Predictive Factors for Successful Treatment of Deep Incisional Surgical Site Infections following Instrumented Spinal Surgeries: Retrospective Review of 1832 Cases
Abstract
:1. Introduction
2. Materials and Methods
3. Results
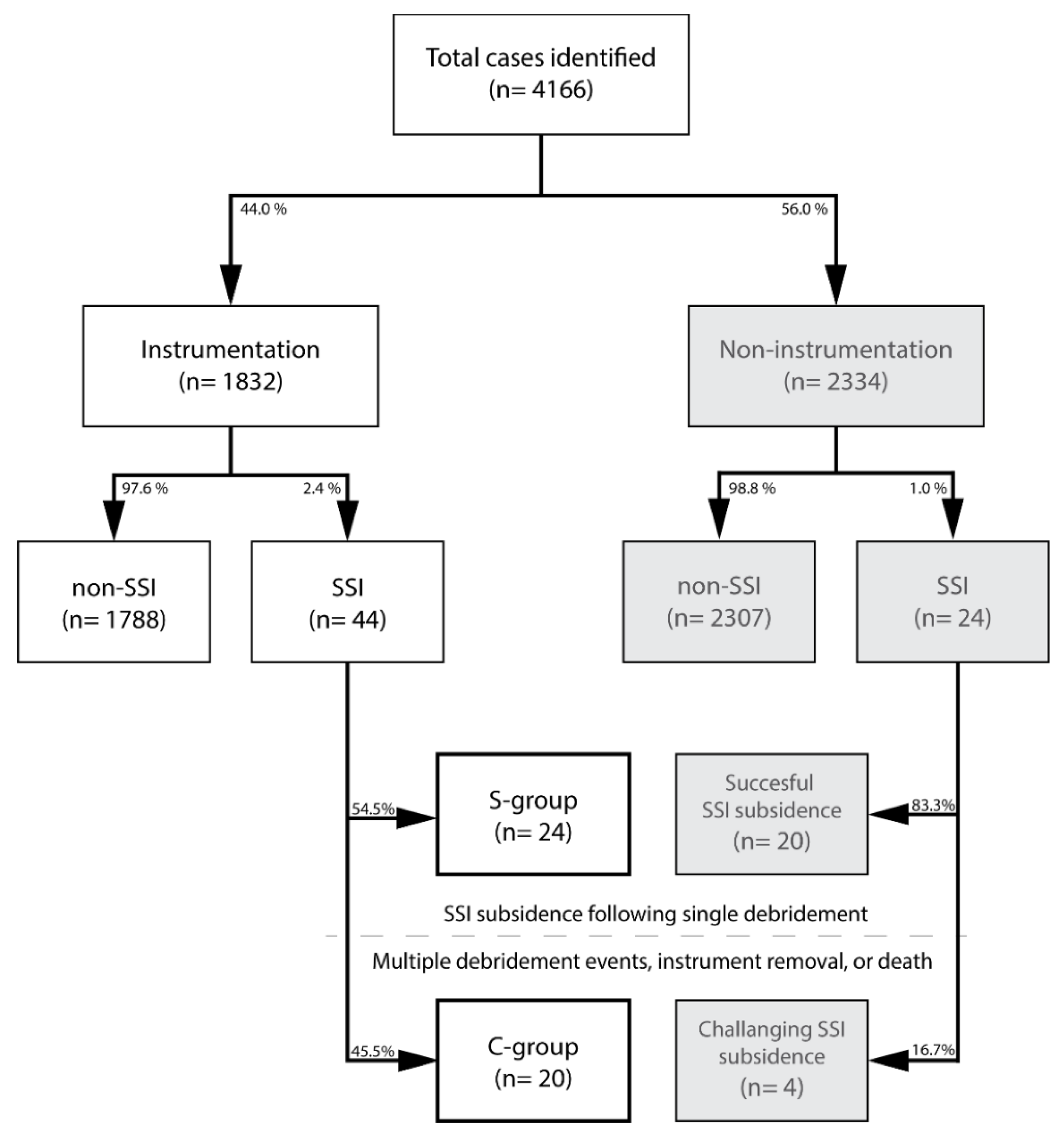
3.1. Patient Selection
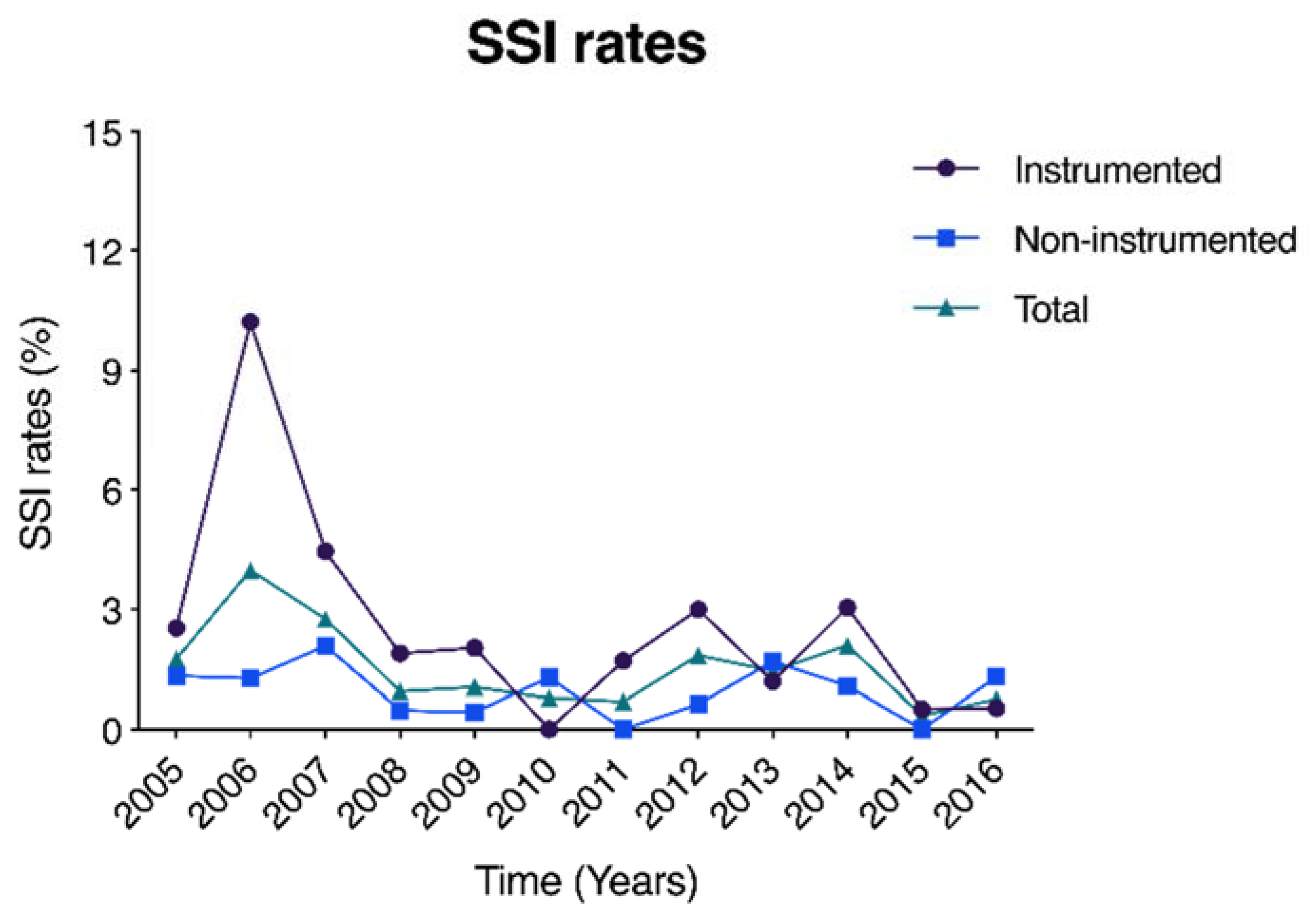
3.2. Surgical Site Infection Characteristics
3.3. Instrumentation SSI Subsidence
3.4. Multivariate Analysis
4. Discussion
4.1. SSI Characteristics
4.2. Prognostic Factors for Successful SSI Treatment
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watanabe, M.; Sakai, D.; Matsuyama, D.; Yamamoto, Y.; Sato, M.; Mochida, J. Risk factors for surgical site infection following spine surgery: Efficacy of intraoperative saline irrigation. J. Neurosurg. Spine 2010, 12, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Pull ter Gunne, A.F.; Mohamed, A.S.; Skolasky, R.L.; van Laarhoven, C.J.; Cohen, D.B. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine 2010, 35, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.A.; McCabe, J.P.; Cammisa, F.P., Jr. Postoperative spinal wound infection: A review of 2391 consecutive index procedures. J. Spinal Disord. 2000, 13, 422–426. [Google Scholar] [CrossRef]
- Fang, A.; Hu, S.S.; Endres, N.; Bradford, D.S. Risk factors for infection after spinal surgery. Spine 2005, 30, 1460–1465. [Google Scholar] [CrossRef]
- Joyce, K.; Sakai, D.; Pandit, A. Preclinical models of vertebral osteomyelitis and associated infections: Current models and recommendations for study design. JOR Spine 2021, 4, e1142. [Google Scholar] [CrossRef] [PubMed]
- Collins, I.; Wilson-MacDonald, J.; Chami, G.; Burgoyne, W.; Vinayakam, P.; Berendt, T.; Fairbank, J. The diagnosis and management of infection following instrumented spinal fusion. Eur. Spine J. 2008, 17, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davne, S.H.; Myers, D.L. Complications of lumbar spinal fusion with transpedicular instrumentation. Spine 1992, 17, S184–S189. [Google Scholar] [CrossRef]
- Sponseller, P.D.; LaPorte, D.M.; Hungerford, M.W.; Eck, K.; Bridwell, K.H.; Lenke, L.G. Deep wound infections after neuromuscular scoliosis surgery: A multicenter study of risk factors and treatment outcomes. Spine 2000, 25, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Jenis, L.G.; Hsu, W.K.; O’Brien, J.R.; Whang, P.G. Recent advances in the prevention and management of complications associated with routine lumbar spine surgery. Instr. Course Lect. 2014, 63, 263–270. [Google Scholar] [PubMed]
- Kasliwal, M.K.; Tan, L.A.; Traynelis, V.C. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surg. Neurol. Int. 2013, 4, S392–S403. [Google Scholar] [CrossRef] [PubMed]
- Habiba, S.; Nygaard, O.P.; Brox, J.I.; Hellum, C.; Austevoll, I.M.; Solberg, T.K. Risk factors for surgical site infections among 1772 patients operated on for lumbar disc herniation: A multicentre observational registry-based study. Acta Neurochir. 2017, 159, 1113–1118. [Google Scholar] [CrossRef]
- Tominaga, H.; Setoguchi, T.; Kawamura, H.; Kawamura, I.; Nagano, S.; Abematsu, M.; Tanabe, F.; Ishidou, Y.; Yamamoto, T.; Komiya, S. Risk factors for unavoidable removal of instrumentation after surgical site infection of spine surgery: A retrospective case-control study. Medicine 2016, 95, e5118. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.; Vasquez, G.; Harrop, J.; Maltenfort, M.; Stein, N.; Kaliyadan, G.; Klibert, F.; Epstein, R.; Sharan, A.; Vaccaro, A.; et al. Risk factors for surgical site infections following spinal fusion procedures: A case-control study. Clin. Infect. Dis. 2011, 53, 686–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, R.; Huo, X.; Xiong, W.; Kang, L.; Xue, Y. Incidence of Surgical Site Infection After Spine Surgery: A Systematic Review and Meta-analysis. Spine 2020, 45, 208–216. [Google Scholar] [CrossRef]
- Maruo, K.; Berven, S.H. Outcome and treatment of postoperative spine surgical site infections: Predictors of treatment success and failure. J. Orthop. Sci. 2014, 19, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Dipaola, C.P.; Saravanja, D.D.; Boriani, L.; Zhang, H.; Boyd, M.C.; Kwon, B.K.; Paquette, S.J.; Dvorak, M.F.; Fisher, C.G.; Street, J.T. Postoperative infection treatment score for the spine (PITSS): Construction and validation of a predictive model to define need for single versus multiple irrigation and debridement for spinal surgical site infection. Spine J. 2012, 12, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Cho, O.H.; Bae, I.G.; Moon, S.M.; Park, S.Y.; Kwak, Y.G.; Kim, B.N.; Yu, S.N.; Jeon, M.H.; Kim, T.; Choo, E.J.; et al. Therapeutic outcome of spinal implant infections caused by Staphylococcus aureus: A retrospective observational study. Medicine 2018, 97, e12629. [Google Scholar] [CrossRef]
- Hersh, A.; Young, R.; Pennington, Z.; Ehresman, J.; Ding, A.; Kopparapu, S.; Cottrill, E.; Sciubba, D.M.; Theodore, N. Removal of instrumentation for postoperative spine infection: Systematic review. J. Neurosurg. Spine 2021, 35, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kelkar, A.; Agarwal, A.G.; Jayaswal, D.; Schultz, C.; Jayaswal, A.; Goel, V.K.; Agarwal, A.K.; Gidvani, S. Implant Retention or Removal for Management of Surgical Site Infection After Spinal Surgery. Glob. Spine J. 2020, 10, 640–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Surgical Site Infection Event (SSI). Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf (accessed on 1 December 2021).
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control 1999, 27, 97–132; quiz 133–134; discussion 196. [Google Scholar] [CrossRef]
- Patel, H.; Khoury, H.; Girgenti, D.; Welner, S.; Yu, H. Burden of Surgical Site Infections Associated with Select Spine Operations and Involvement of Staphylococcus aureus. Surg. Infect. 2017, 18, 461–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClelland, S., 3rd; Takemoto, R.C.; Lonner, B.S.; Andres, T.M.; Park, J.J.; Ricart-Hoffiz, P.A.; Bendo, J.A.; Goldstein, J.A.; Spivak, J.M.; Errico, T.J. Analysis of Postoperative Thoracolumbar Spine Infections in a Prospective Randomized Controlled Trial Using the Centers for Disease Control Surgical Site Infection Criteria. Int. J. Spine Surg. 2016, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Jabbar, A.; Berven, S.H.; Hu, S.S.; Chou, D.; Mummaneni, P.V.; Takemoto, S.; Ames, C.; Deviren, V.; Tay, B.; Weinstein, P.; et al. Surgical site infections in spine surgery: Identification of microbiologic and surgical characteristics in 239 cases. Spine 2013, 38, E1425–E1431. [Google Scholar] [CrossRef] [PubMed]
- Torres, K.; Sampathkumar, P. Predictors of methicillin-resistant Staphylococcus aureus colonization at hospital admission. Am. J. Infect. Control 2013, 41, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Wakatake, H.; Fujitani, S.; Kodama, T.; Kawamoto, E.; Yamada, H.; Yanai, M.; Morisawa, K.; Takemura, H.; Lefor, A.T.; Taira, Y. Positive clinical risk factors predict a high rate of methicillin-resistant Staphylococcus aureus colonization in emergency department patients. Am. J. Infect. Control 2012, 40, 988–991. [Google Scholar] [CrossRef]
- Fukuta, Y.; Cunningham, C.A.; Harris, P.L.; Wagener, M.M.; Muder, R.R. Identifying the risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) infection among patients colonized with MRSA on admission. Infect. Control Hosp. Epidemiol. 2012, 33, 1219–1225. [Google Scholar] [CrossRef]
- Blumberg, T.J.; Woelber, E.; Bellabarba, C.; Bransford, R.; Spina, N. Predictors of increased cost and length of stay in the treatment of postoperative spine surgical site infection. Spine J. 2018, 18, 300–306. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ando, K.; Ito, K.; Tsushima, M.; Morozumi, M.; Tanaka, S.; Machino, M.; Ota, K.; Ishiguro, N.; Imagama, S. Prediction of surgical site infection in spine surgery from tests of nasal MRSA colonization and drain tip culture. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 1053–1057. [Google Scholar] [CrossRef]
- Kalra, L.; Camacho, F.; Whitener, C.J.; Du, P.; Miller, M.; Zalonis, C.; Julian, K.G. Risk of methicillin-resistant Staphylococcus aureus surgical site infection in patients with nasal MRSA colonization. Am. J. Infect. Control 2013, 41, 1253–1257. [Google Scholar] [CrossRef]
- Poe-Kochert, C.; Shimberg, J.L.; Thompson, G.H.; Son-Hing, J.P.; Hardesty, C.K.; Mistovich, R.J. Surgical site infection prevention protocol for pediatric spinal deformity surgery: Does it make a difference? Spine Deform. 2020, 8, 931–938. [Google Scholar] [CrossRef]
- Suzuki, S. A View on 20 Years of Antimicrobial Resistance in Japan by Two National Surveillance Systems: The National Epidemiological Surveillance of Infectious Diseases and Japan Nosocomial Infections Surveillance. Antibiotics 2021, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: The battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total (n = 68) | No Instrumentation (n = 24) | Instrumentation (n = 44) | S-Group (n = 24) | C-Group (n = 20) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Micro-Organism | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Gram-negative | Corynebacteriaceae | Corynebacterium | 1 | 1% | 0 | 0% | 1 | 2% | 0 | 0% | 1 | 5% |
| Enterobacteriaceae | Proteus mirabilis | 1 | 1% | 0 | 0% | 1 | 2% | 0 | 0% | 1 | 5% | |
| Enterobacter cloaca | 1 | 1% | 0 | 0% | 1 | 2% | 1 | 4% | 0 | 0% | ||
| Escherichia coli | 1 | 1% | 0 | 0% | 1 | 2% | 0 | 0% | 1 | 5% | ||
| Moraxellaceae | Acinetobacter baumannii | 1 | 1% | 0 | 0% | 1 | 2% | 0 | 0% | 1 | 5% | |
| Gram-positive | Enterococcaceae | Enterococcus faecalis | 1 | 1% | 0 | 0% | 1 | 2% | 1 | 4% | 0 | 0% |
| Bacillaceae | Bacillus cereus | 1 | 1% | 0 | 0% | 1 | 2% | 0 | 0% | 1 | 5% | |
| Staphylococcaceae | Staphylococcus capitis | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Staphylococcus capitis-MR | 7 | 10% | 3 | 13% | 4 | 9% | 4 | 17% | 0 | 0% | ||
| Staphylococcus epidermidis | 3 | 4% | 1 | 4% | 2 | 5% | 2 | 8% | 0 | 0% | ||
| Staphylococcus epidermidis-MR | 11 | 16% | 5 | 21% | 6 | 14% | 5 | 21% | 1 | 5% | ||
| Staphylococcus schleiferi | 1 | 1% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% | ||
| Staphylococcus lugdunensis | 1 | 1% | 0 | 0% | 1 | 2% | 0 | 0% | 1 | 5% | ||
| Staphylococcus aureus | 6 | 9% | 1 | 4% | 5 | 11% | 3 | 13% | 2 | 10% | ||
| Staphylococcus aureus-MR | 26 | 38% | 10 | 42% | 16 | 36% | 4 | 17% | 12 | 60% | ||
| Other | Unknown | 9 | 13% | 3 | 13% | 6 | 14% | 6 | 25% | 0 | 0% | |
| Cases with polymicrobial infection | 3 | 4% | 0 | 0% | 3 | 7% | 2 | 8% | 1 | 5% | ||
| Methicillin-resistant species | 44 | 64.7% | 18 | 75% | 26 | 59% | 14 | 58% | 12 | 60% | ||
| Total | S-Group | C-Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | (unit) | Mean | ±sd | n | Mean | (±sd) | n | Mean | (±sd) | n | p-Value |
| Age | (years) | 50.7 | ±20.1 | 44 | 20.0 | ±20.0 | 24 | 56.2 | ±19.4 | 20 | 0.056 * |
| Fixated vertebrae | (vertebrae) | 4.7 | ±3.4 | 44 | 5.3 | ±4.0 | 24 | 4.0 | ±2.3 | 20 | 0.642 * |
| Time onset SSI | (days post-surgery) | 34.7 | ±107.1 | 44 | 15.9 | ±8.5 | 24 | 57.3 | ±157.7 | 20 | 0.986 * |
| WBC count | (WBC/μL) | 10,034.1 | ±3964.5 | 44 | 8370.8 | ±2007.7 | 24 | 12,030.0 | ±4796.3 | 20 | 0.002 ** |
| CRP levels | (mg/dL) | 10.2 | ±9.3 | 44 | 6.4 | ±8.0 | 24 | 14.8 | ±8.9 | 20 | 0.001 * |
| Time until debridement | (days) | 2.4 | ±2.7 | 44 | 2.2 | ±2.8 | 24 | 2.6 | ±2.5 | 20 | 0.388 * |
| Debridement time | (min) | 99.8 | ±38.2 | 43 | 97.8 | ±34.5 | 24 | 102.4 | ±43.1 | 19 | 0.695 ** |
| Blood loss | (g) | 352.2 | ±329.5 | 40 | 388.4 | ±365.0 | 22 | 308.0 | ±284.1 | 18 | 0.545 * |
| Volume saline irrigation | (mL) | 14,848.8 | ±8686.7 | 43 | 13,541.7 | ±5633.6 | 24 | 16,500.0 | ±11,417.6 | 19 | 0.649 * |
| Time of closed suction drain | (days) | 5.9 | ±2.6 | 33 | 5.6 | ±2.2 | 21 | 6.6 | ±3.1 | 12 | 0.363 * |
| n | % | N | n | % | N | n | % | N | p-value *** | ||
| Sex | (male: female) | 18:26 | 44 | 12:12 | 6:14 | 0.227 | |||||
| Medical history A | 15 | 34.9% | 43 | 9 | 37.5% | 24 | 6 | 31.6% | 19 | 0.755 | |
| Trauma | 20 | 45.5% | 44 | 6 | 25.0% | 24 | 14 | 70.0% | 20 | 0.006 | |
| Continious closed irrigation | 3 | 6.8% | 44 | 1 | 4.2% | 24 | 2 | 10.0% | 20 | 0.583 | |
| Bacteremia | 25 | 56.8% | 44 | 13 | 54.2% | 24 | 12 | 60.0% | 20 | 0.766 | |
| Vancomycin powder | 7 | 15.9% | 44 | 4 | 16.7% | 24 | 3 | 15.0% | 20 | >0.999 | |
| Antibiotic sensitivity B | 25 | 65.8% | 38 | 16 | 88.9% | 18 | 9 | 45.0% | 20 | 0.006 | |
| Gram-positive species | 34 | 77.3% | 44 | 17 | 70.8% | 24 | 17 | 85.0% | 20 | 0.306 | |
| CoNS | 14 | 31.8% | 44 | 11 | 45.8% | 24 | 3 | 15.0% | 20 | 0.050 | |
| S. Aureus | 21 | 47.7% | 44 | 7 | 29.2% | 24 | 14 | 70.0% | 20 | 0.014 | |
| Gram-negative species | 5 | 11.4% | 44 | 1 | 4.2% | 24 | 4 | 20.0% | 20 | 0.161 | |
| MRSA frequency | 16 | 36.4% | 44 | 4 | 16.7% | 24 | 12 | 60.0% | 20 | 0.005 | |
| Prognostic Factor | OR | 95% CI | p Value |
|---|---|---|---|
| WBC count | 1.00 | (1.00–1.00) | 0.134 |
| CRP levels | 1.11 | (1.00–1.24) | 0.054 |
| Trauma | 1.26 | (0.12–12.86) | 0.845 |
| Antibiotic sensitivity | 0.08 | (0.01–1.30) | 0.082 |
| S. Aureus | 0.56 | (0.02–15.23) | 0.728 |
| MRSA | 14.52 | (0.26–798.52) | 0.191 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroiwa, M.; Schol, J.; Sakai, D.; Horikita, N.; Hiyama, A.; Katoh, H.; Yamamoto, Y.; Sato, M.; Watanabe, M. Predictive Factors for Successful Treatment of Deep Incisional Surgical Site Infections following Instrumented Spinal Surgeries: Retrospective Review of 1832 Cases. Diagnostics 2022, 12, 551. https://doi.org/10.3390/diagnostics12020551
Kuroiwa M, Schol J, Sakai D, Horikita N, Hiyama A, Katoh H, Yamamoto Y, Sato M, Watanabe M. Predictive Factors for Successful Treatment of Deep Incisional Surgical Site Infections following Instrumented Spinal Surgeries: Retrospective Review of 1832 Cases. Diagnostics. 2022; 12(2):551. https://doi.org/10.3390/diagnostics12020551
Chicago/Turabian StyleKuroiwa, Masahiro, Jordy Schol, Daisuke Sakai, Natsumi Horikita, Akihiko Hiyama, Hiroyuki Katoh, Yukihiro Yamamoto, Masato Sato, and Masahiko Watanabe. 2022. "Predictive Factors for Successful Treatment of Deep Incisional Surgical Site Infections following Instrumented Spinal Surgeries: Retrospective Review of 1832 Cases" Diagnostics 12, no. 2: 551. https://doi.org/10.3390/diagnostics12020551
APA StyleKuroiwa, M., Schol, J., Sakai, D., Horikita, N., Hiyama, A., Katoh, H., Yamamoto, Y., Sato, M., & Watanabe, M. (2022). Predictive Factors for Successful Treatment of Deep Incisional Surgical Site Infections following Instrumented Spinal Surgeries: Retrospective Review of 1832 Cases. Diagnostics, 12(2), 551. https://doi.org/10.3390/diagnostics12020551









