Beyond the Prognostic Value of 2-[18F]FDG PET/CT in Prostate Cancer: A Case Series and Literature Review Focusing on the Diagnostic Value and Impact on Patient Management
Abstract
1. Introduction
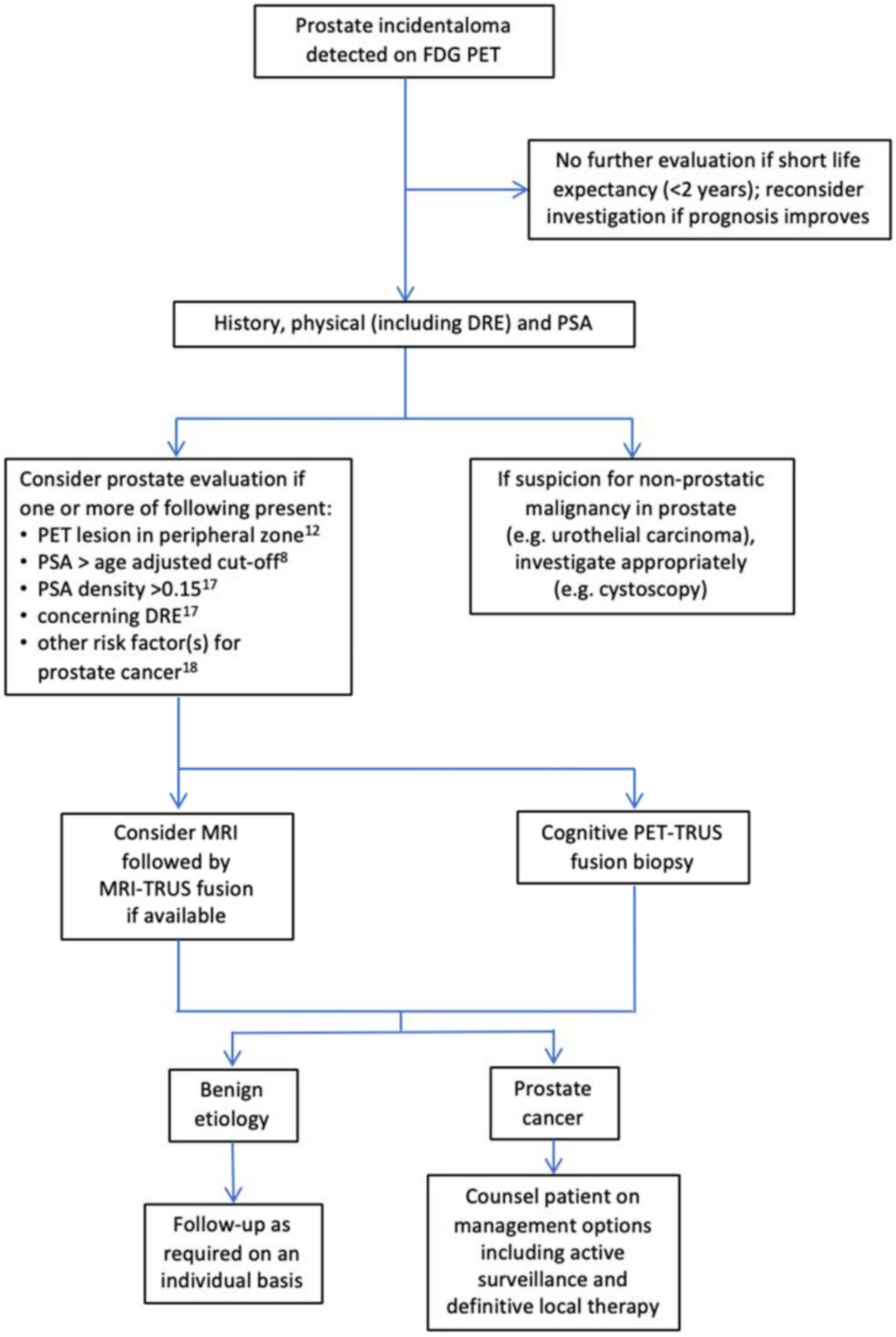
2. FDG PET in the Incidental Detection of the Primary PCa
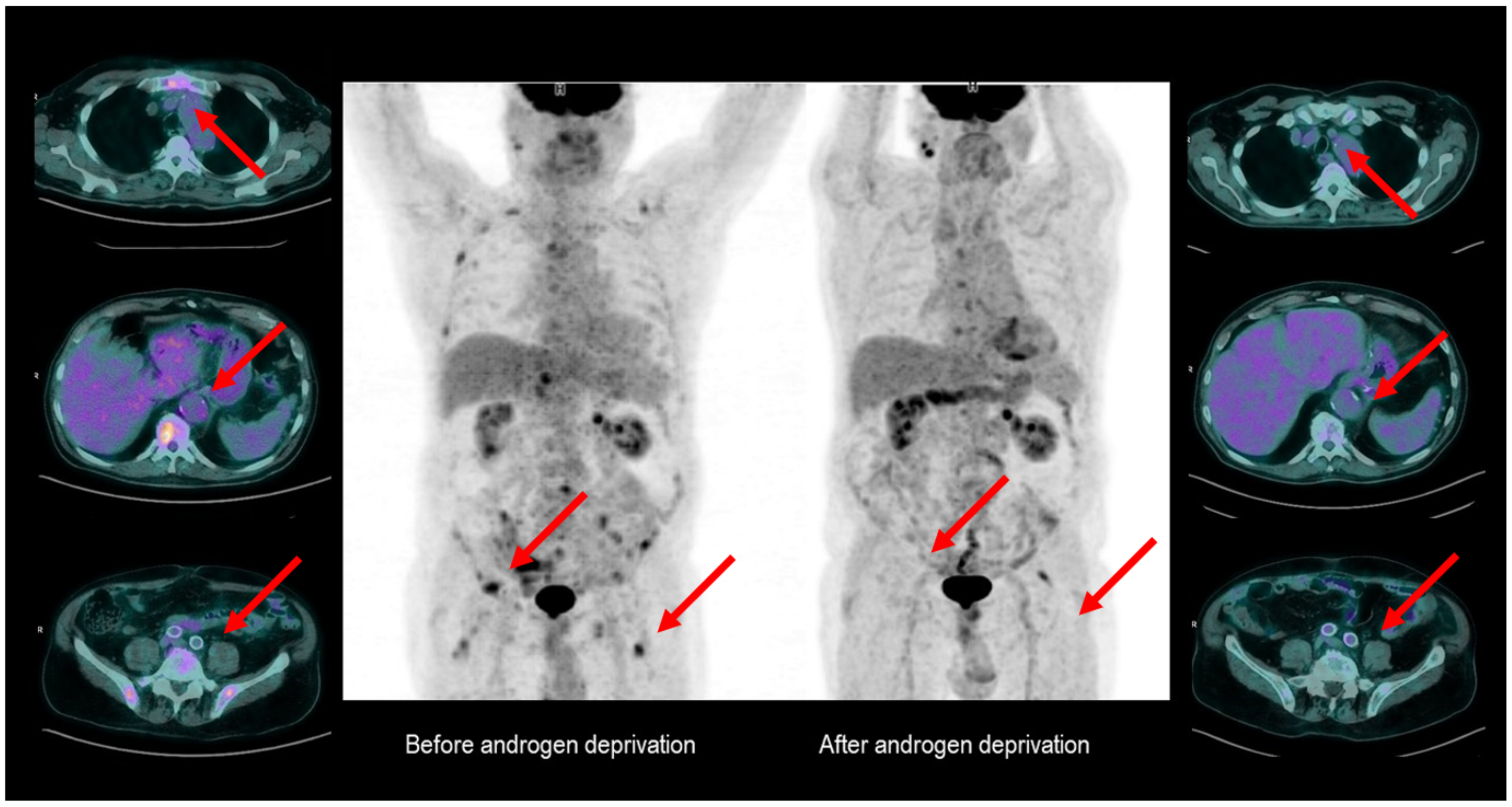
3. FDG PET as a Potential Tool for Therapy Monitoring in Hormone-Sensitive PCa
4. FDG PET as a Tool to Improve Systemic Treatment Selection in the Castration-Resistant Phase of the Disease
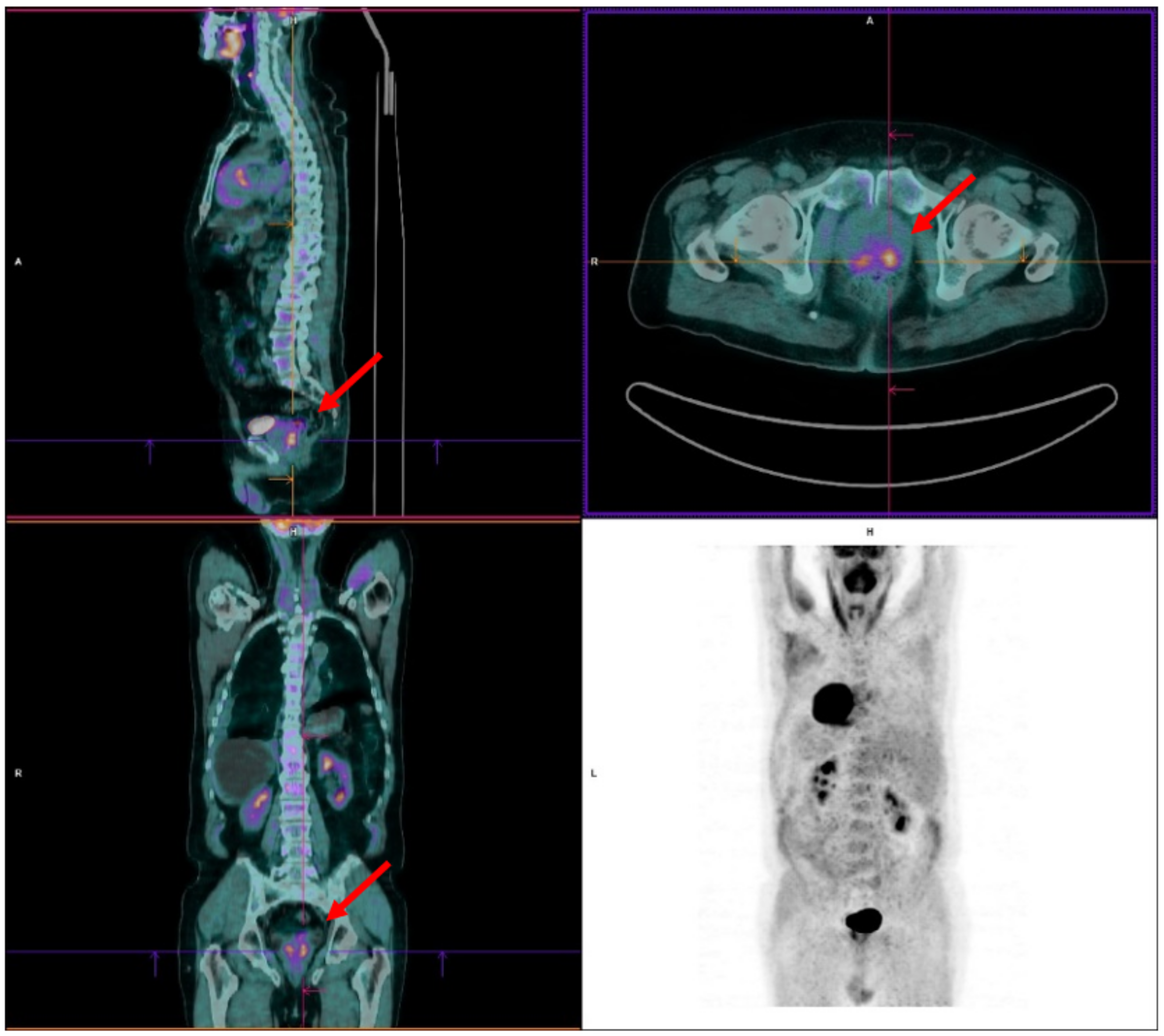
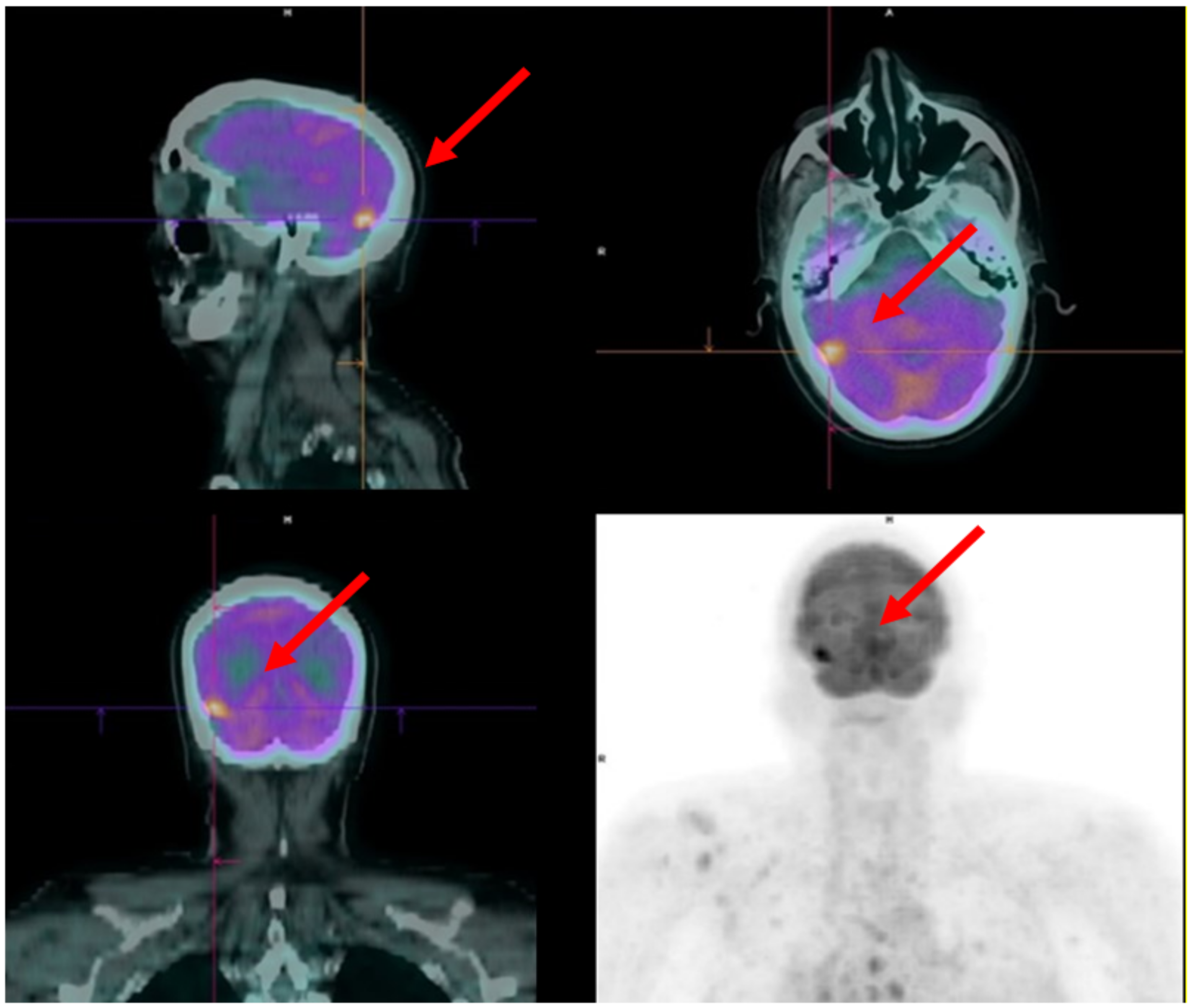
4.1. Case 1
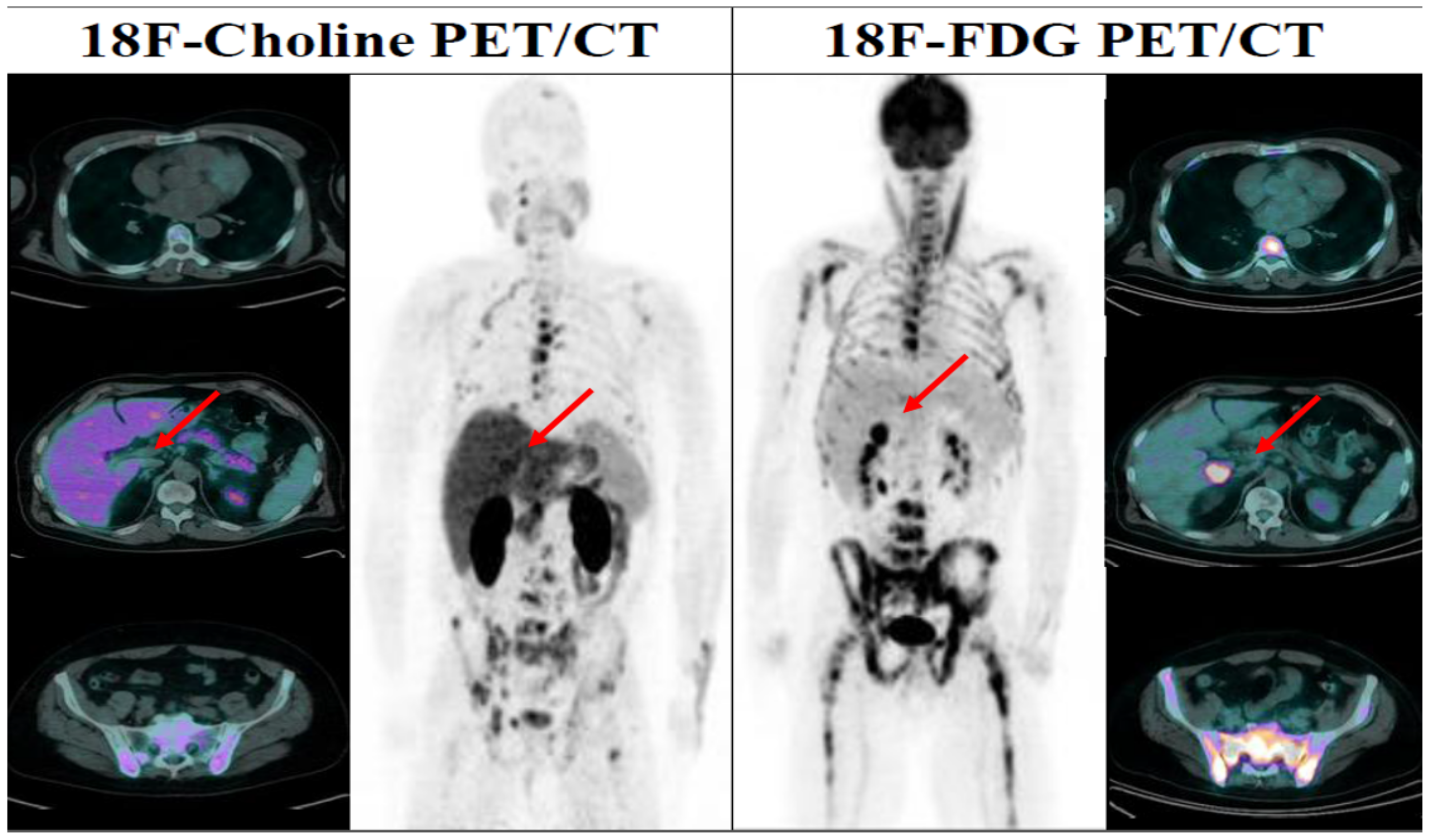
4.2. Case 2
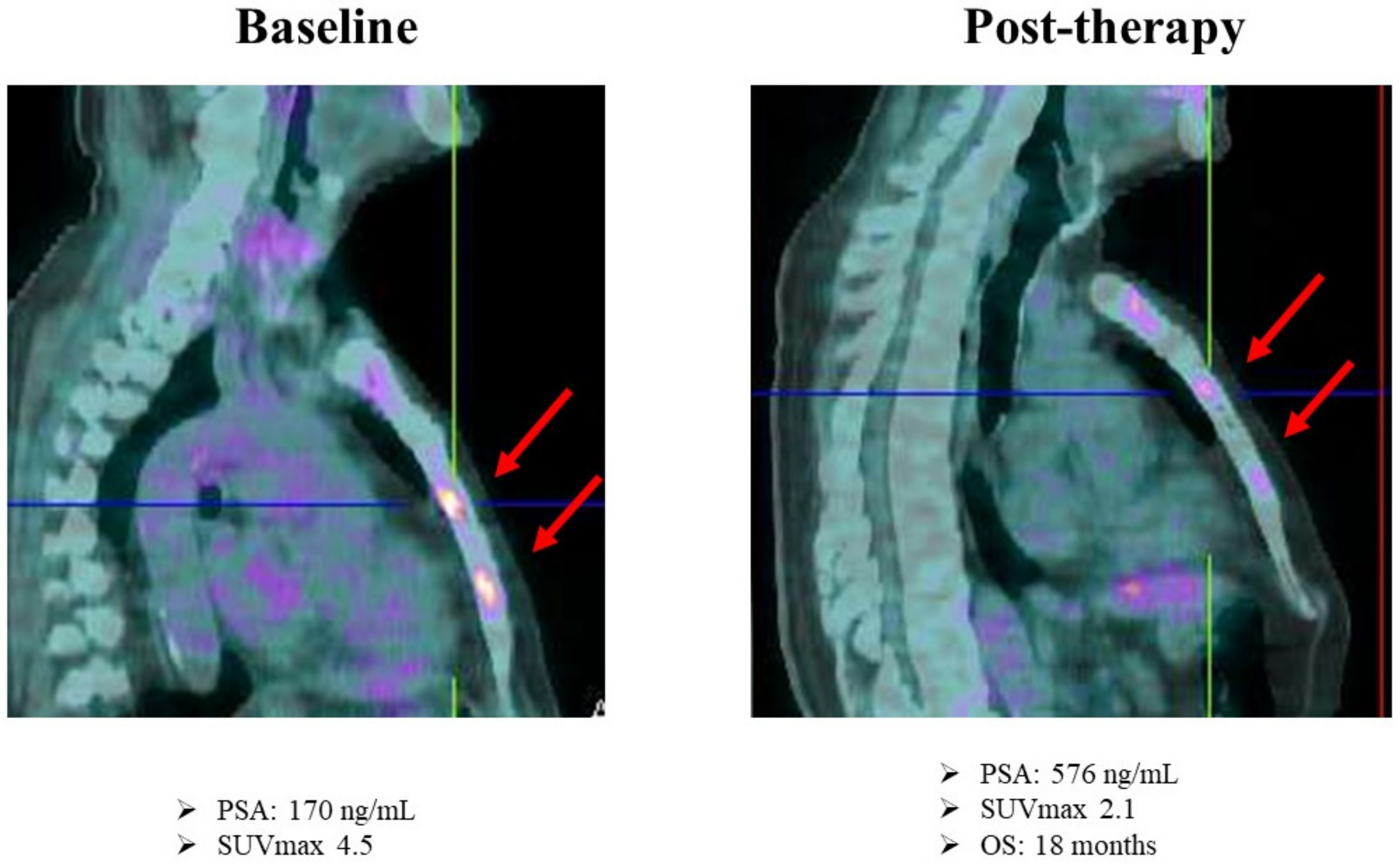
5. FDG PET as a Potential Tool for Therapy Monitoring in the Castration-Resistant Phase of the Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S.; ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Facchini, G.; Perri, F.; Misso, G.; D’Aniello, C.; Scarpati, G.; Rossetti, S.; Pepa, C.D.; Pisconti, S.; Unteregger, G.; Cossu, A.; et al. Optimal Management of Prostate Cancer Based on Its Natural Clinical History. Curr. Cancer Drug Targets 2018, 18, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, M.; Apfelbeck, M.; Pfitzinger, P.; Bischoff, R.; Lellig, E.; Rath, L.; Schlenker, B.; Stief, C.G.; Clevert, D.A. Multiparametric magnetic resonance imaging and multiparametric magnetic resonance imaging-guided biopsy in the diagnostic pathway of prostate cancer. Radiologe 2020, 60, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Cimadamore, A.; Cheng, M.; Santoni, M.; Lopez-Beltran, A.; Battelli, N.; Massari, F.; Galosi, A.B.; Scarpelli, M.; Montironi, R. New Prostate Cancer Targets for Diagnosis, Imaging, and Therapy: Focus on Prostate-Specific Membrane Antigen. Front. Oncol. 2018, 8, 653. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Azad, A.; Hofman, M.S. Guiding management of therapy in prostate cancer: Time to switch from conventional imaging to PSMA PET? Ther. Adv. Med. Oncol. 2019, 11, 1–14. [Google Scholar] [CrossRef]
- Giovacchini, G.; Giovannini, E.; Leoncini, R.; Riondato, M.; Ciarmiello, A. PET and PET/CT with radiolabeled choline in prostate cancer: A critical reappraisal of 20 years of clinical studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1751–1776. [Google Scholar] [CrossRef]
- Laudicella, R.; Albano, D.; Alongi, P.; Argiroffi, G.; Bauckneht, M.; Baldari, S.; Bertagna, F.; Boero, M.; Vincentis, G.; Sole, A.D.; et al. 18F-Facbc in Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1348. [Google Scholar] [CrossRef]
- Jadvar, H. PET of Glucose Metabolism and Cellular Proliferation in Prostate Cancer. J. Nucl. Med. 2016, 57 (Suppl. 3), 25S–29S. [Google Scholar] [CrossRef][Green Version]
- Jadvar, H. Is There Use for FDG-PET in Prostate Cancer? Semin. Nucl. Med. 2016, 46, 502–506. [Google Scholar] [CrossRef]
- Hofer, C.; Laubenbacher, C.; Block, T.; Breul, J.; Hartung, R.; Schwaiger, M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur. Urol. 1999, 36, 31–35. [Google Scholar] [CrossRef]
- Jadvar, H. FDG PET in prostate cancer. PET Clin. 2009, 4, 155–161. [Google Scholar] [CrossRef]
- Jadvar, H.; Desai, B.; Ji, L.; Conti, P.S.; Dorff, T.B.; Groshen, S.G.; Pinski, J.K.; Quinn, D.I. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J. Nucl. Med. 2013, 54, 1195–1201. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Zhu, Y.; Shi, Y.; Xu, L.; Huang, G.; Liu, J. The added value of 18F-FDG PET/CT compared to 68Ga-PSMA PET/CT in patients with castration-resistant prostate. J. Nucl. Med. 2021, 63, 69–75. [Google Scholar] [CrossRef]
- Bauckneht, M.; Bertagna, F.; Donegani, M.I.; Durmo, R.; Miceli, A.; De Biasi, V.; Laudicella, R.; Fornarini, G.; Berruti, A.; Baldari, S.; et al. The prognostic power of 18F-FDG PET/CT extends to estimating systemic treatment response duration in metastatic castration-resistant prostate cancer (mCRPC) patients. Prostate Cancer Prostatic Dis. 2021, 24, 1198–1207. [Google Scholar] [CrossRef]
- Watanabe, H.; Kanematsu, M.; Kondo, H.; Kako, N.; Yamamoto, N.; Yamada, T.; Goshima, S.; Hoshi, H.; Bae, K.T. Preoperative detection of prostate cancer: A comparison with 11C-choline PET, 18F-fluorodeoxyglucose PET and MR imaging. J. Magn. Reson. Imaging 2010, 31, 1151–1156. [Google Scholar] [CrossRef]
- Minamimoto, R.; Senda, M.; Jinnouchi, S.; Terauchi, T.; Yoshida, T.; Murano, T.; Fukuda, H.; Iinuma, T.; Uno, K.; Nishizawa, S.; et al. The current status of an FDG-PET cancer screening program in Japan, based on a 4-year (2006–2009) nationwide survey. Ann. Nucl. Med. 2013, 27, 46–57. [Google Scholar] [CrossRef]
- Minamimoto, R.; Uemura, H.; Sano, F.; Terao, H.; Nagashima, Y.; Yamanaka, S.; Shizukuishi, K.; Tateishi, U.; Kubota, Y.; Inoue, T. The potential of FDG-PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann. Nucl. Med. 2011, 25, 21–27. [Google Scholar] [CrossRef]
- Effert, P.J.; Bares, R.; Handt, S.; Wolff, J.M.; Büll, U.; Jakse, G. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18 fluorine-labeled deoxyglucose. J. Urol. 1996, 155, 994–998. [Google Scholar] [CrossRef]
- Laubenbacher, C.; Hofer, C.; Avril, N.; Block, T.; Ziegler, S.; Herz, M.; Kruschke, C.; Hartung, R.; Schwaiger, M. F-18 FDG PET for differentiation of local recurrent prostate cancer and scar. J. Nucl. Med. 1995, 36, 198. [Google Scholar]
- Bertagna, F.; Sadeghi, R.; Giovanella, L.; Treglia, G. Incidental uptake of 18F-fluorodeoxyglucose in the prostate gland. Systematic review and meta-analysis on prevalence and risk of malignancy. Nuklearmedizin 2014, 53, 249–258. [Google Scholar] [CrossRef]
- Mannas, M.P.; Lee, T.; Pourghiasian, M.; Wilson, D.C.; Black, P.C. Incidentalomas of the prostate detected by 18-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. Can. Urol. Assoc. J. 2020, 14, E180–E184. [Google Scholar] [CrossRef]
- Oyama, N.; Akino, H.; Suzuki, Y.; Kanamaru, H.; Miwa, Y.; Tsuka, H.; Sadato, N.; Yonekura, Y.; Okada, K. Prognostic value of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography imaging for patients with prostate cancer. Mol. Imaging Biol. 2020, 4, 99–104. [Google Scholar] [CrossRef]
- Kichloo, A.; Amir, R.; Aljadah, M.; Wani, F.; Solanki, S.; Singh, J.; Chugh, S.S. FDG-PET Versus PSMA-PET: A Patient with Prostate Cancer. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620941313. [Google Scholar] [CrossRef]
- Meziou, S.; Ringuette Goulet, C.; Hovington, H.; Lefebvre, V.; Lavallée, É.; Bergeron, M.; Brisson, H.; Champagne, A.; Neveu, B.; Lacombe, D.; et al. GLUT1 expression in high-risk prostate cancer: Correlation with 18F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. 2020, 23, 441–448. [Google Scholar] [CrossRef]
- McEwan, L.M.; Wong, D.; Yaxley, J. Flourodeoxyglucose positron emission tomography scan may be helpful in the case of ductal variant prostate cancer when prostate specific membrane antigen ligand positron emission tomography scan is negative. J. Med. Imaging Radiat. Oncol. 2017, 61, 503–505. [Google Scholar] [CrossRef]
- Spratt, D.E.; Gavane, S.; Tarlinton, L.; Fareedy, S.B.; Doran, M.G.; Zelefsky, M.J.; Osborne, J.R. Utility of FDG-PET in clinical neuroendocrine prostate cancer. Prostate 2014, 74, 1153–1159. [Google Scholar] [CrossRef]
- Jadvar, H.; Xiankui, L.; Shahinian, A.; Park, R.; Tohme, M.; Pinski, J.; Conti, P.S. Glucose metabolism of human prostate cancer mouse xenografts. Mol. Imaging 2005, 4, 91–97. [Google Scholar] [CrossRef]
- Oyama, N.; Akino, H.; Suzuki, Y.; Kanamaru, H.; Ishida, H.; Tanase, K.; Sadato, N.; Yonekura, Y.; Okada, K. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl. Med. Commun. 2011, 22, 963–969. [Google Scholar] [CrossRef]
- Jadvar, H.; Desai, B.; Quinn, D.; Dorff, T.; Pinski, J.; Conti, P.; Groshen, S. Treatment response assessment of metastatic prostate cancer with FDG PET/CT. J. Nucl. Med. 2011, 52 (Suppl. 1), 1908. [Google Scholar]
- Jadvar, H.; Velez, E.M.; Desai, B.; Ji, L.; Colletti, P.M.; Quinn, D.I. Prediction of Time to Hormonal Treatment Failure in Metastatic Castration-Sensitive Prostate Cancer with 18F-FDG PET/CT. J. Nucl. Med. 2019, 60, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.V.; Alves, M.G.; Marques, R.; Moreira, P.I.; Oliveira, P.F.; Maia, C.J.; Socorro, S. Androgen-responsive and nonresponsive prostate cancer cells present a distinct glycolytic metabolism profile. Int. J. Biochem. Cell Biol. 2012, 44, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Ding, S.M.; Cao, G.; Wang, S.J.; Zheng, X.H.; Li, G.H. miR-132 mediates a metabolic shift in prostate cancer cells by targeting Glut1. FEBS Open Bio 2016, 6, 735–741. [Google Scholar] [CrossRef]
- Haberkorn, U.; Bellemann, M.E.; Altmann, A.; Gerlach, L.; Morr, I.; Oberdorfer, F.; Brix, G.; Doll, J.; Blatter, J.; van Kaick, G. PET 2-fluoro-2-deoxyglucose uptake in rat prostate adenocarcinoma during chemotherapy with gemcitabine. J. Nucl. Med. 1997, 38, 1215–1221. [Google Scholar]
- Fox, J.J.; Gavane, S.C.; Blanc-Autran, E.; Nehmeh, S.; Gönen, M.; Beattie, B.; Vargas, H.A.; Schöder, H.; Humm, J.L.; Fine, S.W.; et al. Positron Emission Tomography/Computed Tomography-Based Assessments of Androgen Receptor Expression and Glycolytic Activity as a Prognostic Biomarker for Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2018, 4, 217–224. [Google Scholar] [CrossRef]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of Baseline and Post-Therapy 18F-FDG PET in the Prognostic Stratification of Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients Treated with Radium-223. Cancers 2019, 12, 31. [Google Scholar] [CrossRef]
- Bauckneht, M.; Rebuzzi, S.E.; Signori, A.; Donegani, M.I.; Murianni, V.; Miceli, A.; Borea, R.; Raffa, S.; Damassi, A.; Ponzano, M.; et al. The Prognostic Role of Baseline Metabolic Tumor Burden and Systemic Inflammation Biomarkers in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223: A Proof of Concept Study. Cancers 2020, 12, 3213. [Google Scholar] [CrossRef]
- Bauckneht, M.; Rebuzzi, S.E.; Signori, A.; Frantellizzi, V.; Murianni, V.; Lodi Rizzini, E.; Mascia, M.; Lavelli, V.; Donegani, M.I.; Ponzano, M.; et al. The prognostic power of inflammatory indices and clinical factors in metastatic castration-resistant prostate cancer patients treated with radium-223 (BIO-Ra study). Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1063–1074. [Google Scholar] [CrossRef]
- Bauckneht, M.; Lai, R.; D’Amico, F.; Miceli, A.; Donegani, M.I.; Campi, C.; Schenone, D.; Raffa, S.; Chiola, S.; Lanfranchi, F.; et al. Opportunistic skeletal muscle metrics as prognostic tools in metastatic castration-resistant prostate cancer patients candidates to receive Radium-223. Ann. Nucl. Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef]
- Morris, M.J.; Heller, G.; Bryce, A.H.; Armstrong, A.J.; Beltran, H.; Hahn, O.M.; McGary, E.C.; Mehan, P.T.; Goldkorn, A.; Roth, B.J.; et al. Alliance A031201: A phase III trial of enzalutamide (ENZ) versus enzalutamide, abiraterone, and prednisone (ENZ/AAP) for metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 2019, 37 (Suppl. 15), 5008. [Google Scholar] [CrossRef]
- McKay, R.R.; Xie, W.; Ye, H.; Fennessy, F.M.; Zhang, Z.; Lis, R.; Calagua, C.; Rathkopf, D.; Laudone, V.P.; Bubley, G.J.; et al. Results of a Randomized Phase II Trial of Intense Androgen Deprivation Therapy prior to Radical Prostatectomy in Men with High-Risk Localized Prostate Cancer. J. Urol. 2021, 206, 80–87. [Google Scholar] [CrossRef]
- Andrews, J.R.; Ahmed, M.E.; Karnes, R.J.; Kwon, E.; Bryce, A.H. Systemic treatment for metastatic castrate resistant prostate cancer: Does seqence matter? Prostate 2020, 80, 399–406. [Google Scholar] [CrossRef]
- Miyake, H.; Sugiyama, T.; Aki, R.; Matsushita, Y.; Tamura, K.; Motoyama, D.; Ito, T.; Otsuka, A. Comparison of Alternative Androgen Receptor-Axis-Targeted Agent (ARATA) and Docetaxel as Second-Line Therapy for Patients with Metastatic Castration-Resistant Prostate Cancer with Progression after Initial ARATA in Real-World Clinical Practice in Japan. Clin. Genitourin. Cancer 2018, 16, 219–225. [Google Scholar] [CrossRef]
- Oh, W.K.; Cheng, W.Y.; Miao, R.; Vekeman, F.; Gauthier-Loiselle, M.; Duh, M.S.; Drea, E.; Szatrowski, T.P. Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor-targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting. Urol. Oncol. 2018, 36, 500.e1–500.e9. [Google Scholar] [CrossRef]
- Matsubara, N.; Yamada, Y.; Tabata, K.I.; Satoh, T.; Kamiya, N.; Suzuki, H.; Kawahara, T.; Uemura, H.; Yano, A.; Kawakami, S.; et al. Comparison of Sequential Treatment with Androgen Receptor-Targeted Agent Followed by Another Androgen Receptor-Targeted Agent versus Androgen Receptor-Targeted Agent Followed by Docetaxel in Chemotherapy-Naive Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, e1073–e1080. [Google Scholar] [CrossRef]
- Oh, W.K.; Miao, R.; Vekeman, F.; Sung, J.; Cheng, W.Y.; Gauthier-Loiselle, M.; Dhawan, R.; Duh, M.S. Real-world Characteristics and Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Chemotherapy versus Androgen Receptor-Targeted Therapy after Failure of First-Line Androgen Receptor-Targeted Therapy in the Community Setting. Clin. Genitourin. Cancer 2018, 16, 50–57. [Google Scholar] [CrossRef]
- Bauckneht, M.; Morbelli, S.; Miceli, A.; Rebuzzi, S.E.; Fornarini, G. Neuroendocrine Differentiation of Prostate Cancer Is Not Systematically Associated with Increased 18F-FDG Uptake. Diagnostics 2021, 11, 468. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Bolton, R.C.D.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Suman, S.; Parghane, R.V.; Joshi, A.; Prabhash, K.; Bakshi, G.; Talole, S.; Banerjee, S.; Basu, S. Therapeutic efficacy, prognostic variables and clinical outcome of 177Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: Prognostic implications of high FDG uptake on dual tracer PET-CT vis-à-vis Gleason score in such cohort. Br. J. Radiol. 2019, 92, 20190380. [Google Scholar] [CrossRef]
- Van der Zande, K.; Oyen, W.; Zwart, W.; Bergman, A.M. Radium-223 Treatment of Patients with Metastatic Castration Resistant Prostate Cancer: Biomarkers for Stratification and Response Evaluation. Cancers 2021, 13, 4346. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Fosbøl, M.Ø.; Petersen, P.M.; Kjaer, A.; Mortensen, J. 223Ra Therapy of Advanced Metastatic Castration-Resistant Prostate Cancer: Quantitative Assessment of Skeletal Tumor Burden for Prognostication of Clinical Outcome and Hematologic Toxicity. J. Nucl. Med. 2018, 59, 596–602. [Google Scholar] [CrossRef]
- Kairemo, K.; Joensuu, T. Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer-Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT. Diagnostics 2015, 5, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Letellier, A.; Johnson, A.C.; Kit, N.H.; Savigny, J.F.; Batalla, A.; Parienti, J.J.; Aide, N. Uptake of Radium-223 Dichloride and Early [18F]NaF PET Response Are Driven by Baseline [18F]NaF Parameters: A Pilot Study in Castration-Resistant Prostate Cancer Patients. Mol. Imaging Biol. 2018, 20, 482–491. [Google Scholar] [CrossRef]
- García Vicente, A.M.; Amo-Salas, M.; Cassinello Espinosa, J.; Gómez Díaz, R.; Soriano Castrejón, Á. Interim and end-treatment 18F-Fluorocholine PET/CT and bone scan in prostate cancer patients treated with Radium 223 dichloride. Sci. Rep. 2021, 11, 7389. [Google Scholar] [CrossRef]
- Grubmüller, B.; Rasul, S.; Baltzer, P.; Fajkovic, H.; D’Andrea, D.; Berndl, F.; Maj-Hes, A.; Grubmüller, K.H.; Mitterhauser, M.; Wadsak, W.; et al. Response assessment using [68 Ga]Ga-PSMA ligand PET in patients undergoing systemic therapy for metastatic castration-resistant prostate cancer. Prostate 2020, 80, 74–82. [Google Scholar] [CrossRef]
- Velez, E.M.; Desai, B.; Ji, L.; Quinn, D.I.; Colletti, P.M.; Jadvar, H. Comparative prognostic implication of treatment response assessments in mCRPC: PERCIST 1.0, RECIST 1.1, and PSA response criteria. Theranostics 2020, 10, 3254–3262. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borea, R.; Favero, D.; Miceli, A.; Donegani, M.I.; Raffa, S.; Gandini, A.; Cremante, M.; Marini, C.; Sambuceti, G.; Zanardi, E.; et al. Beyond the Prognostic Value of 2-[18F]FDG PET/CT in Prostate Cancer: A Case Series and Literature Review Focusing on the Diagnostic Value and Impact on Patient Management. Diagnostics 2022, 12, 581. https://doi.org/10.3390/diagnostics12030581
Borea R, Favero D, Miceli A, Donegani MI, Raffa S, Gandini A, Cremante M, Marini C, Sambuceti G, Zanardi E, et al. Beyond the Prognostic Value of 2-[18F]FDG PET/CT in Prostate Cancer: A Case Series and Literature Review Focusing on the Diagnostic Value and Impact on Patient Management. Diagnostics. 2022; 12(3):581. https://doi.org/10.3390/diagnostics12030581
Chicago/Turabian StyleBorea, Roberto, Diletta Favero, Alberto Miceli, Maria Isabella Donegani, Stefano Raffa, Annalice Gandini, Malvina Cremante, Cecilia Marini, Gianmario Sambuceti, Elisa Zanardi, and et al. 2022. "Beyond the Prognostic Value of 2-[18F]FDG PET/CT in Prostate Cancer: A Case Series and Literature Review Focusing on the Diagnostic Value and Impact on Patient Management" Diagnostics 12, no. 3: 581. https://doi.org/10.3390/diagnostics12030581
APA StyleBorea, R., Favero, D., Miceli, A., Donegani, M. I., Raffa, S., Gandini, A., Cremante, M., Marini, C., Sambuceti, G., Zanardi, E., Morbelli, S., Fornarini, G., Rebuzzi, S. E., & Bauckneht, M. (2022). Beyond the Prognostic Value of 2-[18F]FDG PET/CT in Prostate Cancer: A Case Series and Literature Review Focusing on the Diagnostic Value and Impact on Patient Management. Diagnostics, 12(3), 581. https://doi.org/10.3390/diagnostics12030581








