Evaluation of Computer-Aided Detection (CAD) in Screening Automated Breast Ultrasound Based on Characteristics of CAD Marks and False-Positive Marks
Abstract
:1. Introduction
2. Materials and Methods
2.1. ABUS Acquisitions
2.2. CAD System
2.3. Study Design
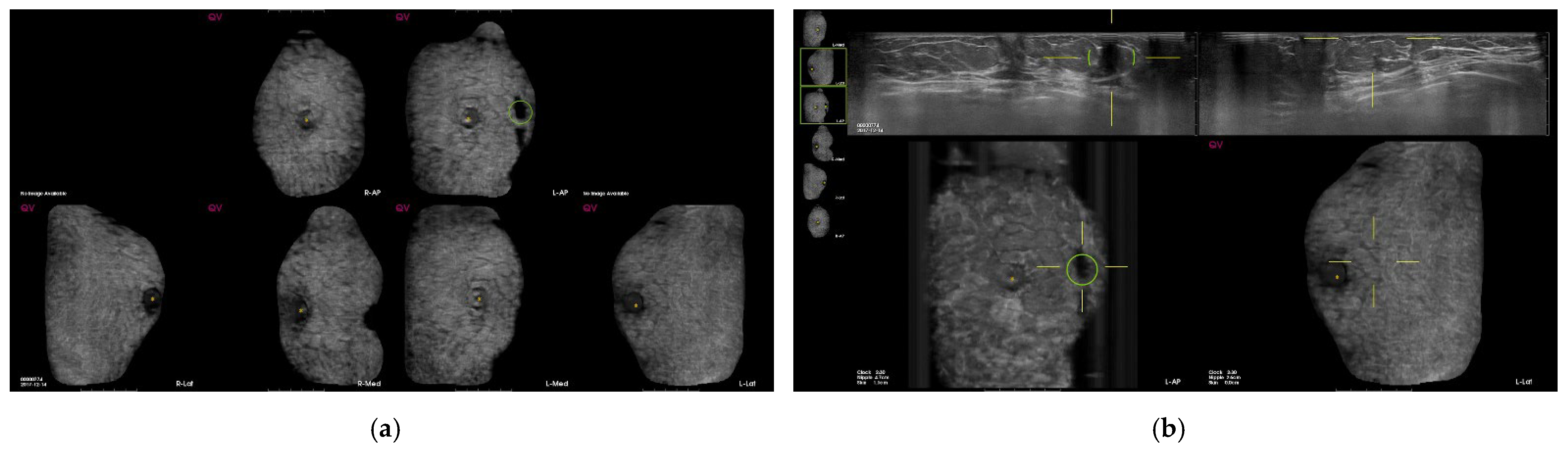
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elmore, J.G.; Armstrong, K.; Lehman, C.D.; Fletcher, S.W. Screening for breast cancer. JAMA 2005, 293, 1245–1256. [Google Scholar] [CrossRef]
- Siu, A.L.; Force USPST. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 279–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeffinger, K.C.; Fontham, E.T.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.C.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update from the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef]
- Morimoto, T.; Nagao, T.; Okazaki, K.; Kira, M.; Nakagawa, Y.; Tangoku, A. Current status of breast cancer screening in the world. Breast Cancer 2009, 16, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.P.; Shen, Z.Z.; Liu, T.J.; Agarwal, G.; Tajima, T.; Paik, N.S.; Sandelin, K.; Derossis, A.; Cody, H.; Foulkes, W.D. Is breast cancer the same disease in Asian and Western countries? World J. Surg. 2010, 34, 2308–2324. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, N.; Mariapun, S.; Eriksson, M.; Tapia, J.; Kwan, P.Y.; Ho, W.K.; Harun, F.; Rahmat, K.; Czene, K.; Taib, N.A.M.; et al. Differences in mammographic density between Asian and Caucasian populations: A comparative analysis. Breast Cancer Res. Treat. 2017, 161, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic density and breast cancer risk: Current understanding and future prospects. Breast Cancer Res. 2011, 13, 223. [Google Scholar] [CrossRef] [Green Version]
- Teh, W.; Wilson, A. The role of ultrasound in breast cancer screening. A consensus statement by the European Group for Breast Cancer Screening. Eur. J. Cancer 1998, 34, 449–450. [Google Scholar] [CrossRef]
- Mao, Y.-J.; Lim, H.-J.; Ni, M.; Yan, W.-H.; Wong, D.W.-C.; Cheung, J.C.-W. Breast Tumour Classification Using Ultrasound Elastography with Machine Learning: A Systematic Scoping Review. Cancers 2022, 14, 367. [Google Scholar] [CrossRef]
- Brem, R.F.; Tabár, L.; Duffy, S.W.; Inciardi, M.F.; Guingrich, J.A.; Hashimoto, B.E.; Lander, M.R.; Lapidus, R.L.; Peterson, M.K.; Rapelyea, J.A.; et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: The SomoInsight Study. Radiology 2015, 274, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, V.; Giuliano, C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin. Imaging 2013, 37, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Nothacker, M.; Duda, V.; Hahn, M.; Warm, M.; Degenhardt, F.; Madjar, H.; Weinbrenner, S.; Albert, U.-S. Early detection of breast cancer: Benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer 2009, 9, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenkel, E.; Heckmann, M.; Heinrich, M.; Schwab, S.; Uder, M.; Schulz-Wendtland, R.; Bautz, W.A.; Janka, R. Automated Breast Ultrasound: Lesion Detection and BI-RADS™ Classification—A Pilot Study; RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgebenden Verfahren; Georg Thieme Verlag KG Stuttgart: New York, NY, USA, 2008; pp. 804–808. [Google Scholar]
- Wilczek, B.; Wilczek, H.E.; Rasouliyan, L.; Leifland, K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur. J. Radiol. 2016, 85, 1554–1563. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Mordang, J.J.; van Zelst, J.; Grivegnée, A.; Gubern-Mérida, A.; Melendez, J.; Mann, R.M.; Zhang, W.; Platel, B.; Karssemeijer, N. Computer-aided detection of breast cancers using Haar-like features in automated 3D breast ultrasound. Med. Phys. 2015, 42, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- van Zelst, J.C.; Tan, T.; Clauser, P.; Domingo, A.; Dorrius, M.D.; Drieling, D.; Golatta, M.; Gras, F.; de Jong, M.; Pijnappel, R.; et al. Dedicated computer-aided detection software for automated 3D breast ultrasound; an efficient tool for the radiologist in supplemental screening of women with dense breasts. Eur. Radiol. 2018, 28, 2996–3006. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Rim, J.; Kim, S.M.; La Yun, B.; Park, S.Y.; Ahn, H.S.; Kim, B.; Jang, M. False-negative results on computer-aided detection software in preoperative automated breast ultrasonography of breast cancer patients. Ultrasonography 2021, 40, 83. [Google Scholar] [CrossRef] [Green Version]
- Van Zelst, J.; Tan, T.; Platel, B.; De Jong, M.; Steenbakkers, A.; Mourits, M.; Grivegnee, A.; Borelli, C.; Karssemeijer, N.; Mann, R.M.; et al. Improved cancer detection in automated breast ultrasound by radiologists using computer aided detection. Eur. J. Radiol. 2017, 89, 54–59. [Google Scholar] [CrossRef]
- Kim, W.H.; Lee, S.H.; Chang, J.M.; Cho, N.; Moon, W.K. Background echotexture classification in breast ultrasound: Inter-observer agreement study. Acta Radiol. 2017, 58, 1427–1433. [Google Scholar] [CrossRef]
- Jia, M.; Lin, X.; Zhou, X.; Yan, H.; Chen, Y.; Liu, P.; Bao, L.; Li, A.; Basu, P.; Qiao, Y.; et al. Diagnostic performance of automated breast ultrasound and handheld ultrasound in women with dense breasts. Breast Cancer Res. Treat. 2020, 181, 589–597. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Zhou, Y.; Mao, F.; Lin, Y.; Shen, S.; Sun, Q.; Ouyang, Z. Diagnostic value of an automated breast volume scanner compared with a hand-held ultrasound: A meta-analysis. Gland Surg. 2019, 8, 698–711. [Google Scholar] [CrossRef]
- Vourtsis, A.; Kachulis, A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1,886 women. Eur. Radiol. 2018, 28, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Dean, J.; Comulada, W.S.; Lee, S.J. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur. Radiol. 2010, 20, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zelst, J.C.M.; Mann, R.M. Automated Three-dimensional Breast US for Screening: Technique, Artifacts, and Lesion Characterization. Radiographics 2018, 38, 663–683. [Google Scholar] [CrossRef]
- Berg, W.A.; Bandos, A.I.; Mendelson, E.B.; Lehrer, D.; Jong, R.A.; Pisano, E.D. Ultrasound as the Primary Screening Test for Breast Cancer: Analysis from ACRIN 6666. J. Natl. Cancer Inst. 2016, 108, djv367. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, N.; Suzuki, A.; Sobue, T.; Kawai, M.; Yamamoto, S.; Zheng, Y.-F.; Shiono, Y.N.; Saito, H.; Kuriyama, S.; Tohno, E.; et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomised controlled trial. Lancet 2016, 387, 341–348. [Google Scholar] [CrossRef]
- Jiang, Y. Concurrent-Read CaD helps streamline automated Breast ultrasound (aBus) interpretation. Breast Imaging 2018, 11, 40–42. [Google Scholar]
- Yang, S.; Gao, X.; Liu, L.; Shu, R.; Yan, J.; Zhang, G.; Xiao, Y.; Ju, Y.; Zhao, N.; Song, H. Performance and reading time of automated breast US with or without computer-aided detection. Radiology 2019, 292, 540–549. [Google Scholar] [CrossRef]
- Jiang, Y.; Inciardi, M.F.; Edwards, A.V.; Papaioannou, J. Interpretation time using a concurrent-read computer-aided detection system for automated breast ultrasound in breast cancer screening of women with dense breast tissue. Am. J. Roentgenol. 2018, 211, 452–461. [Google Scholar] [CrossRef]
- Ciurea, A.I.; Ciortea, C.A.; Dudea, S.M. Pros and Cons for Automated Breast Ultrasound (ABUS): A Narrative Review. J. Pers. Med. 2021, 11, 703. [Google Scholar]
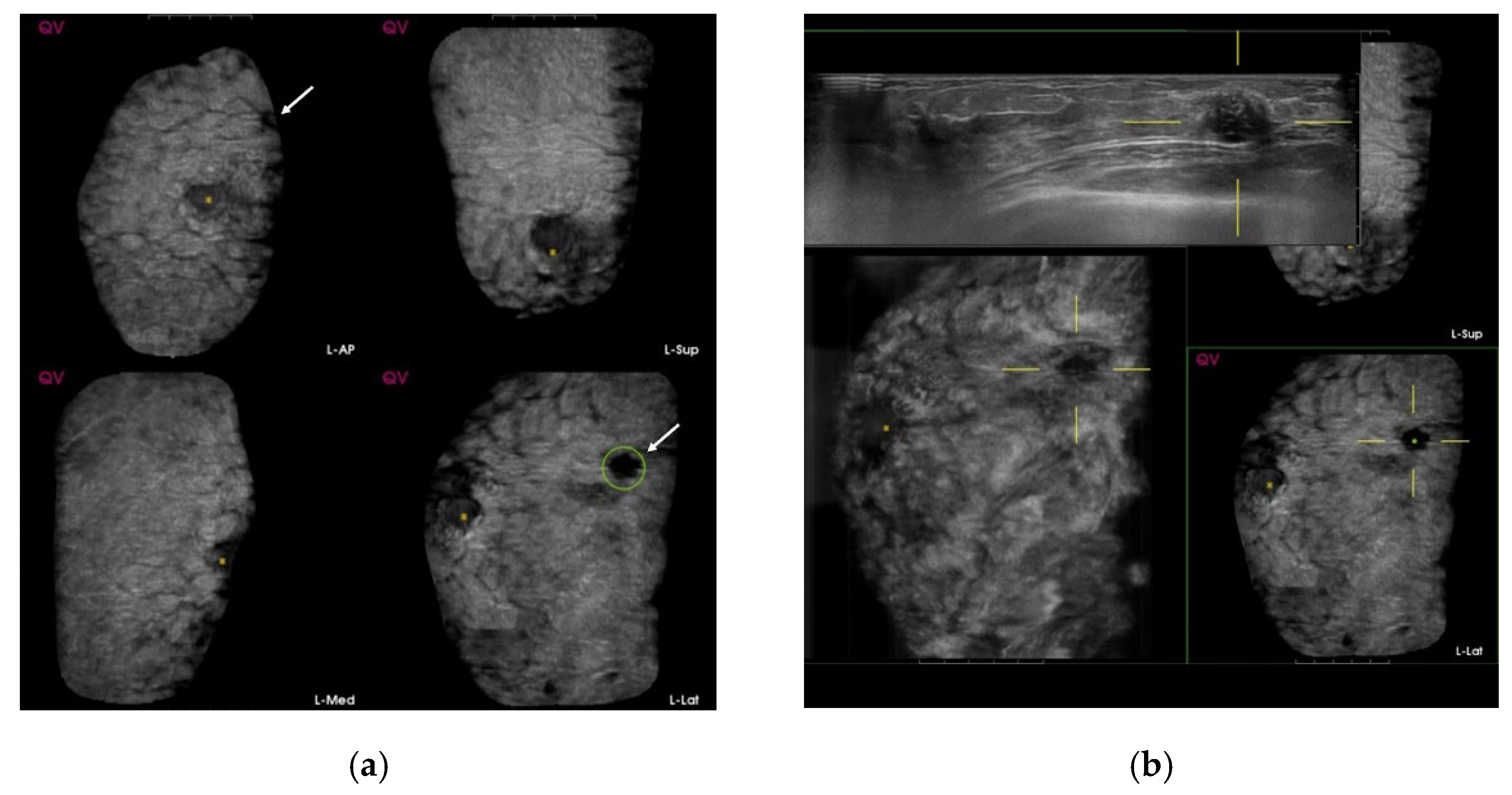
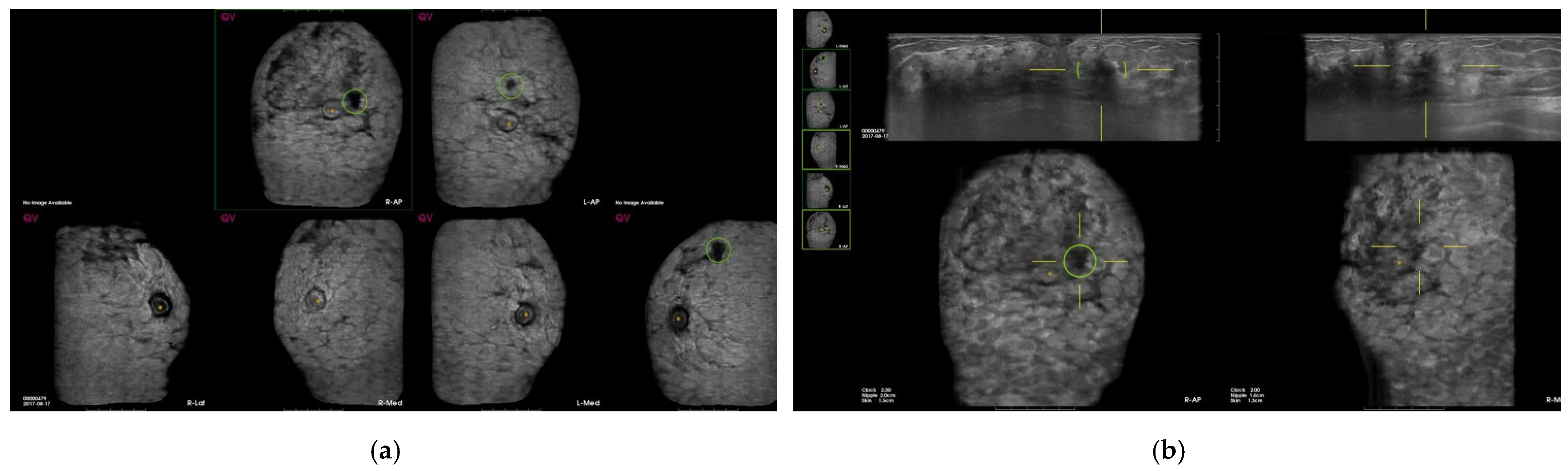
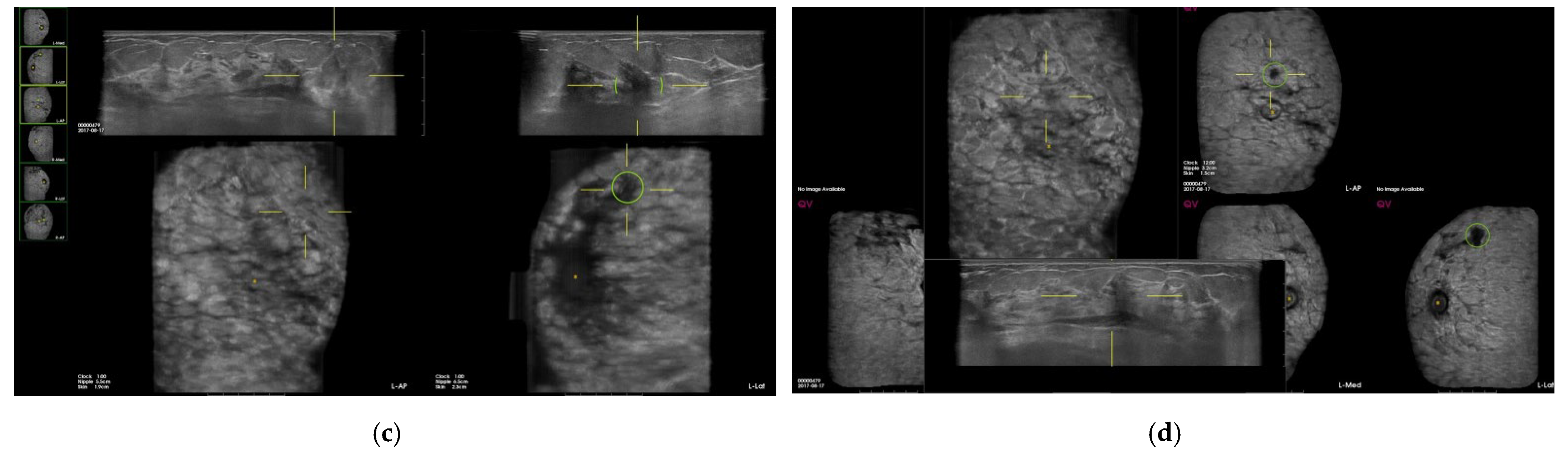
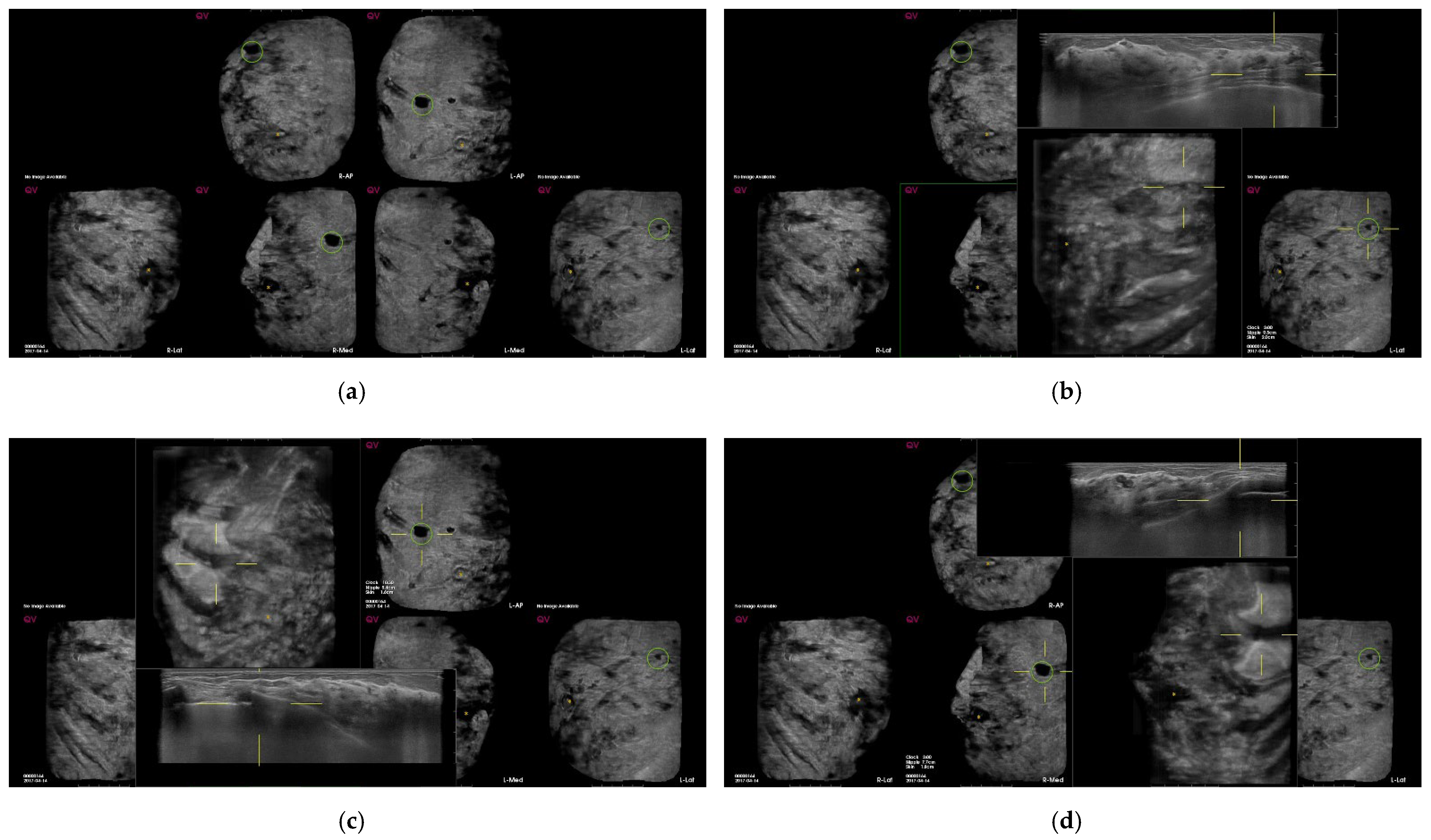
| Total (n = 846) | Benign | Malig | SEN | SPE | PPV | NPV | Accuracy | p-Value |
|---|---|---|---|---|---|---|---|---|
| CAD | ||||||||
| CAD (−) | 496 | 2 | 60 | 59 | 0.9 | 99.6 | 59 | 0.407 |
| CAD (+) | 345 | 3 | ||||||
| Mark No. 1 # | 324 | 2 | ||||||
| Mark No. 2 | 20 | - | ||||||
| Mark No. 3 | 1 | 1 | ||||||
| ABUS Category | ||||||||
| <4 | 827 | 0 | 100 | 98.3 | 26.3 | 100 | 0.6 | <0.0001 |
| =>4 | 14 | 5 | ||||||
| Lesion type | ||||||||
| non-mass | 10 | 1 | ||||||
| mass | 56 | 4 | ||||||
| Non-mass (n = 11) | ||||||||
| CAD (−) | 3 | 1 | - | 30 | - | 75 | 27.3 | 0.364 |
| CAD (+) | 7 | 0 | ||||||
| Mark No. 1 | 6 | - | ||||||
| Mark No. 2 | 1 | - | ||||||
| Mark No. 3 | - | - | ||||||
| Mass (n = 60) | ||||||||
| CAD (−) | 27 | 1 | 75 | 48.2 | 9.4 | 96.4 | 50 | 0.616 |
| CAD (+) | 29 | 3 | ||||||
| Mark No. 1 | 23 | 2 | ||||||
| Mark No. 2 | 5 | - | ||||||
| Mark No. 3 | 1 | 1 | ||||||
| Tissue Composition | ||||||||
| 1–2 | 665 | 3 | 40 | 79.1 | 1.1 | 99.6 | 78.8 | 0.284 |
| 3–4 | 176 | 2 | ||||||
| Tissue Composition (1–2, n = 668) | ||||||||
| CAD (−) | 409 | 1 | 66.7 | 61.5 | 0.8 | 99.8 | 61.5 | 0.563 |
| CAD (+) | 256 | 2 | ||||||
| Mark No. 1 | 241 | 1 | ||||||
| Mark No. 2 | 14 | - | ||||||
| Mark No. 3 | 1 | 1 | ||||||
| Tissue Composition (3–4, n = 178) | ||||||||
| CAD (−) | 87 | 1 | 50 | 49.4 | 1.1 | 98.9 | 49.4 | 1 |
| CAD (+) | 89 | 1 | ||||||
| Mark No. 1 | 83 | 1 | ||||||
| Mark No. 2 | 6 | - | ||||||
| Mark No. 3 | - | - | ||||||
| Total (n = 1032) | ||||||||
| CAD (−) | 496 | 2 | 60 | 48.3 | 0.6 | 99.6 | 48.4 | 1 |
| CAD (+) | 531 | 3 | ||||||
| Mark Number 1 € | 220 | 3 | ||||||
| Mark Number 2 | 79 | - | ||||||
| Mark Number 3 | 36 | - | ||||||
| Mark Number 4 | 6 | - | ||||||
| Mark Number 5 | 3 | - | ||||||
| Mark Number 6 | 1 | - |
| Mark No. # | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2, 3 | Total (1,2,3) | p-Value * | |
| Size | 0.702 | ||||
| mean ± SD | 12.2 ± 7.6 | 11.5 ± 6.3 | 18.9 ± 17.6 | 13 ± 9.9 | |
| median(IQR) | 10 (7, 14.5) | 10 (7, 13) | 15 (8, 20) | 10 (7, 14) | |
| Mass type | 0.743 | ||||
| non-mass | 28 (87.5) | 25 (80.7) | 7 (87.5) | 32 (82) | |
| mass | 4 (12.5) | 6 (19.4) | 1 (12.5) | 7 (18) | |
| Tissue composition | 0.004 | ||||
| 1, 2 | 410 (82.3) | 242 (74.2) | 16 (72.7) | 258 (74.1) | |
| 3, 4 | 88 (17.7) | 84 (25.8) | 6 (27.3) | 90 (25.9) | |
| Reading time | <0.0001 | ||||
| CAD with ABUS = ABUS | 16 (3.2) | 39 (12.1) | 10 (50) | 49 (14.3) | |
| CAD with ABUS > ABUS | - | 279 (86.4) | 10 (50) | 289 (84.3) | |
| CAD with ABUS < ABUS | 482 (96.8) | 5 (1.6) | - | 5 (1.5) | |
| Characteristics of All CAD Mark (n = 1032) | ||
|---|---|---|
| Mean and median No. of CAD marks per patient | ||
| mean ± SD | 0.8 ± 1 | |
| median (IQR) | 1 (0, 1) | |
| No. of CAD mark per patient | n | % |
| 0 (498) | 498 | 48.3 |
| 1 (1 × 223) | 223 | 21.6 |
| 2 (2 × 79) | 158 | 15.3 |
| 3 (3 × 36) | 108 | 10.5 |
| 4 (4 × 6) | 24 | 2.3 |
| 5 (5 × 3) | 15 | 1.5 |
| 6 (6 × 1) | 6 | 0.6 |
| Characteristics of CAD marks per lesion (n = 534) | n | % |
| Suspicious | 4 | 0.8 |
| Benign | 71 | 13.3 |
| Fat | 35 | 6.6 |
| Benign mass | 19 | 3.6 |
| Cyst | 9 | 1.7 |
| Fibrosis/heterogenous parenchyma | 8 | 1.5 |
| False-positive marks for pseudolesions | 459 | 86 |
| Marginal shadowing | 209 | 39.1 |
| Cooper’s ligament shadowing | 143 | 26.8 |
| Periareolar shadowing | 64 | 12 |
| Rib | 37 | 6.9 |
| Skin lesion | 6 | 1.1 |
| Mark No. # | |||||
|---|---|---|---|---|---|
| All | 1 | 2, 3 | p-Value | p-Value * | |
| Mark Location | 0.337 | 0.002 | |||
| right | 262 (57.1) | 251 (56.7) | 11 (68.8) | ||
| left | 197 (42.9) | 192 (43.3) | 5 (31.3) | ||
| Mark Location | 0.806 | 0.026 | |||
| antero-posterior | 142 (29.9) | 131 (29.6) | 11 (34.4) | ||
| medial | 147 (31) | 137 (30.9) | 10 (31.3) | ||
| lateral | 186 (39.2) | 175 (39.5) | 11 (34.4) | ||
| Mark Site | 0.674 | <0.0001 | |||
| upper | 377 (82.1) | 362 (81.7) | 15 (93.8) | ||
| mid | 30 (6.5) | 30 (6.8) | - | ||
| lower | 52 (11.3) | 51 (11.5) | 1 (6.3) | ||
| Mark Site | 0.572 | <0.0001 | |||
| inner | 101 (22) | 96 (21.7) | 5 (31.3) | ||
| mid | 139 (30.3) | 134 (30.3) | 5 (31.3) | ||
| outer | 219 (47.7) | 213 (48.1) | 6 (37.5) | ||
| Tissue Composition | 0.843 | <0.0001 | |||
| 1, 2 | 305 (66.5) | 294 (66.4) | 11 (68.8) | ||
| 3, 4 | 154 (33.6) | 149 (33.6) | 5 (31.3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kang, B.J.; Kim, S.H.; Park, G.E. Evaluation of Computer-Aided Detection (CAD) in Screening Automated Breast Ultrasound Based on Characteristics of CAD Marks and False-Positive Marks. Diagnostics 2022, 12, 583. https://doi.org/10.3390/diagnostics12030583
Lee J, Kang BJ, Kim SH, Park GE. Evaluation of Computer-Aided Detection (CAD) in Screening Automated Breast Ultrasound Based on Characteristics of CAD Marks and False-Positive Marks. Diagnostics. 2022; 12(3):583. https://doi.org/10.3390/diagnostics12030583
Chicago/Turabian StyleLee, Jeongmin, Bong Joo Kang, Sung Hun Kim, and Ga Eun Park. 2022. "Evaluation of Computer-Aided Detection (CAD) in Screening Automated Breast Ultrasound Based on Characteristics of CAD Marks and False-Positive Marks" Diagnostics 12, no. 3: 583. https://doi.org/10.3390/diagnostics12030583
APA StyleLee, J., Kang, B. J., Kim, S. H., & Park, G. E. (2022). Evaluation of Computer-Aided Detection (CAD) in Screening Automated Breast Ultrasound Based on Characteristics of CAD Marks and False-Positive Marks. Diagnostics, 12(3), 583. https://doi.org/10.3390/diagnostics12030583






