The NILS Study Protocol: A Retrospective Validation Study of an Artificial Neural Network Based Preoperative Decision-Making Tool for Noninvasive Lymph Node Staging in Women with Primary Breast Cancer (ISRCTN14341750)
Abstract
:1. Introduction
2. Materials and Methods
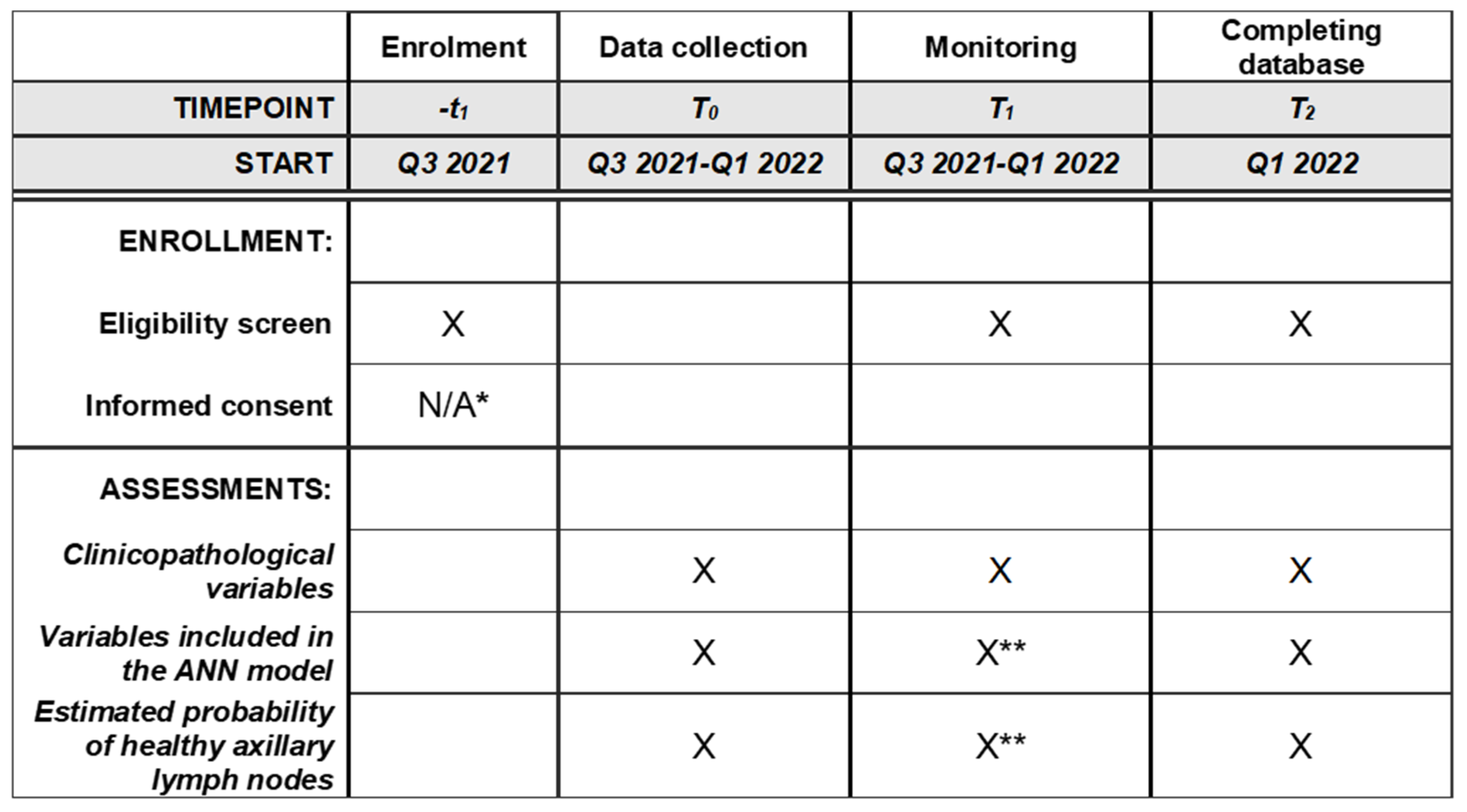
2.1. Study Design
2.2. Definition of pN0
2.3. Ethical Approval
2.4. Study Population
2.5. Participating Centers
2.6. Power Calculation and Statistical Plan
2.7. Inclusion Criteria
- Accepted study participation (e.g., did not opt out);
- Female sex;
- Invasive BC;
- Negative preoperative assessment of the axilla, clinically and by ultrasound;
- Scheduled for primary surgery.
2.8. Exclusion Criteria
- Did not accept study inclusion (i.e., opted out);
- Male sex;
- Children and adolescents (age < 18 years);
- Previous ipsilateral breast/axillary surgery;
- Synchronous distant metastases at diagnosis;
- Previous primary neoadjuvant therapy;
- Preoperatively verified axillary lymph node metastases by cytology or histology;
- Positive preoperative assessment of the axilla, clinically and by ultrasound;
- No surgical axillary staging;
- Upfront axillary nodal dissection.
2.9. Endpoints
2.9.1. Primary Endpoint
2.9.2. Secondary Endpoint
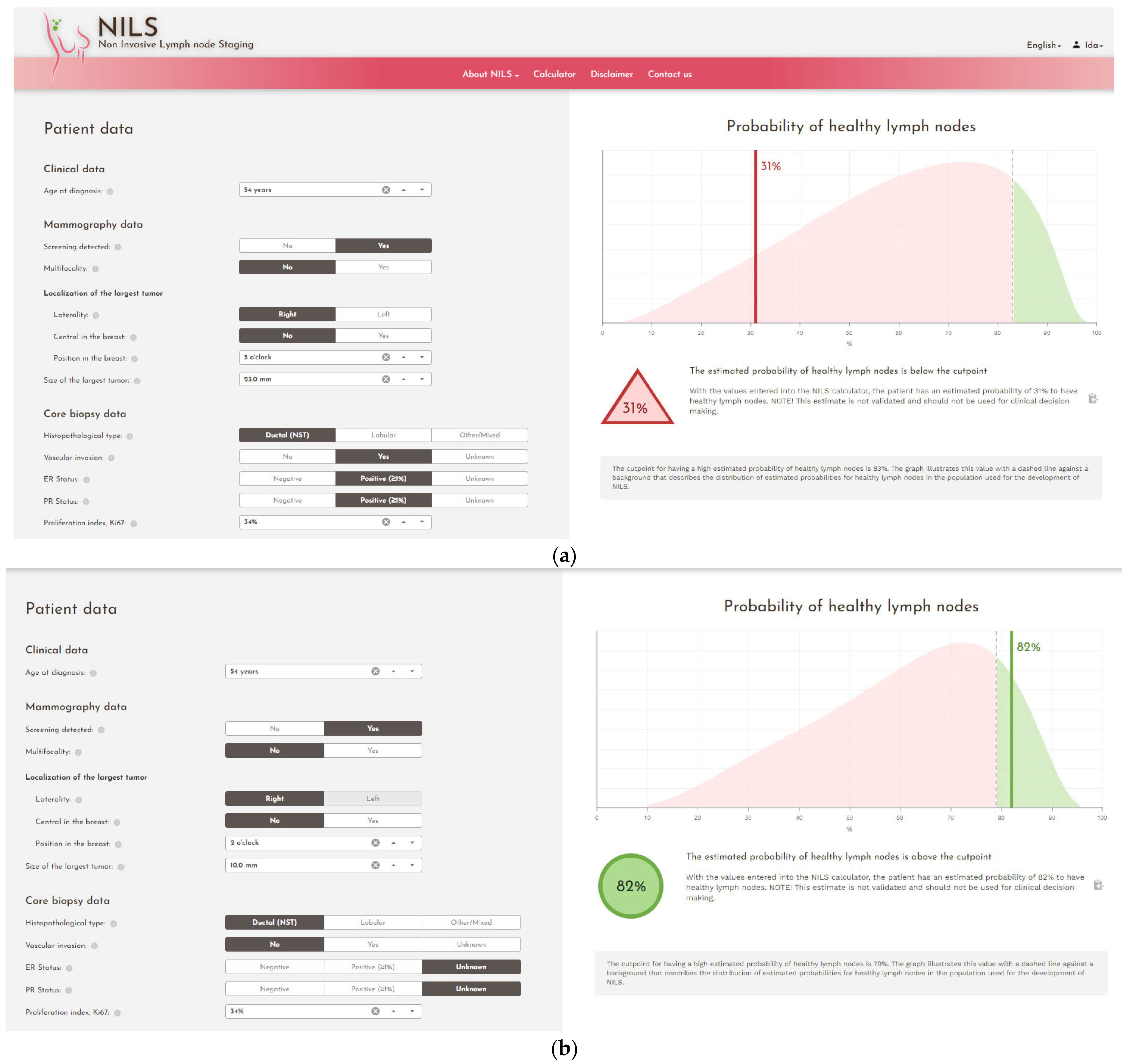
2.10. Data Analysis
3. Discussion
3.1. Significance
3.2. Current Status
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220, Erratum in: Ann. Oncol. 2019, 30, 1674. Erratum in: Ann. Oncol. 2021, 32, 284.. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- De Boniface, J.; Schmidt, M.; Engel, J.; Smidt, M.L.; Offersen, B.V.; Reimer, T. What Is the Best Management of cN0pN1(sn) Breast Cancer Patients. Breast Care 2018, 13, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimer, T.; Stachs, A.; Nekljudova, V.; Loibl, S.; Hartmann, S.; Wolter, K.; Hildebrandt, G.; Gerber, B. Restricted Axillary Staging in Clinically and Sonographically Node-Negative Early Invasive Breast Cancer (c/iT1–2) in the Context of Breast Conserving Therapy: First Results Following Commencement of the Intergroup-Sentinel-Mamma (INSEMA) Trial. Geburtshilfe Frauenheilkd. 2017, 77, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, A.; Zahergivar, A.; Iranpour, P.; Akrami, M.; Kazemi, S. Diagnostic Accuracy of Axillary Ultrasonography Compared with Intra-operative Pathological Findings in Patients with Breast Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 3615–3621. [Google Scholar] [CrossRef] [Green Version]
- Skarping, I.; Förnvik, D.; Zackrisson, S.; Borgquist, S.; Rydén, L. Predicting pathological axillary lymph node status with ultrasound following neoadjuvant therapy for breast cancer. Breast Cancer Res. Treat. 2021, 189, 131–144. [Google Scholar] [CrossRef]
- Esen, G.; Gurses, B.; Yilmaz, M.H.; Ilvan, S.; Ulus, S.; Celik, V.; Farahmand, M.; Calay, O.O. Gray scale and power Doppler US in the preoperative evaluation of axillary metastases in breast cancer patients with no palpable lymph nodes. Eur. Radiol. 2005, 15, 1215–1223. [Google Scholar] [CrossRef]
- Lee, B.; Lim, A.K.; Krell, J.; Satchithananda, K.; Coombes, R.C.; Lewis, J.S.; Stebbing, J. The Efficacy of Axillary Ultrasound in the Detection of Nodal Metastasis in Breast Cancer. Am. J. Roentgenol. 2013, 200, W314–W320. [Google Scholar] [CrossRef]
- Riedel, F.; Schaefgen, B.; Sinn, H.-P.; Feisst, M.; Hennigs, A.; Hug, S.; Binnig, A.; Gomez, C.; Harcos, A.; Stieber, A.; et al. Diagnostic accuracy of axillary staging by ultrasound in early breast cancer patients. Eur. J. Radiol. 2020, 135, 109468. [Google Scholar] [CrossRef]
- Alvarez, S.; Añorbe, E.; Alcorta, P.; López, F.; Alonso, I.; Cortés, J. Role of Sonography in the Diagnosis of Axillary Lymph Node Metastases in Breast Cancer: A Systematic Review. Am. J. Roentgenol. 2006, 186, 1342–1348. [Google Scholar] [CrossRef] [Green Version]
- Deurloo, E.; Tanis, P.; Gilhuijs, K.; Muller, S.; Kröger, R.; Peterse, J.; Rutgers, E.; Olmos, R.V.; Kool, L.S. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur. J. Cancer 2003, 39, 1068–1073. [Google Scholar] [CrossRef]
- Brackstone, M.; Baldassarre, F.G.; Perera, F.E.; Cil, T.; Mac Gregor, M.C.; Dayes, I.S.; Engel, J.; Horton, J.K.; King, T.A.; Kornecki, A.; et al. Management of the Axilla in Early-Stage Breast Cancer: Ontario Health (Cancer Care Ontario) and ASCO Guideline. J. Clin. Oncol. 2021, 39, 3056–3082. [Google Scholar] [CrossRef]
- Sackey, H.; Magnuson, A.; Sandelin, K.; Liljegren, G.; Bergkvist, L.; Fülep, Z.; Celebioglu, F.; Frisell, J. Arm lymphoedema after axillary surgery in women with invasive breast cancer. Br. J. Surg. 2014, 101, 390–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.; Euhus, D.M.; Mayo, H.G.; Balch, C.M. Axillary Node Interventions in Breast Cancer. JAMA 2013, 310, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Dihge, L.; Ohlsson, M.; Edén, P.; Bendahl, P.-O.; Rydén, L. Artificial neural network models to predict nodal status in clinically node-negative breast cancer. BMC Cancer 2019, 19, 610. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Moons, K.G.M. Reporting of artificial intelligence prediction models. Lancet 2019, 393, 1577–1579. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Krag, D.N.; Anderson, S.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Ashikaga, T.; Weaver, D.L.; Miller, B.J.; Jalovec, L.M.; Frazier, T.G.; et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007, 8, 881–888. [Google Scholar] [CrossRef]
- Pesek, S.; Ashikaga, T.; Krag, L.E.; Krag, D. The False-Negative Rate of Sentinel Node Biopsy in Patients with Breast Cancer: A Meta-Analysis. World J. Surg. 2012, 36, 2239–2251. [Google Scholar] [CrossRef] [Green Version]
- Keelan, S.; Heeney, A.; Downey, E.; Hegarty, A.; Roche, T.; Power, C.; Ni Mhuircheartaigh, N.; Duke, D.; Kerr, J.; Hambly, N.; et al. Breast cancer patients with a negative axillary ultrasound may have clinically significant nodal metastasis. Breast Cancer Res. Treat. 2021, 187, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kuijs, V.J.L.; Moossdorff, M.; Schipper, R.J.; Beets-Tan, R.G.H.; Heuts, E.M.; Keymeulen, K.B.M.I.; Smidt, M.L.; Lobbes, M.B.I. The role of MRI in axillary lymph node imaging in breast cancer patients: A systematic review. Insights Imaging 2015, 6, 203–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruckmann, N.M.; Sawicki, L.M.; Kirchner, J.; Martin, O.; Umutlu, L.; Herrmann, K.; Fendler, W.; Bittner, A.-K.; Hoffmann, O.; Mohrmann, S.; et al. Prospective evaluation of whole-body MRI and 18F-FDG PET/MRI in N and M staging of primary breast cancer patients. Eur. J. Pediatr. 2020, 47, 2816–2825. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.A.; Avendano, D.; Zapata, P.; Riedl, C.C.; Pinker, K. Lymph Node Imaging in Patients with Primary Breast Cancer: Concurrent Diagnostic Tools. Oncologist 2019, 25, e231–e242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, R.-J.; Paiman, E.; Beets-Tan, R.G.H.; Nelemans, P.J.; De Vries, B.; Heuts, E.M.; Van De Vijver, K.K.; Keymeulen, K.B.; Brans, B.; Smidt, M.L.; et al. Diagnostic Performance of Dedicated Axillary T2- and Diffusion-weighted MR Imaging for Nodal Staging in Breast Cancer. Radiology 2015, 275, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botsikas, D.; Bagetakos, I.; Picarra, M.; Barisits, A.C.D.C.A.; Boudabbous, S.; Montet, X.; Lam, G.T.; Mainta, I.; Kalovidouri, A.; Becker, M. What is the diagnostic performance of 18-FDG-PET/MR compared to PET/CT for the N- and M- staging of breast cancer? Eur. Radiol. 2018, 29, 1787–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Roozendaal, L.M.; Schipper, R.J.; Van De Vijver, K.K.B.T.; Haekens, C.M.; Lobbes, M.B.I.; Tjan-Heijnen, V.C.G.; De Boer, M.; Smidt, M.L. The impact of the pathological lymph node status on adjuvant systemic treatment recommendations in clinically node negative breast cancer patients. Breast Cancer Res. Treat. 2014, 143, 469–476. [Google Scholar] [CrossRef]
- Nguyen, S.; Polat, D.; Karbasi, P.; Moser, D.; Wang, L.; Hulsey, K.; Çobanoğlu, M.C.; Dogan, B.; Montillo, A. Preoperative Prediction of Lymph Node Metastasis from Clinical DCE MRI of the Primary Breast Tumor Using a 4D CNN. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Switzerland, 2020; Volume 12262, pp. 326–334. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Huang, C.-S.; Shih, C.-C.; Chang, R.-F. Axillary lymph node metastasis status prediction of early-stage breast cancer using convolutional neural networks. Comput. Biol. Med. 2020, 130, 104206. [Google Scholar] [CrossRef]
- Zheng, X.; Yao, Z.; Huang, Y.; Yu, Y.; Wang, Y.; Liu, Y.; Mao, R.; Li, F.; Xiao, Y.; Wang, Y.; et al. Deep learning radiomics can predict axillary lymph node status in early-stage breast cancer. Nat. Commun. 2020, 11, 1236. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Cattell, R.; Duanmu, H.; Huang, P.; Li, H.; Vanguri, R.; Liu, M.Z.; Jambawalikar, S.; Ha, R.; Wang, F.; et al. Convolutional Neural Network Detection of Axillary Lymph Node Metastasis Using Standard Clinical Breast MRI. Clin. Breast Cancer 2019, 20, e301–e308. [Google Scholar] [CrossRef]
- Hu, X.; Xue, J.; Peng, S.; Yang, P.; Yang, Z.; Yang, L.; Dong, Y.; Yuan, L.; Wang, T.; Bao, G. Preoperative Nomogram for Predicting Sentinel Lymph Node Metastasis Risk in Breast Cancer: A Potential Application on Omitting Sentinel Lymph Node Biopsy. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lan, Y.; Zhang, L.; Wu, T.; Cui, H.; Li, Z.; Sun, P.; Tian, P.; Tian, J.; Li, X. Nomograms for Predicting Axillary Lymph Node Status Reconciled with Preoperative Breast Ultrasound Images. Front. Oncol. 2021, 11, 567648. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Dai, Y.; Lin, F.; Ma, H.; Duan, S.; Xie, H.; Zhao, W.; Hong, N. Radiomics Nomogram of DCE-MRI for the Prediction of Axillary Lymph Node Metastasis in Breast Cancer. Front. Oncol. 2020, 10, 2305. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wu, Y.; Bao, F.; Zhou, J.; Wan, J.; Tian, J.; Lin, Y.; Wang, M. Mammography-based radiomics nomogram: A potential biomarker to predict axillary lymph node metastasis in breast cancer. Br. J. Radiol. 2020, 93. [Google Scholar] [CrossRef] [PubMed]
- Dihge, L.; Bendahl, P.; Rydén, L. Nomograms for preoperative prediction of axillary nodal status in breast cancer. Br. J. Surg. 2017, 104, 1494–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojarad, S.; Venturini, B.; Fulgenzi, P.; Papaleo, R.; Brisigotti, M.; Monti, F.; Canuti, D.; Ravaioli, A.; Woo, L.; Dlay, S.; et al. Prediction of nodal metastasis and prognosis of breast cancer by ANN-based assessment of tumour size and p53, Ki-67 and steroid receptor expression. Anticancer Res. 2013, 33, 3925–3933. [Google Scholar]
- Fu, Y.; Jiang, J.; Chen, S.; Qiu, F. Establishment of risk prediction nomogram for ipsilateral axillary lymph node metastasis in T1 breast cancer. J. Zhejiang Univ. (Med. Sci.) 2021, 50, 81–89. [Google Scholar] [CrossRef]
- Meretoja, T.J.; Heikkilä, P.S.; Mansfield, A.; Cserni, G.; Ambrozay, E.; Boross, G.; Zgajnar, J.; Perhavec, A.; Gazic, B.; Arisio, R.; et al. A Predictive Tool to Estimate the Risk of Axillary Metastases in Breast Cancer Patients with Negative Axillary Ultrasound. Ann. Surg. Oncol. 2014, 21, 2229–2236. [Google Scholar] [CrossRef]
- Bevilacqua, J.L.B.; Kattan, M.W.; Fey, J.V.; Cody, H.S.; Borgen, P.I.; Van Zee, K.J. Doctor, What Are My Chances of Having a Positive Sentinel Node? A Validated Nomogram for Risk Estimation. J. Clin. Oncol. 2007, 25, 3670–3679. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skarping, I.; Dihge, L.; Bendahl, P.-O.; Huss, L.; Ellbrant, J.; Ohlsson, M.; Rydén, L. The NILS Study Protocol: A Retrospective Validation Study of an Artificial Neural Network Based Preoperative Decision-Making Tool for Noninvasive Lymph Node Staging in Women with Primary Breast Cancer (ISRCTN14341750). Diagnostics 2022, 12, 582. https://doi.org/10.3390/diagnostics12030582
Skarping I, Dihge L, Bendahl P-O, Huss L, Ellbrant J, Ohlsson M, Rydén L. The NILS Study Protocol: A Retrospective Validation Study of an Artificial Neural Network Based Preoperative Decision-Making Tool for Noninvasive Lymph Node Staging in Women with Primary Breast Cancer (ISRCTN14341750). Diagnostics. 2022; 12(3):582. https://doi.org/10.3390/diagnostics12030582
Chicago/Turabian StyleSkarping, Ida, Looket Dihge, Pär-Ola Bendahl, Linnea Huss, Julia Ellbrant, Mattias Ohlsson, and Lisa Rydén. 2022. "The NILS Study Protocol: A Retrospective Validation Study of an Artificial Neural Network Based Preoperative Decision-Making Tool for Noninvasive Lymph Node Staging in Women with Primary Breast Cancer (ISRCTN14341750)" Diagnostics 12, no. 3: 582. https://doi.org/10.3390/diagnostics12030582
APA StyleSkarping, I., Dihge, L., Bendahl, P.-O., Huss, L., Ellbrant, J., Ohlsson, M., & Rydén, L. (2022). The NILS Study Protocol: A Retrospective Validation Study of an Artificial Neural Network Based Preoperative Decision-Making Tool for Noninvasive Lymph Node Staging in Women with Primary Breast Cancer (ISRCTN14341750). Diagnostics, 12(3), 582. https://doi.org/10.3390/diagnostics12030582






