Assessment of Liver Fat: Dual-Energy CT versus Conventional CT with and without Contrast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. DECT and Multi-Material Decomposition (MMD)
2.3. Computed Tomography Acquisition
2.4. Image Analysis
2.5. Statistical Analysis
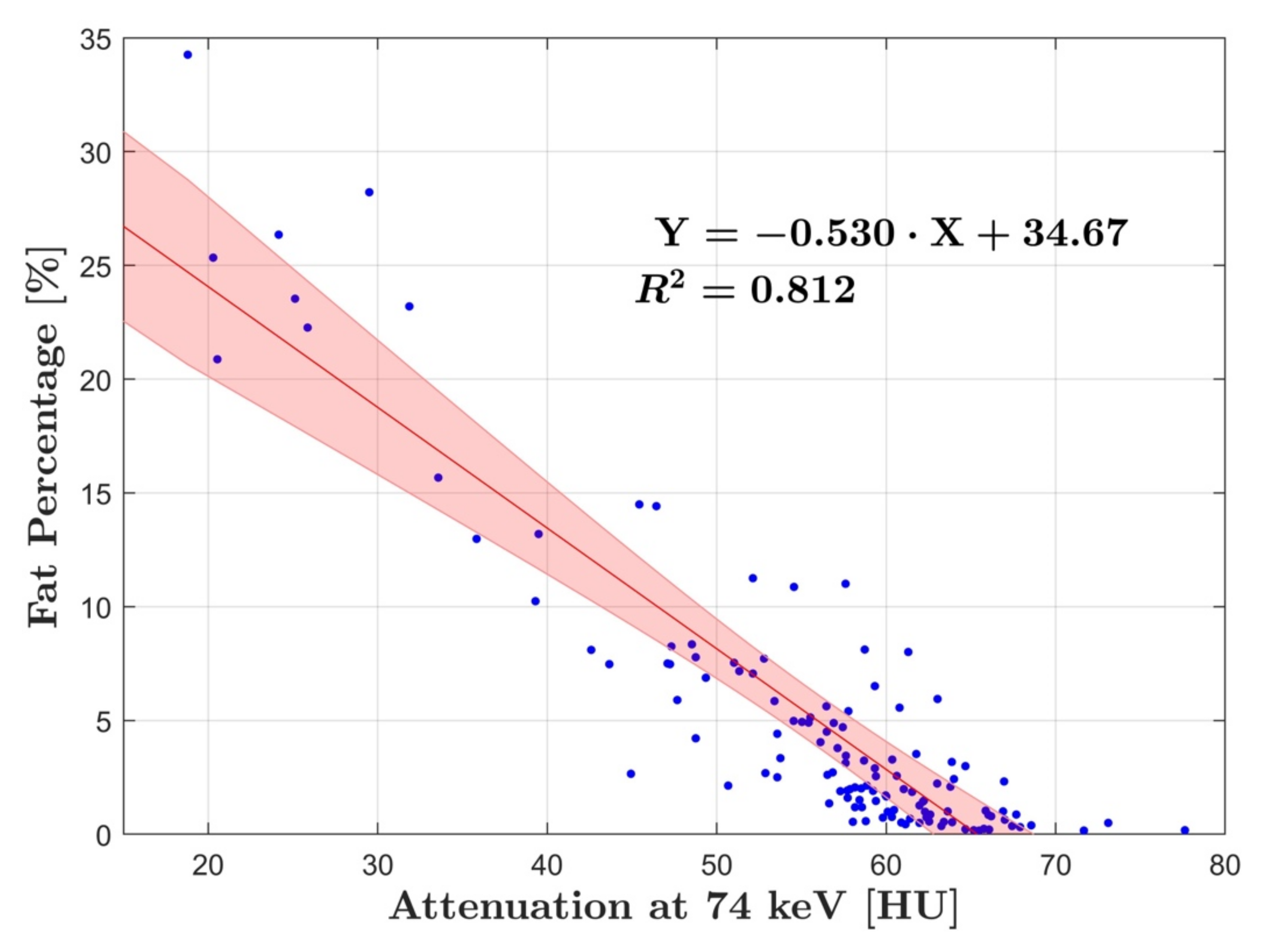
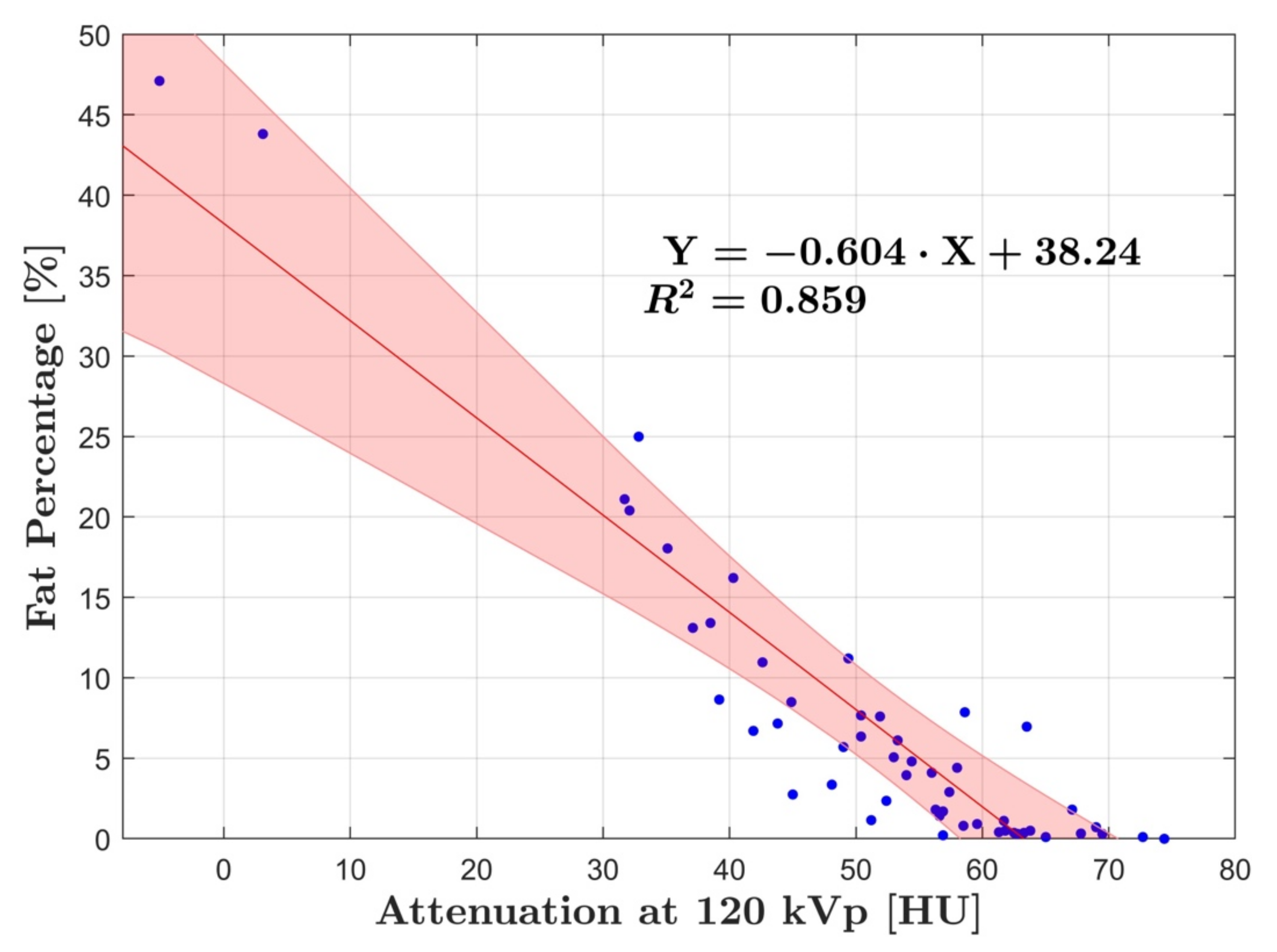
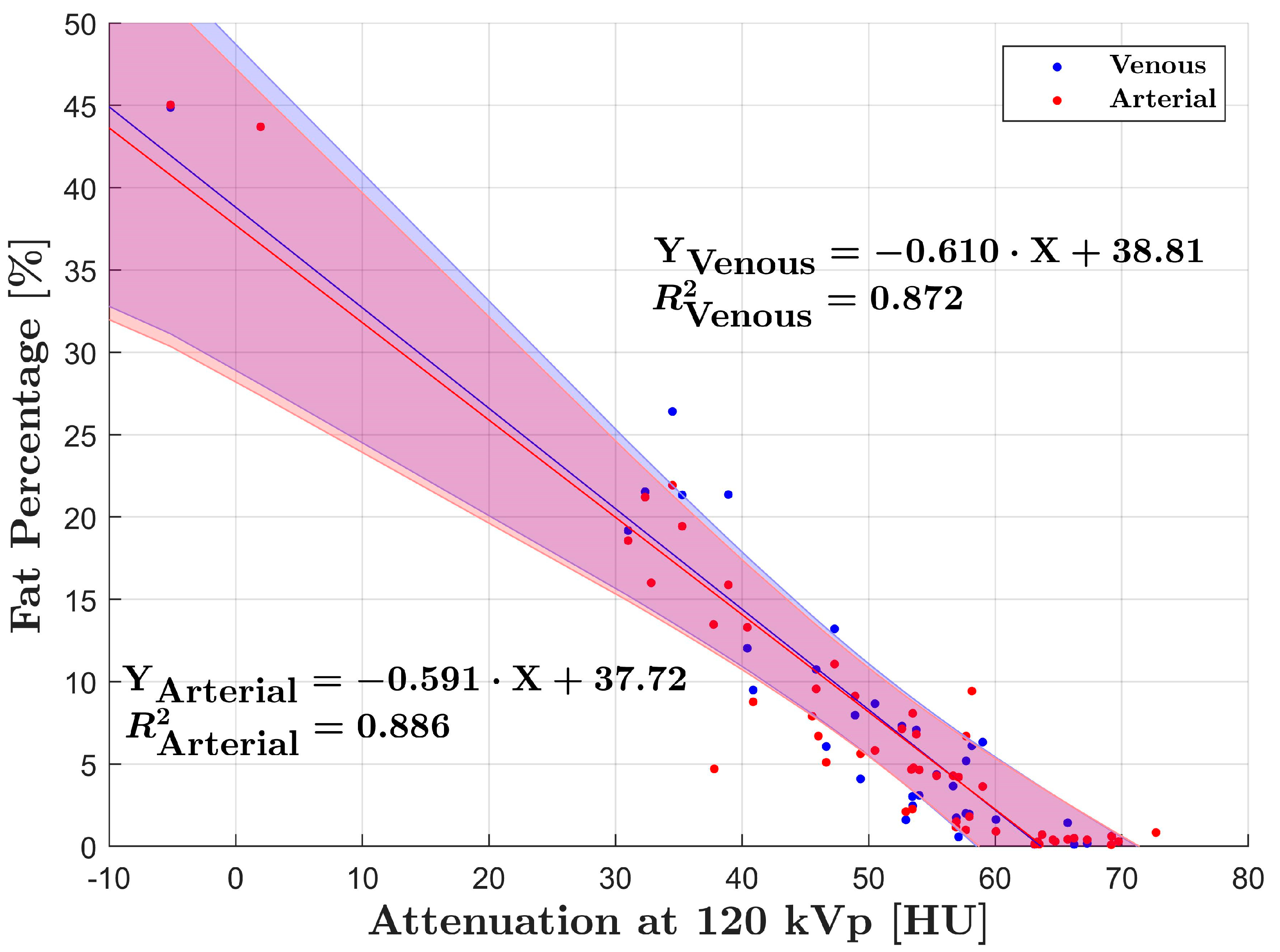
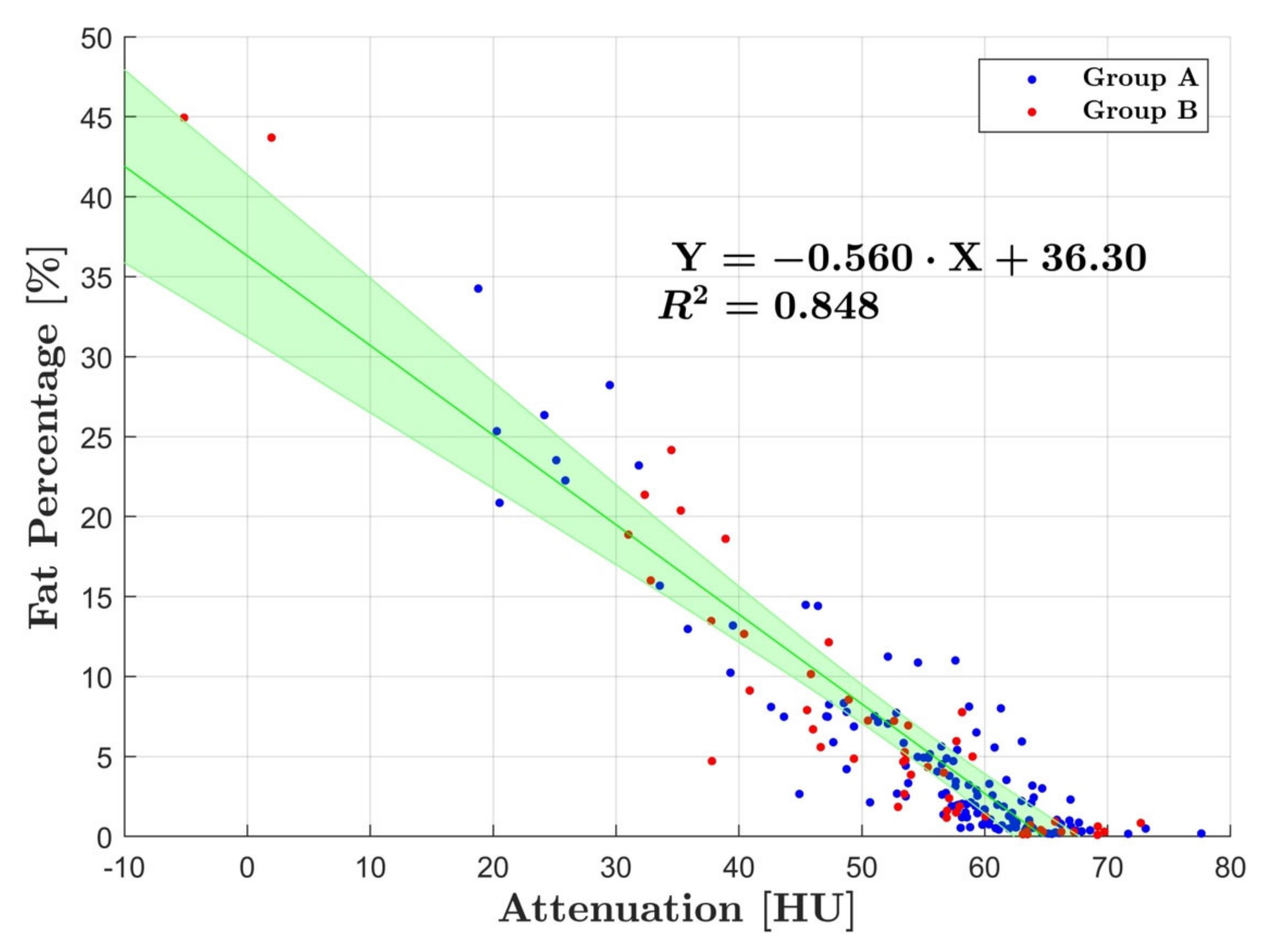
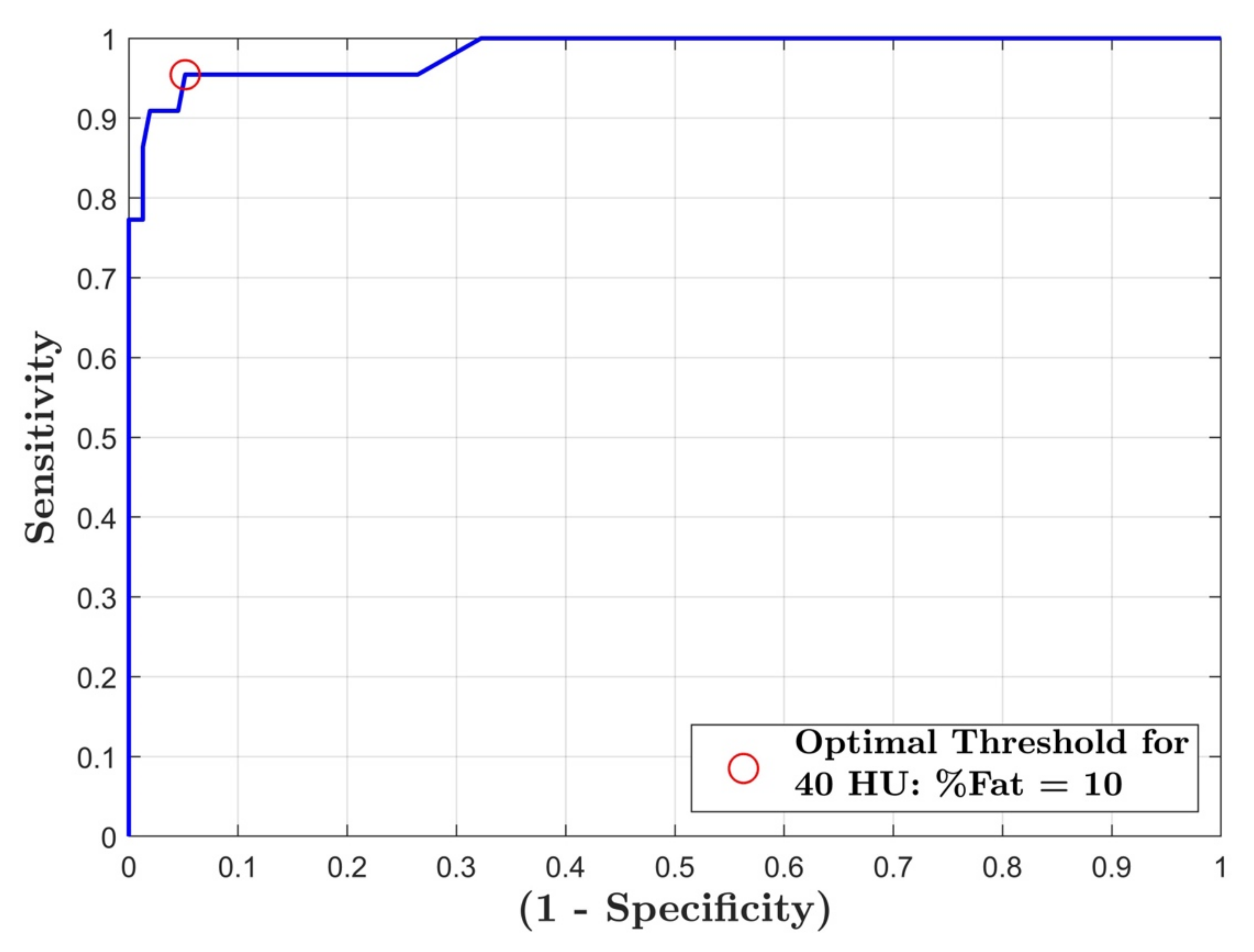
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuoka, H.C.; Chalasani, N. Nonalcoholic fatty liver disease: An emerging threat to obese and diabetic individuals. Ann. N. Y. Acad. Sci. 2013, 1281, 106–122. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. N. Y. 2015, 11, 167–175. [Google Scholar] [PubMed]
- Zhang, Y.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging—A clinical overview of ultrasound, CT, and M R imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef] [Green Version]
- Kodama, Y.; Ng, C.S.; Wu, T.T.; Ayers, G.D.; Curley, S.A.; Abdalla, E.K.; Vauthey, J.N.; Charnsangavej, C. Comparison of CT methods for determining the fat content of the liver. Am. J. Roentgenol. 2007, 188, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Pickhardt, P.J.; Park, S.H.; Hahn, L.; Lee, S.-G.; Bae, K.T.; Yu, E.S. Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: Implications for the investigation of the natural history of incidental steatosis. Eur. Radiol. 2012, 22, 1075–1082. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, P.N.; Kim, K.W.; Lee, S.W.; Yoon, S.E.; Park, S.W.; Ha, H.K.; Lee, M.-G.; Hwang, S.; Lee, S.-G.; et al. Macrovesicular hepatic steatosis in living liver donors: Use of CT for quantitative and qualitative assessment. Radiology 2006, 239, 105–112. [Google Scholar] [CrossRef]
- Lee, D.H. Imaging evaluation of non-alcoholic fatty liver disease: Focused on quantification. Clin. Mol. Hepatol. 2017, 23, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Artz, N.S.; Hines, C.D.G.; Brunner, S.T.; Agni, R.M.; Kühn, J.-P.; Roldan-Alzate, A.; Chen, G.-H.; Reeder, S.B. Quantification of hepatic steatosis with dual-energy computed tomography: Comparison with tissue reference standards and quantitative magnetic resonance imaging in the ob/ob mouse. Investig. Radiol. 2012, 47, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Taudorf, M.; Ulriksen, P.S.; Achiam, M.P.; Resch, T.A.; Nielsen, M.B.; Lönn, L.B.; Hansen, K.L. Gastrointestinal Applications of Iodine Quantification Using Dual-Energy CT: A Systematic Review. Diagnostics 2020, 10, 814. [Google Scholar] [CrossRef]
- Goo, H.W.; Goo, J.M. Dual-energy CT: New horizon in medical imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yu, L.; Primak, A.N.; McCollough, C.H. Quantitative imaging of element composition and mass fraction using dual-energy CT: Three-material decomposition. Med. Phys. 2009, 36, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Wait, J.M.S.; Cody, D.; Jones, A.K.; Rong, J.; Baladandayuthapani, V.; Kappadath, S.C. Performance Evaluation of Material Decomposition with Rapid-Kilovoltage-Switching Dual-Energy CT and Implications for Assessing Bone Mineral Density. Am. J. Roentgenol. 2015, 204, 1234–1241. [Google Scholar] [CrossRef]
- Ren, L.; McCollough, C.H.; Yu, L. Three-Material Decomposition in Multi-energy CT: Impact of Prior Information on Noise and Bias. Proc. SPIE—Int. Soc. Opt. Eng. 2018, 10573, 105731G. [Google Scholar] [CrossRef] [Green Version]
- Hyodo, T.; Yada, N.; Hori, M.; Maenishi, O.; Lamb, P.; Sasaki, K.; Onoda, M.; Kudo, M.; Mochizuki, T.; Murakami, T. Multimaterial Decomposition Algorithm for the Quantification of Liver Fat Content by Using Fast-Kilovolt-Peak Switching Dual-Energy CT: Clinical Evaluation. Radiology 2017, 283, 108–118. [Google Scholar] [CrossRef]
- Hur, B.Y.; Lee, J.M.; Hyunsik, W.; Lee, K.B.; Joo, I.; Han, J.K.; Choi, B.I. Quantification of the fat fraction in the liver using dual-energy computed tomography and multimaterial decomposition. J. Comput. Assist. Tomogr. 2014, 38, 845–852. [Google Scholar] [CrossRef]
- Gauntt, D.M. A suggested method for setting up GSI profiles on the GE Revolution CT scanner. J. Appl. Clin. Med. Phys. 2019, 20, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, Z.; Chen, C.; Hong, N. Value of virtual monochromatic spectral images with metal artifact reduction algorithm in dual-energy computed tomography-guided microcoil localization of pulmonary nodules. Medicine 2018, 97, e11562. [Google Scholar] [CrossRef]
- Couinaud, C. Liver anatomy: Portal (and suprahepatic) or biliary segmentation. Dig. Surg. 1999, 16, 459–467. [Google Scholar] [CrossRef]
- Boyce, C.J.; Pickhardt, P.J.; Kim, D.H.; Taylor, A.J.; Winter, T.C.; Bruce, R.J.; Lindstrom, M.J.; Hinshaw, J.L. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am. J. Roentgenol. 2010, 194, 623–628. [Google Scholar] [CrossRef]
- El-Badry, A.M.; Breitenstein, S.; Jochum, W.; Washington, K.; Paradis, V.; Rubbia-Brandt, L.; Puhan, M.A.; Slankamenac, K.; Graf, R.; Clavien, P.-A. Assessment of hepatic steatosis by expert pathologists: The end of a gold standard. Ann. Surg. 2009, 250, 691–697. [Google Scholar] [CrossRef] [PubMed]
- St. Pierre, T.G.; House, M.J.; Bangma, S.J.; Pang, W.; Bathgate, A.; Gen, E.K.; Ayonrinde, O.T.; Bhathal, P.S.; Clouston, A.; Olynyk, J.K.; et al. Stereological analysis of liver biopsy histology sections as a reference standard for validating non-invasive liver fat fraction measurements by MRI. PLoS ONE 2016, 11, e0160789. [Google Scholar] [CrossRef]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, M.S.; Heba, E.R.; Hooker, C.A.; Bashir, M.R.; Fowler, K.J.; Sandrasegaran, K.; Brunt, E.M.; Kleiner, D.E.; Doo, E.; Van Natta, M.L.; et al. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-Assigned Steatosis Grades of Liver Biopsies from Adults with Nonalcoholic Steatohepatitis. Gastroenterology 2017, 153, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, R.J.; Stamm, E.R.; Lewin, J.M.; Hendrick, R.E.; Archer, P.G. Diagnosis of fatty infiltration of the liver on contrast enhanced CT: Limitations of liver-minus-spleen attenuation difference measurements. Abdom. Imaging 1998, 23, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Di Martino, M.; Bosco, S.; Del Monte, M.; De Cecco, C.N.; Lombardo, V.; Piga, M.; Catalano, C. MDCT classification of steatotic liver: A multicentric analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 290–297. [Google Scholar] [CrossRef]
- Starck, G.; Lönn, L.; Cederblad, A.; Forssell-Aronsson, E.; Sjöström, L.; Alpsten, M. A method to obtain the same levels of CT image noise for patients of various sizes, to minimize radiation dose. Br. J. Radiol. 2002, 75, 140–150. [Google Scholar] [CrossRef]
- Hua, B.; Hakkarainen, A.; Zhou, Y.; Lundbom, N.; Yki-Järvinen, H. Fat accumulates preferentially in the right rather than the left liver lobe in non-diabetic subjects. Dig. Liver Dis. 2018, 50, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Bonekamp, S.; Tang, A.; Mashhood, A.; Wolfson, T.; Changchien, C.; Middleton, M.S.; Clark, L.; Gamst, A.; Loomba, R.; Sirlin, C.B. Spatial distribution of MRI-determined hepatic proton density fat fraction in adults with nonalcoholic fatty liver disease. J. Magn. Reson. Imaging 2014, 39, 1525–1532. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.N.; Kumbla, R.A.; Berland, L.L.; Fineberg, N.S.; Morgan, D.E. Material density hepatic steatosis quantification on intravenous contrast-enhanced rapid kilovolt (peak)-switching single-source dual-energy computed tomography. J. Comput. Assist. Tomogr. 2013, 37, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Slavic, S.; Madhav, P.; Profio, M.; Crotty, D.; Nett, E.; Hsieh, J.; Liu, E. Technology White Paper: GSI Xtream on Revolution CT; GE Healthcare: Chicago, IL, USA, 2017; pp. 1–20. [Google Scholar]
- Fischer, M.A.; Gnannt, R.; Raptis, D.; Reiner, C.S.; Clavien, P.-A.; Schmidt, B.; Leschka, S.; Alkadhi, H.; Goetti, R. Quantification of liver fat in the presence of iron and iodine: An ex-vivo dual-energy CT study. Investig. Radiol. 2011, 46, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Left Lobe | Right Lobe 1 | Right Lobe 2 | |
|---|---|---|---|
| Left lobe | 0.003 *§ | 0.04 **§ | 0.43 ** |
| Right lobe 1 | 0.81 *** | 0.19 * | 0.67 *** |
| Right lobe 2 | 0.48 *** | 0.23 ** | 0.08 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.J.; Boesen, M.R.; Hansen, S.L.; Ulriksen, P.S.; Holm, S.; Lönn, L.; Hansen, K.L. Assessment of Liver Fat: Dual-Energy CT versus Conventional CT with and without Contrast. Diagnostics 2022, 12, 708. https://doi.org/10.3390/diagnostics12030708
Xu JJ, Boesen MR, Hansen SL, Ulriksen PS, Holm S, Lönn L, Hansen KL. Assessment of Liver Fat: Dual-Energy CT versus Conventional CT with and without Contrast. Diagnostics. 2022; 12(3):708. https://doi.org/10.3390/diagnostics12030708
Chicago/Turabian StyleXu, Jack Junchi, Mikkel Ranum Boesen, Sofie Lindskov Hansen, Peter Sommer Ulriksen, Søren Holm, Lars Lönn, and Kristoffer Lindskov Hansen. 2022. "Assessment of Liver Fat: Dual-Energy CT versus Conventional CT with and without Contrast" Diagnostics 12, no. 3: 708. https://doi.org/10.3390/diagnostics12030708
APA StyleXu, J. J., Boesen, M. R., Hansen, S. L., Ulriksen, P. S., Holm, S., Lönn, L., & Hansen, K. L. (2022). Assessment of Liver Fat: Dual-Energy CT versus Conventional CT with and without Contrast. Diagnostics, 12(3), 708. https://doi.org/10.3390/diagnostics12030708







