Noninvasive Assessment of Liver Fibrosis with ElastPQ in Patients with Chronic Viral Hepatitis: Comparison Using Histopathological Findings
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
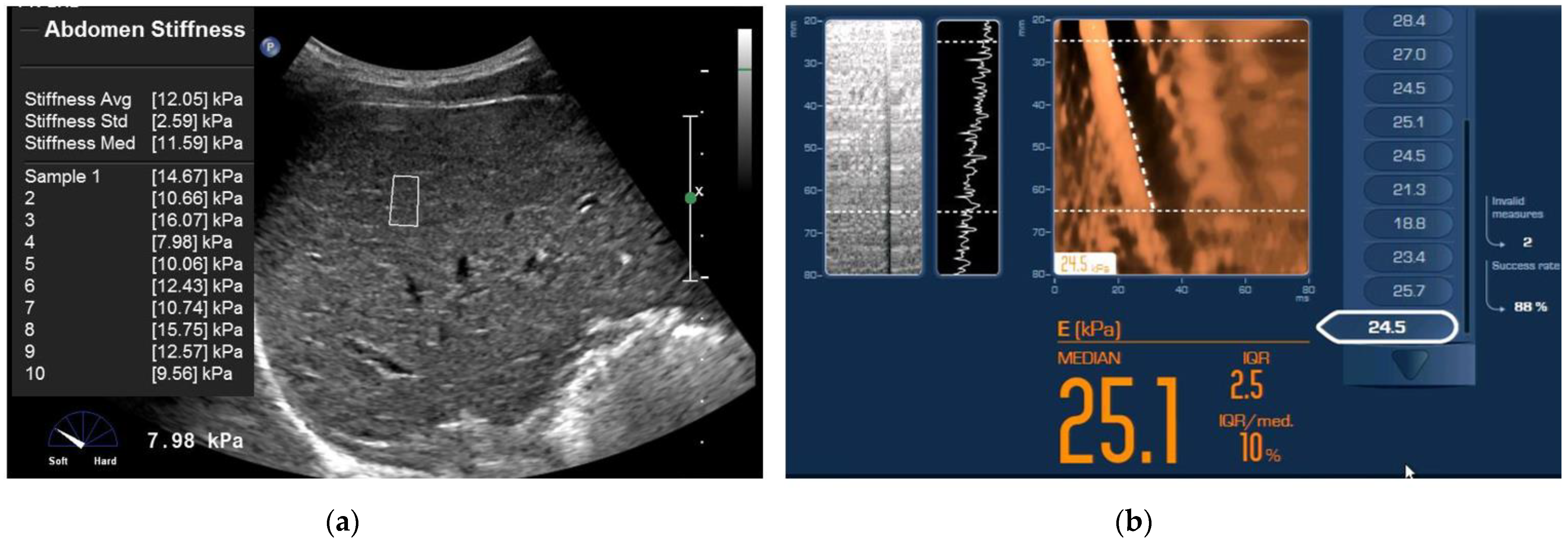
2.2. Point Shear Wave Elastography (ElastPQ)
2.3. Transient Elastography (TE)
2.4. Serological Test
2.5. Histopathological Analysis
2.6. Data and Statistical Analyses
3. Results
3.1. Patient Characteristics
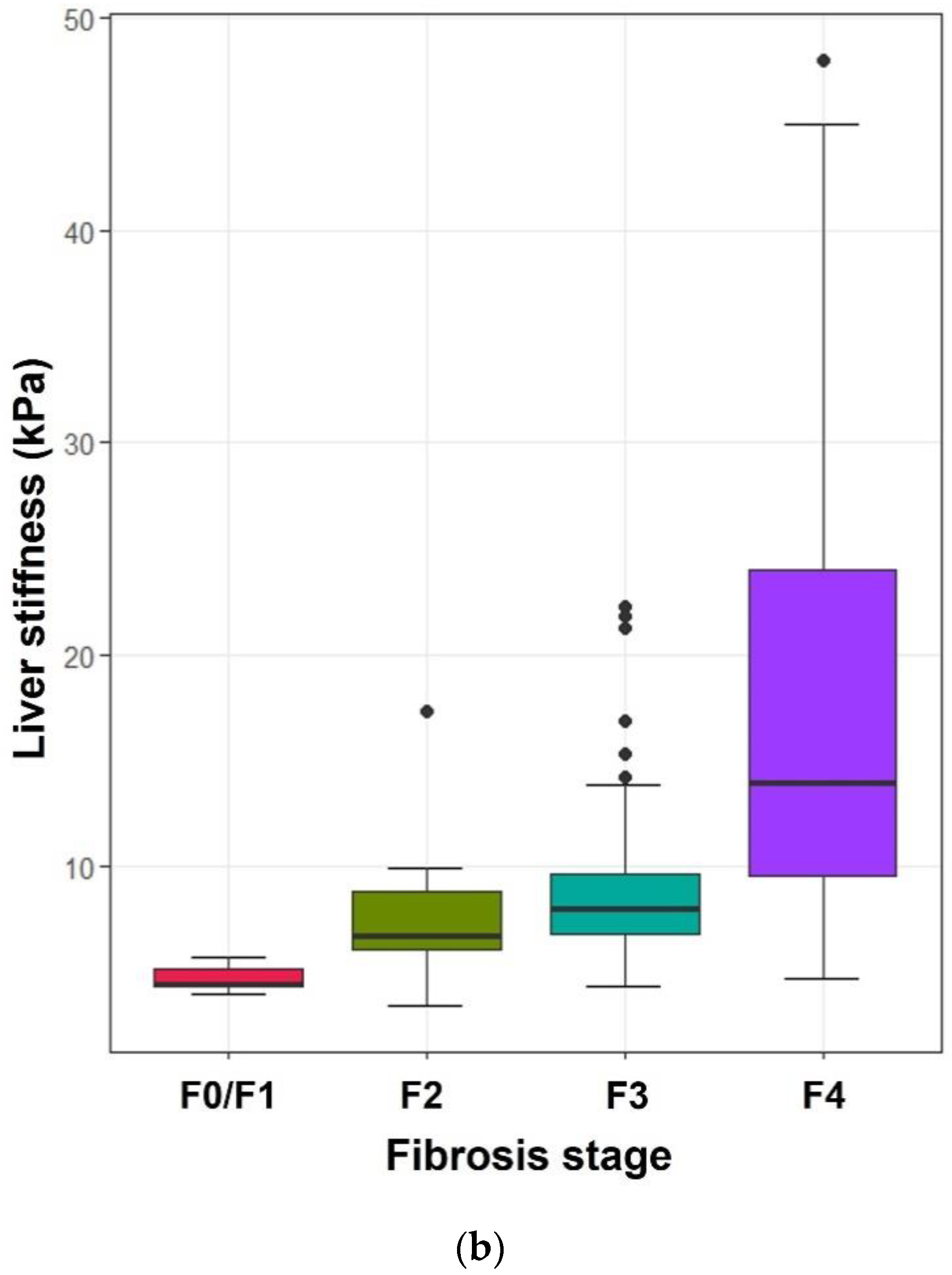
3.2. Relationship between Histologic Fibrosis Stages and the Values on ElastPQ, TE, and APRI
3.3. Diagnostic Accuracy and Cut-Off Values of ElastPQ
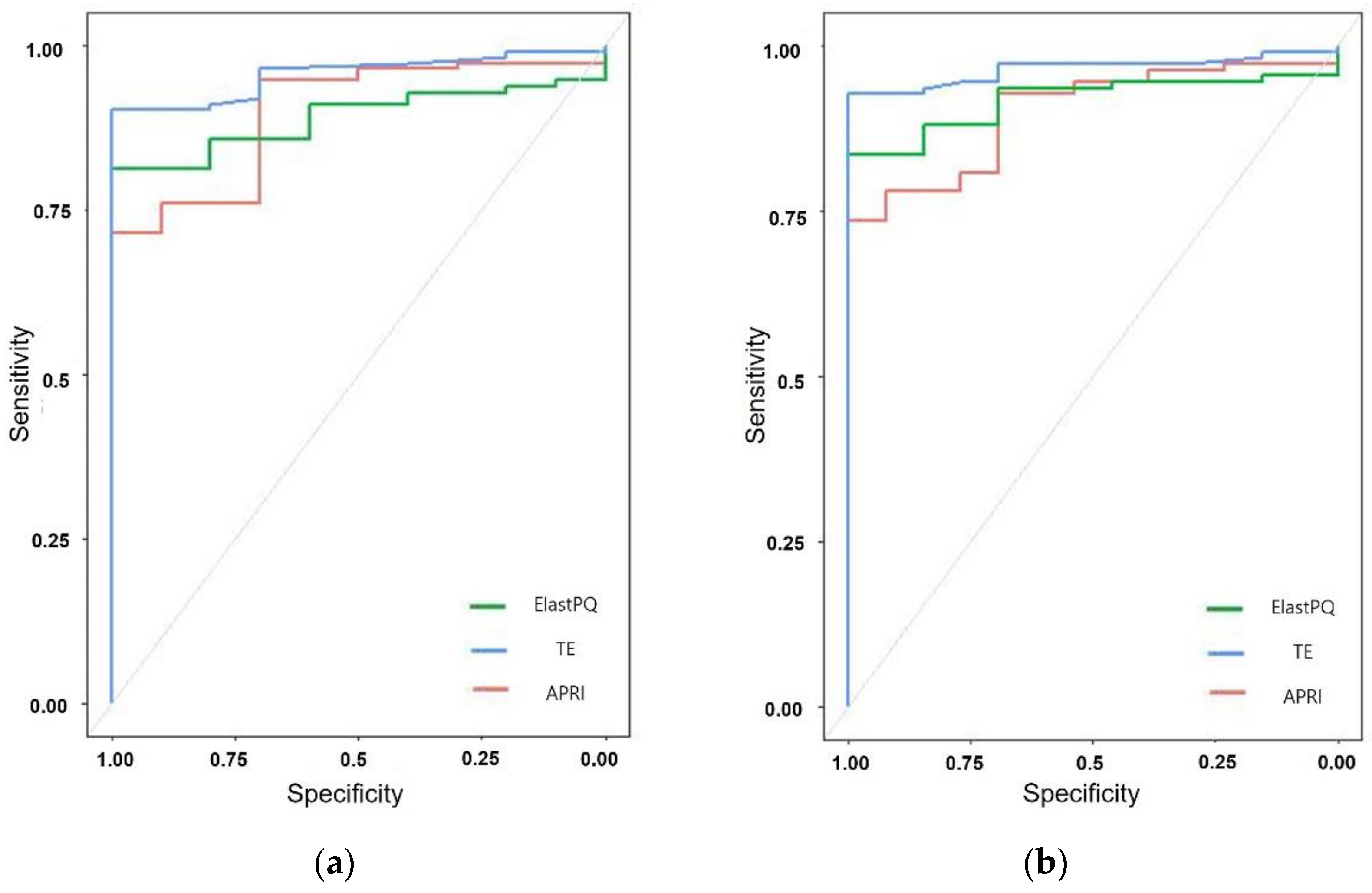
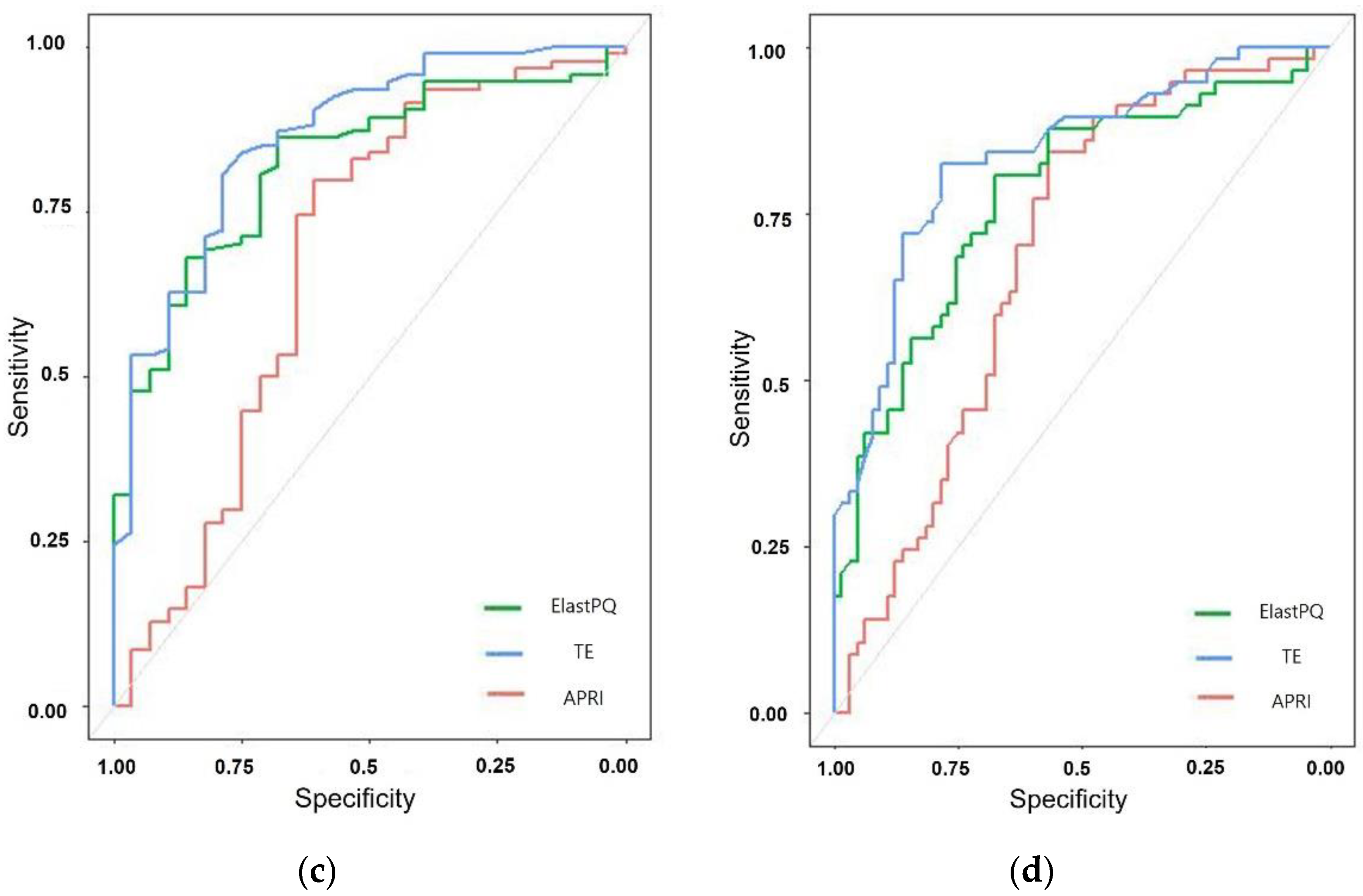
3.4. Comparison of the Diagnostic Performance of ElastPQ, TE, and APRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bucsics, T.; Grasl, B.; Ferlitsch, A.; Schwabl, P.; Mandorfer, M.; Zinober, K.; Stern, R.; Chromy, D.; Scheiner, B.; Sieghart, W.; et al. Point Shear Wave Elastography for Non-invasive Assessment of Liver Fibrosis in Patients with Viral Hepatitis. Ultrasound Med. Biol. 2018, 44, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. National Institutes of Health consensus development conference statement: Management of hepatitis C: 2002—10–12 June 2002. Hepatology 2002, 36, S3–S20. [Google Scholar] [CrossRef]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Van, D.T.; Gavaler, J.S.; Wright, H.; Tzakis, A. Liver biopsy. Its safety and complications as seen at a liver transplant center. Transplantation 1993, 55, 1087–1090. [Google Scholar]
- Huwart, L.; Peeters, F.; Sinkus, R.; Annet, L.; Salameh, N.; Ter Beek, L.C.; Horsmans, Y.; Van Beers, B.E. Liver fibrosis: Non-invasive assessment with MR elastography. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. In Vivo 2003, 19, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Huwart, L.; Sempoux, C.; Salameh, N.; Jamart, J.; Annet, L.; Sinkus, R.; Peeters, F.; Ter Beek, L.C.; Horsmans, Y.; Van Beers, B.E. Liver fibrosis: Noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology 2007, 245, 458–466. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, J.M.; Joo, I.; Lee, E.S.; Sohn, J.Y.; Jang, S.K.; Lee, K.B.; Han, J.K.; Choi, B.I. Hepatic fibrosis: Prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology 2014, 273, 772–782. [Google Scholar] [CrossRef]
- Kennedy, P.; Wagner, M.; Castera, L.; Hong, C.W.; Johnson, C.L.; Sirlin, C.B.; Taouli, B. Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology 2018, 286, 738–763. [Google Scholar] [CrossRef]
- Lee, J.E.; Shin, K.S.; Cho, J.S.; You, S.K.; Min, J.H.; Kim, K.H.; Song, I.S.; Cheon, K.S. Non-invasive Assessment of Liver Fibrosis with ElastPQ: Comparison with Transient Elastography and Serologic Fibrosis Marker Tests, and Correlation with Liver Pathology Results. Ultrasound Med. Biol. 2017, 43, 2515–2521. [Google Scholar] [CrossRef][Green Version]
- Giuffre, M.; Colecchia, A.; Croce, L.S. Elastography: Where are we now? Minerva Gastroenterol. 2021, 67, 109–111. [Google Scholar] [CrossRef]
- Castera, L.; Forns, X.; Alberti, A. Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 2008, 48, 835–847. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Gurusamy, K.S.; Ntaoula, S.; Cholongitas, E.; Davidson, B.R.; Burroughs, A.K. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 2011, 54, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Rust, M.; Ong, M.F.; Martens, S.; Sarrazin, C.; Bojunga, J.; Zeuzem, S.; Herrmann, E. Performance of transient elastography for the staging of liver fibrosis: A meta-analysis. Gastroenterology 2008, 134, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Foucher, J.; Castera, L.; Bernard, P.H.; Adhoute, X.; Laharie, D.; Bertet, J.; Couzigou, P.; De Ledinghen, V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur. J. Gastroenterol. Hepatol. 2006, 18, 411–412. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Nierhoff, J.; Lupsor, M.; Sporea, I.; Fierbinteanu-Braticevici, C.; Strobel, D.; Takahashi, H.; Yoneda, M.; Suda, T.; Zeuzem, S.; et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: A pooled meta-analysis. J. Viral. Hepat. 2012, 19, e212–e219. [Google Scholar] [CrossRef]
- Li, S.M.; Li, G.X.; Fu, D.M.; Wang, Y.; Dang, L.Q. Liver fibrosis evaluation by ARFI and APRI in chronic hepatitis C. World J. Gastroenterol. 2014, 20, 9528–9533. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Sporea, I.; Lupuşoru, R.; Şirli, R.; Popescu, A.; Danila, M.; Pienar, C. The value of ElastPQ for the evaluation of liver stiffness in patients with B and C chronic hepatopathies. Ultrasonics 2017, 77, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Herkner, H.; Sporea, I.; Salzl, P.; Sirli, R.; Neghina, A.M.; Peck-Radosavljevic, M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013, 33, 1138–1147. [Google Scholar] [CrossRef]
- Leong, W.L.; Lai, L.L.; Nik Mustapha, N.R.; Vijayananthan, A.; Rahmat, K.; Mahadeva, S.; Chan, W.K. Comparing point shear wave elastography (ElastPQ) and transient elastography for diagnosis of fibrosis stage in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2020, 35, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Serra, C.; Vukotic, R.; Felicani, C.; Mazzotta, E.; Gitto, S.; Vitale, G.; D’Errico, A.; Andreone, P. Assessment of liver fibrosis with elastography point quantification vs other noninvasive methods. Clin. Gastroenterol. Hepatol. 2019, 17, 510–517.13. [Google Scholar] [CrossRef] [PubMed]
- Baldea, V.; Sporea, I.; Lupusoru, R.; Bende, F.; Mare, R.; Popescu, A.; Sirli, R. Comparative Study between the Diagnostic Performance of Point and 2-D Shear-Wave Elastography for the Non-invasive Assessment of Liver Fibrosis in Patients with Chronic Hepatitis C Using Transient Elastography as Reference. Ultrasound Med. Biol. 2020, 46, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Lee, Y.J.; Chang, W.; Park, J.H.; Lee, K.H.; Kim, Y.H.; Kang, N.K.; Chung, J.W.; Jang, H.Y.; Ahn, S. Diagnostic performance of a point shear wave elastography (pSWE) for hepatic fibrosis in patients with autoimmune liver disease. PLoS ONE 2019, 14, e0212771. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Mare, R.; Lupusoru, R.; Popescu, A.; Danila, M.; Bende, F.; Sirli, R. Comparative study between four ultrasound Shear Waves Elastographic methods for liver fibrosis assessment. Med. Ultrason. 2018, 20, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Maiocchi, L.; Lissandrin, R.; Tinelli, C.; De Silvestri, A.; Filice, C. Accuracy of the ElastPQ® Technique for the Assessment of Liver Fibrosis in Patients with Chronic Hepatitis C: A “Real Life” Single Center Study. J. Gastrointest. Liver Dis. 2016, 25, 331–335. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, Z.-P.; Zheng, J.; Wu, T.; Zheng, R.-Q. Non-invasive assessment of liver fibrosis using two-dimensional shear wave elastography in patients with autoimmune liver diseases. World J. Gastroenterol. 2017, 23, 4839. [Google Scholar] [CrossRef]
- Chang, W.; Lee, J.M.; Yoon, J.H.; Han, J.K.; Choi, B.I.; Yoon, J.H.; Lee, K.B.; Lee, K.W.; Yi, N.J.; Suh, K.S. Liver Fibrosis Staging with MR Elastography: Comparison of Diagnostic Performance between Patients with Chronic Hepatitis B and Those with Other Etiologic Causes. Radiology 2016, 280, 88–97. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar] [CrossRef]
- Ferraioli, G.; De Silvestri, A.; Reiberger, T.; Taylor-Robinson, S.D.; de Knegt, R.J.; Maiocchi, L.; Mare, R.; Bucsics, T.; Atzori, S.; Tinelli, C. Adherence to quality criteria improves concordance between transient elastography and ElastPQ for liver stiffness assessment—A multicenter retrospective study. Dig. Liver Dis. 2018, 50, 1056–1061. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Fouad, R.; Elbaz, T.; Abdel Alem, S.; Elsharkawy, A.; Negm, M.; Khairy, M.; Hassany, M.; Cordie, A.; El Akel, W.; Esmat, G. Evaluation of accuracy of elastography point quantification versus other noninvasive modalities in staging of fibrosis in chronic hepatitis C virus patients. Eur. J. Gastroenterol. Hepatol. 2018, 30, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Kasper, D.; Fitting, D.; Knop, V.; Vermehren, A.; Sprinzl, K.; Hansmann, M.L.; Herrmann, E.; Bojunga, J.; Albert, J. Assessment of liver fibrosis with 2-D shear wave elastography in comparison to transient elastography and acoustic radiation force impulse imaging in patients with chronic liver disease. Ultrasound Med. Biol. 2015, 41, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Yoneda, M.; Imajo, K.; Nakajima, A. Elastography techniques for the assessment of liver fibrosis in non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2020, 21, 4039. [Google Scholar] [CrossRef]
- Giuffrè, M.; Giuricin, M.; Bonazza, D.; Rosso, N.; Giraudi, P.J.; Masutti, F.; Palmucci, S.; Basile, A.; Zanconati, F.; De Manzini, N. Optimization of point-shear wave elastography by skin-to-liver distance to assess liver fibrosis in patients undergoing bariatric surgery. Diagnostics 2020, 10, 795. [Google Scholar] [CrossRef]
- Giuffrè, M.; Fouraki, S.; Comar, M.; Masutti, F.; Crocè, L.S. The importance of transaminases flare in liver elastographycharacterization of the probability of liver fibrosis overestimation by hepatitis C virus-induced cytolysis. Microorganisms 2020, 8, 348. [Google Scholar] [CrossRef]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Furlan, A.; Tublin, M.E.; Yu, L.; Chopra, K.B.; Lippello, A.; Behari, J. Comparison of 2D shear wave elastography, transient elastography, and MR elastography for the diagnosis of fibrosis in patients with nonalcoholic fatty liver disease. Am. J. Roentgenol. 2020, 214, W20–W26. [Google Scholar] [CrossRef]


| Characteristic | Value (n = 122) |
|---|---|
| Patient age (y) | 57.3 ± 11.6 (18–76) |
| Sex | |
| Male | 93 (76) |
| Female | 29 (24) |
| Body mass index (kg/m2) | 23.7 ± 3.1 (15.8–34) |
| ElastPQ (kPa) | 6.1 ± 2.75 (2.5–18.6) |
| Transient elastography (kPa) | 12.6 ± 9.34 (3.5–48) |
| Blood marker | |
| AST (IU/L) | 56.8 ± 68.7 (11–513) |
| ALT (IU/L) | 45.98 ± 53.28 (6–348) |
| ALP (IU/L) | 88.4 ± 44.6 (27–364) |
| Albumin (g/dL) | 3.8 ± 0.5 (0.8–4.9) |
| GGT(IU/L) | 85.2 ± 108.2 (11–654) |
| Platelet count (103/mm3) | 153 ± 61 (11.5–339) |
| APRI | 1.16 ± 1.7 (0.13–14.41) |
| Underlying liver disease | |
| Chronic hepatitis B | 94 (77) |
| Chronic hepatitis C | 20 (16) |
| Fibrosis stage (Metavir score) | |
| F0 * | 10 (8.2) |
| F1 | 3 (2.5) |
| F2 | 15 (12.3) |
| F3 | 37 (30.3) |
| F4 | 57 (46.7) |
| Histologic Fibrosis Stage | ElastPQ | TE | APRI |
|---|---|---|---|
| F0/1 | 3.51 ± 0.14 (3.18–3.94) | 4.71 ± 0.57 (4.0–5.7) | 0.28 ± 0.10 (0.18–0.46) |
| F2 | 4.74 ± 1.27 (4.03–5.45) | 7.63 ± 3.24 (3.5–17.3) | 1.15 ± 0.94 (0.14–2.96) |
| F3 | 5.48 ± 1.76 (2.58–9.39) | 9.47 ± 4.65 (4.4–22.3) | 0.96 ± 1.36 (0.13–7.83) |
| F4 | 7.52 ± 3.09 (3.1–18.6) | 17.71 ± 10.86 (4.7–48.0) | 1.29 ± 1.29 (0.17–5.36) |
| Parameter | Stage ≥ F1 | Stage ≥ F2 | Stage ≥ F3 | Stage F4 |
|---|---|---|---|---|
| AUROC | 0.890 (0.832–0.949) | 0.917 (0.867–0.967) | 0.822 (0.742–0.902) | 0.777 (0.693–0.861) |
| Criterion (kPa) | 3.935 | 3.935 | 3.97 | 5.17 |
| Sensitivity (%) | 81.25 | 83.49 | 86.17 | 80.7 |
| Specificity (%) | 100 | 100 | 67.86 | 67.69 |
| Fibrosis Assessment Method | AUROC | Cut-Off Value (kPa) | Sensitivity (%) | Specificity (%) | p Value |
|---|---|---|---|---|---|
| Stage ≥ F2 | |||||
| ElastPQ | 0.917 (0.867–0.967) | 3.935 | 83.49 | 100 | <0.001 |
| TE | 0.964 (0.914–0.990) | 5.7 | 92.66 | 100 | <0.001 |
| APRI | 0.896 (0.828–0.944) | 0.46 | 71.56 | 100 | <0.001 |
| Stage = F4 | |||||
| ElastPQ | 0.777 (0.693–0.861) | 5.17 | 80.70 | 67.69 | <0.001 |
| TE | 0.836 (0.764–0.908) | 8.9 | 82.46 | 78.46 | <0.001 |
| APRI | 0.689 (0.594–0.784) | 0.47 | 84.21 | 56.92 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choo, D.; Shin, K.S.; Min, J.H.; You, S.-k.; Kim, K.-H.; Lee, J.E. Noninvasive Assessment of Liver Fibrosis with ElastPQ in Patients with Chronic Viral Hepatitis: Comparison Using Histopathological Findings. Diagnostics 2022, 12, 706. https://doi.org/10.3390/diagnostics12030706
Choo D, Shin KS, Min JH, You S-k, Kim K-H, Lee JE. Noninvasive Assessment of Liver Fibrosis with ElastPQ in Patients with Chronic Viral Hepatitis: Comparison Using Histopathological Findings. Diagnostics. 2022; 12(3):706. https://doi.org/10.3390/diagnostics12030706
Chicago/Turabian StyleChoo, Dongmin, Kyung Sook Shin, Ji Hye Min, Sun-kyoung You, Kyung-Hee Kim, and Jeong Eun Lee. 2022. "Noninvasive Assessment of Liver Fibrosis with ElastPQ in Patients with Chronic Viral Hepatitis: Comparison Using Histopathological Findings" Diagnostics 12, no. 3: 706. https://doi.org/10.3390/diagnostics12030706
APA StyleChoo, D., Shin, K. S., Min, J. H., You, S.-k., Kim, K.-H., & Lee, J. E. (2022). Noninvasive Assessment of Liver Fibrosis with ElastPQ in Patients with Chronic Viral Hepatitis: Comparison Using Histopathological Findings. Diagnostics, 12(3), 706. https://doi.org/10.3390/diagnostics12030706






