, F.M.; Stellato, L.; Leoncini, E.; Borthakur, A.; Denaro, V. T1 ρ Magnetic resonance imaging quantification of early lumbar intervertebral disc degeneration in healthy young adults. Spine 2012, 37, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; McArdle, E.; Fenty, M.; Witschey, W.; Elliott, M.; Sochor, M.; Reddy, R.; Borthakur, A. Validation of sodium magnetic resonance imaging of intervertebral disc. Spine 2010, 35, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Noebauer-Huhmann, I.-M.; Juras, V.; Pfirrmann, C.W.A.; Szomolanyi, P.; Zbyn, S.; Messner, A.; Wimmer, J.; Weber, M.; Friedrich, K.M.; Stelzeneder, D.; et al. Sodium MR Imaging of the Lumbar Intervertebral Disk at 7 T: Correlation with T2 Mapping and Modified Pfirrmann Score at 3 T—Preliminary Results. Radiology 2012, 265, 555–564. [Google Scholar] [CrossRef]
- Moon, C.H.; Jacobs, L.; Kim, J.H.; Sowa, G.; Vo, N.; Kang, J.; Bae, K.T. Part 2: Quantitative proton T2 and sodium magnetic resonance imaging to assess intervertebral disc degeneration in a rabbit model. Spine 2012, 37, E1113–E1119. [Google Scholar] [CrossRef] [PubMed]
- Bihan, D.L. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed. 1995, 8, 375–386. [Google Scholar] [CrossRef]
- Cohen, Y.; Assaf, Y. High b-value q-space analyzed diffusion-weighted MRS and MRI in neuronal tissues—A technical review. NMR Biomed. 2002, 15, 516–542. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef]
- Hori, M.; Fukunaga, I.; Masutani, Y.; Taoka, T.; Kamagata, K.; Suzuki, Y.; Aoki, S. Visualizing non-Gaussian diffusion: Clinical application of q-space imaging and diffusional kurtosis imaging of the brain and spine. Magn. Reson. Med. Sci. 2012, 11, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, K.; Hikishima, K.; Nakahara, J.; Tsuji, O.; Hata, J.; Konomi, T.; Nagai, T.; Shibata, S.; Kaneko, S.; Iwanami, A.; et al. Application of q-Space Diffusion MRI for the Visualization of White Matter. J. Neurosci. 2016, 36, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Suzuki, Y.; Hata, J.; Hori, M.; Sasaki, H.; Akai, H.; Mori, H.; Kunimatsu, A.; Masutani, Y.; Aoki, S.; et al. Non-Gaussian diffusion-weighted imaging for assessing diurnal changes in intervertebral disc microstructure. J. Magn. Reson. Imaging 2014, 40, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, W.; Chen, W.; Fang, J.; Li, J. The study of the intervertebral disc microstructure in matured rats with diffusion kurtosis imaging. Magn. Reson. Imaging 2017, 42, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, D.; Fujita, N.; Hata, J.; Komaki, Y.; Suzuki, S.; Nagura, T.; Fujiyoshi, K.; Watanabe, K.; Tsuji, T.; Okano, H.; et al. Quantitative analysis of intervertebral disc degeneration using Q-space imaging in a rat model. J. Orthop. Res. 2020, 38, 2220–2229. [Google Scholar] [CrossRef]
- Ward, K.M.; Aletras, A.H.; Balaban, R.S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef]
- van Zijl, P.C.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Schleich, C.; Müller-Lutz, A.; Eichner, M.; Schmitt, B.; Matuschke, F.; Bittersohl, B.; Zilkens, C.; Wittsack, H.J.; Antoch, G.; Miese, F. Glycosaminoglycan Chemical Exchange Saturation Transfer of Lumbar Intervertebral Discs in Healthy Volunteers. Spine 2016, 41, 146–152. [Google Scholar] [CrossRef]
- Wada, T.; Togao, O.; Tokunaga, C.; Funatsu, R.; Yamashita, Y.; Kobayashi, K.; Nakamura, Y.; Honda, H. Glycosaminoglycan chemical exchange saturation transfer in human lumbar intervertebral discs: Effect of saturation pulse and relationship with low back pain. J. Magn. Reson. Imaging 2017, 45, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Pulickal, T.; Boos, J.; Konieczny, M.; Sawicki, L.M.; Müller-Lutz, A.; Bittersohl, B.; Gerß, J.; Eichner, M.; Wittsack, H.J.; Antoch, G.; et al. MRI identifies biochemical alterations of intervertebral discs in patients with low back pain and radiculopathy. Eur. Radiol. 2019, 29, 6443–6446. [Google Scholar] [CrossRef] [PubMed]
- Bez, M.; Zhou, Z.; Sheyn, D.; Tawackoli, W.; Giaconi, J.C.; Shapiro, G.; Ben David, S.; Gazit, Z.; Pelled, G.; Li, D.; et al. Molecular pain markers correlate with pH-sensitive MRI signal in a pig model of disc degeneration. Sci. Rep. 2018, 8, 17363. [Google Scholar] [CrossRef] [PubMed]
- Pelled, G.; Salas, M.M.; Han, P.; Gill, H.E.; Lautenschlager, K.A.; Lai, T.T.; Shawver, C.M.; Hoch, M.B.; Goff, B.J.; Betts, A.M.; et al. Intradiscal quantitative chemical exchange saturation transfer MRI signal correlates with discogenic pain in human patients. Sci. Rep. 2021, 11, 19195. [Google Scholar] [CrossRef] [PubMed]
- Proctor, W.G.; Yu, F.C. The Dependence of a Nuclear Magnetic Resonance Frequency upon Chemical Compound. Phys. Rev. 1950, 77, 717. [Google Scholar] [CrossRef]
- Castillo, M.; Kwock, L.; Mukherji, S.K. Clinical applications of proton MR spectroscopy. AJNR Am. J. Neuroradiol. 1996, 17, 1–15. [Google Scholar] [PubMed]
- Keshari, K.R.; Lotz, J.C.; Link, T.M.; Hu, S.; Majumdar, S.; Kurhanewicz, J. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine 2008, 33, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Freml, L.; Park, S.S.; Brennan, T.J. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J. Pain 2007, 8, 59–66. [Google Scholar] [CrossRef]
- Stover, J.D.; Farhang, N.; Berrett, K.C.; Gertz, J.; Lawrence, B.; Bowles, R.D. CRISPR Epigenome Editing of AKAP150 in DRG Neurons Abolishes Degenerative IVD-Induced Neuronal Activation. Mol. Ther. 2017, 25, 2014–2027. [Google Scholar] [CrossRef] [PubMed]
- Gornet, M.G.; Peacock, J.; Claude, J.; Schranck, F.W.; Copay, A.G.; Eastlack, R.K.; Benz, R.; Olshen, A.; Lotz, J.C. Magnetic resonance spectroscopy (MRS) can identify painful lumbar discs and may facilitate improved clinical outcomes of lumbar surgeries for discogenic pain. Eur. Spine J. 2019, 28, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Letertre, M.P.M.; Giraudeau, P.; de Tullio, P. Nuclear Magnetic Resonance Spectroscopy in Clinical Metabolomics and Personalized Medicine: Current Challenges and Perspectives. Front. Mol. Biosci. 2021, 8, 698337. [Google Scholar] [CrossRef] [PubMed]
- Aringhieri, G.; Zampa, V.; Tosetti, M. Musculoskeletal MRI at 7 T: Do we need more or is it more than enough? Eur. Radiol. Exp. 2020, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Sher, I.; Daly, C.; Oehme, D.; Chandra, R.V.; Sher, M.; Ghosh, P.; Smith, J.; Goldschlager, T. Novel Application of the Pfirrmann Disc Degeneration Grading System to 9.4T MRI: Higher Reliability Compared to 3T MRI. Spine 2019, 44, E766–E773. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Tanner, C.M.; Khurana, S.; Hayward, C.; Welsh, J.; Date, E.; Truong, T.; Rossi, M.; Hagle, C. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine 2000, 25, 1373–1380, discussion 1381. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Don, A.S.; Hurwitz, E.L.; Cuellar, J.M.; Carrino, J.A.; Herzog, R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: A ten-year matched cohort study. Spine 2009, 34, 2338–2345. [Google Scholar] [CrossRef]
- Konno, S.I.; Sekiguchi, M. Association between brain and low back pain. J. Orthop. Sci. 2018, 23, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Galbusera, F.; Casaroli, G.; Bassani, T. Artificial intelligence and machine learning in spine research. JOR Spine 2019, 2, e1044. [Google Scholar] [CrossRef] [PubMed]

| Sequences | Measurement Target | Advantages | Disadvantages | Relation to Discogenic LBP |
|---|---|---|---|---|
| T2-weighted imaging | Water content and disc morphology | Most commonly used and well established | Not for quantitative evaluation Reproducibility | △ (Modic change and HIZ) |
| T2 mapping | Water content and to what extent water is “bound” or “free” | Allows for quantitative evaluation of water and collagen content | Not only sensitive to PG content | △ |
| Diffusion-weighted MRI with ADC mapping | Diffusion of water molecules | Allows for evaluation of nutrient availability | Based on the theoretical assumption that the diffusion of water molecules shows a normal distribution | × |
| T1ρ mapping | PG content | Allows for detection of early stages of degeneration | Lack of standardization for imaging settings | △ |
| Sodium MRI | PG content based on sodium concentration | High correlation with PG content | Low SNR Need for specialized hardware | △ |
| Q-space imaging | Restricted diffusion of water molecules | Allows for detection of the degree of restricted diffusion of actual water molecules | Need for longer scan time Little available literature | × |
| gagCEST quantitative CEST | GAG content pH changes | Allows for detection of endogenous GAG content No need for specialized hardware | Low SNR Need for longer scan time | △ |
| MR Spectroscopy | Chemical composition of the trace metabolites | Different characteristics from other MRI sequences | Low SNR Does not allow for identification of the source of each signal | △ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamagawa, S.; Sakai, D.; Nojiri, H.; Sato, M.; Ishijima, M.; Watanabe, M. Imaging Evaluation of Intervertebral Disc Degeneration and Painful Discs—Advances and Challenges in Quantitative MRI. Diagnostics 2022, 12, 707. https://doi.org/10.3390/diagnostics12030707
Tamagawa S, Sakai D, Nojiri H, Sato M, Ishijima M, Watanabe M. Imaging Evaluation of Intervertebral Disc Degeneration and Painful Discs—Advances and Challenges in Quantitative MRI. Diagnostics. 2022; 12(3):707. https://doi.org/10.3390/diagnostics12030707
Chicago/Turabian StyleTamagawa, Shota, Daisuke Sakai, Hidetoshi Nojiri, Masato Sato, Muneaki Ishijima, and Masahiko Watanabe. 2022. "Imaging Evaluation of Intervertebral Disc Degeneration and Painful Discs—Advances and Challenges in Quantitative MRI" Diagnostics 12, no. 3: 707. https://doi.org/10.3390/diagnostics12030707
APA StyleTamagawa, S., Sakai, D., Nojiri, H., Sato, M., Ishijima, M., & Watanabe, M. (2022). Imaging Evaluation of Intervertebral Disc Degeneration and Painful Discs—Advances and Challenges in Quantitative MRI. Diagnostics, 12(3), 707. https://doi.org/10.3390/diagnostics12030707








