A Rare Case of Hepatic Vanishing Bile Duct Syndrome Occurring after Combination Therapy with Nivolumab and Cabozantinib in a Patient with Renal Carcinoma
Abstract
:1. Introduction
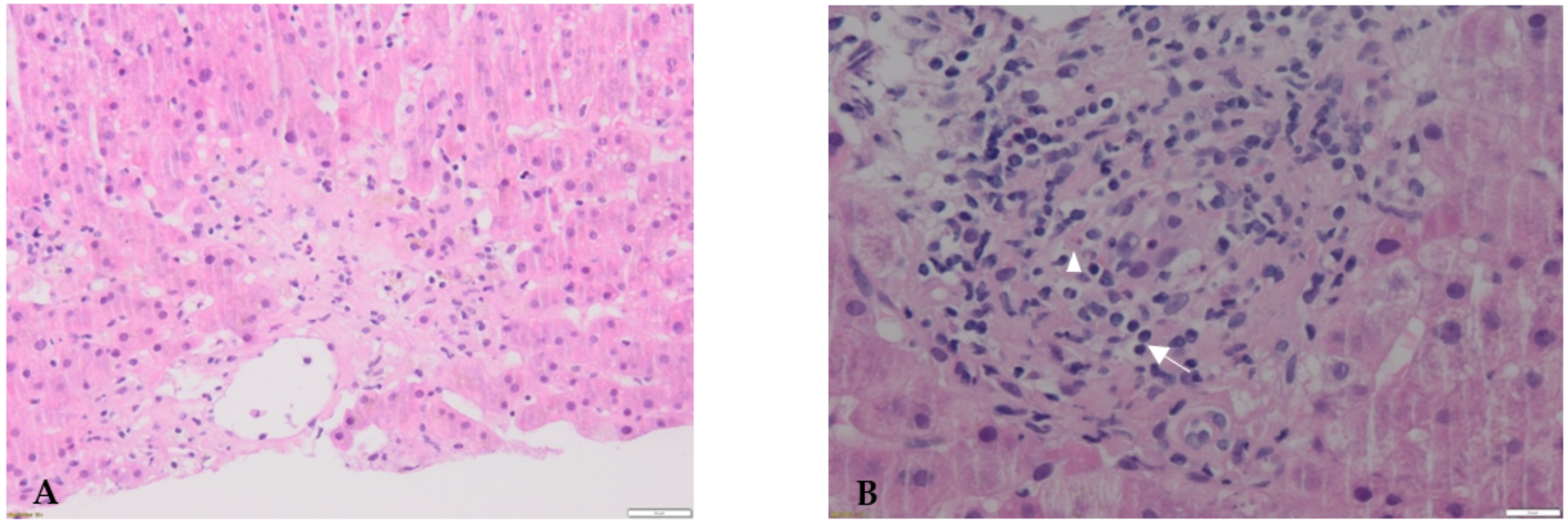
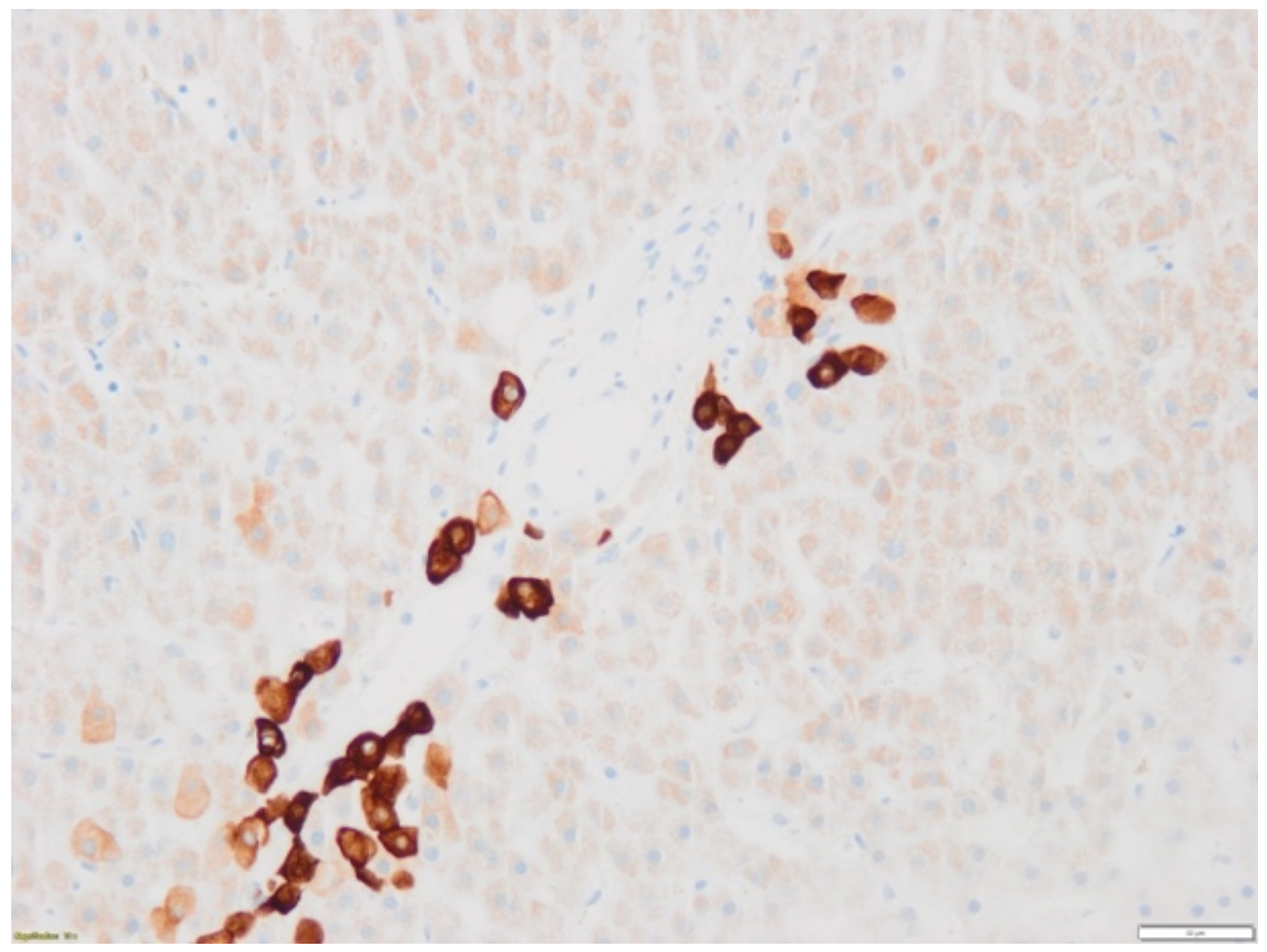
2. Case Report
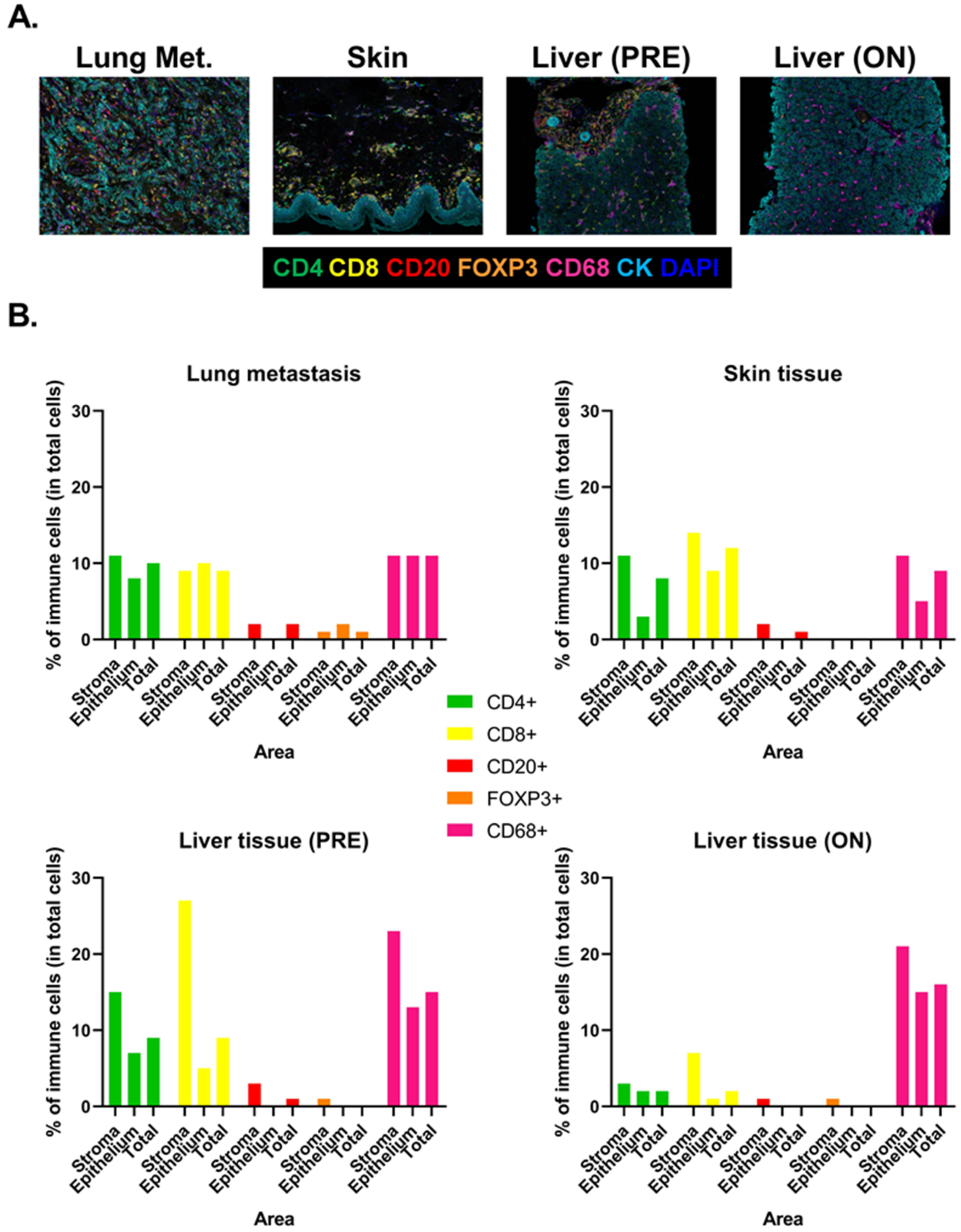
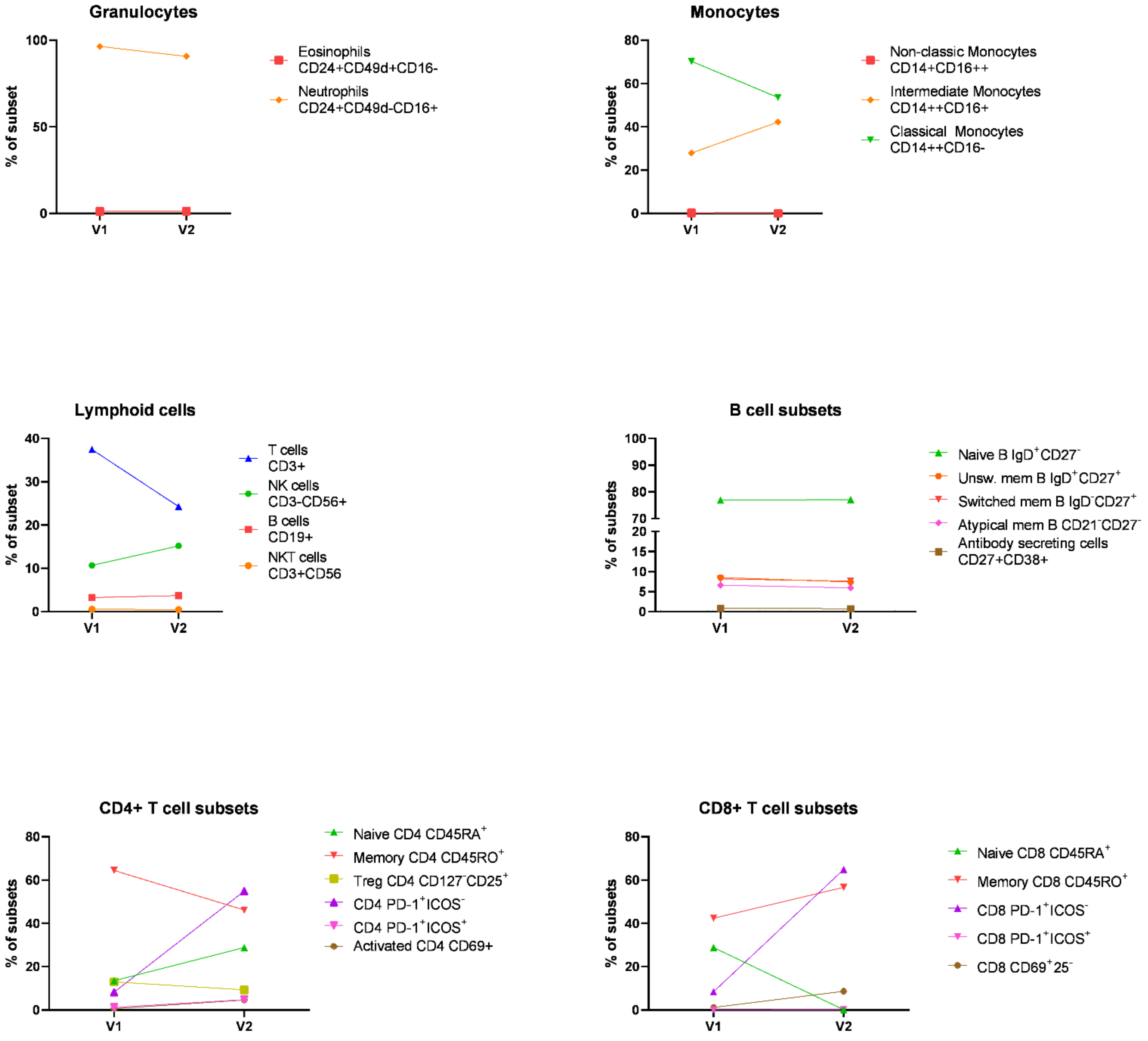
3. Discussion
3.1. Case
3.2. Biomarkers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; Feldman, D.R.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Hessel, C.; Halabi, S.; Sanford, B.; Michaelson, M.D.; Hahn, O.; Walsh, M.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur. J. Cancer 2018, 94, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rini, B.I.; Battle, D.; Figlin, R.A.; George, D.J.; Hammers, H.; Hutson, T.; Jonasch, E.; Joseph, R.W.; McDermott, D.F.; Motzer, R.J.; et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J. Immunother. Cancer 2019, 7, 354. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vida, A.; Hutson, T.E.; Bellmunt, J.; Strijbos, M.H. New treatment options for metastatic renal cell carcinoma. ESMO Open 2017, 2, e000185. [Google Scholar] [CrossRef] [Green Version]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.R.; Morganroth, J.; Shah, D.R. Hepatotoxicity of tyrosine kinase inhibitors: Clinical and regulatory perspectives. Drug Saf. 2013, 36, 491–503. [Google Scholar] [CrossRef]
- Doherty, G.J.; Duckworth, A.M.; Davies, S.E.; Mells, G.F.; Brais, R.; Harden, S.V.; Parkinson, C.A.; Corrie, P.G. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open 2017, 2, e000268. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Yang, X.; Yi, C.; Zhu, H. Adverse Events of Concurrent Immune Checkpoint Inhibitors and Antiangiogenic Agents: A Systematic Review. Front. Pharmacol. 2019, 10, 1173. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; Committee, E.G. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Tomita, Y.; Motzer, R.J.; Choueiri, T.K.; Rini, B.I.; Miyake, H.; Oya, M.; Albiges, L.; Fujii, Y.; Umeyama, Y.; Wang, J.; et al. Efficacy of avelumab plus axitinib (A + Ax) versus sunitinib (S) by number of IMDC risk factors and tumor sites at baseline in advanced renal cell carcinoma (aRCC): Extended follow-up results from JAVELIN Renal 101. J. Clin. Oncol. 2021, 39, 302. [Google Scholar] [CrossRef]
- Reau, N.S.; Jensen, D.M. Vanishing bile duct syndrome. Clin. Liver Dis. 2008, 12, 203–217. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; McLean, L.; Buckle, A.; Siva, S.; Tran, B. Vanishing bile duct syndrome associated with pazopanib after progression on pembrolizumab. Can. J. Urol. 2020, 27, 10339–10341. [Google Scholar]
- Elisei, R.; Schlumberger, M.J.; Muller, S.P.; Schoffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef] [Green Version]
- Ghatalia, P.; Je, Y.; Mouallem, N.E.; Nguyen, P.L.; Trinh, Q.D.; Sonpavde, G.; Choueiri, T.K. Hepatotoxicity with vascular endothelial growth factor receptor tyrosine kinase inhibitors: A meta-analysis of randomized clinical trials. Crit. Rev. Oncol. Hematol. 2015, 93, 257–276. [Google Scholar] [CrossRef]
- Matsubara, T.; Nishida, T.; Higaki, Y.; Tomita, R.; Shimakoshi, H.; Shimoda, A.; Osugi, N.; Sugimoto, A.; Takahashi, K.; Nakamatsu, D.; et al. Nivolumab Induces Sustained Liver Injury in a Patient with Malignant Melanoma. Intern. Med. 2018, 57, 1789–1792. [Google Scholar] [CrossRef] [Green Version]
- Reddy, H.G.; Schneider, B.J.; Tai, A.W. Immune Checkpoint Inhibitor-Associated Colitis and Hepatitis. Clin. Transl. Gastroenterol. 2018, 9, 180. [Google Scholar] [CrossRef]
- Thorsteinsdottir, T.; Løitegård, T.; Reims, H.M.; Porojnicu, A.C. Fatal Cholestatic Liver Injury during Treatment with PD1 Immune Checkpoint Inhibitor for Malignant Melanoma: A Case Report. Case Rep. Oncol. 2020, 13, 659–663. [Google Scholar] [CrossRef]
- Masetti, C.; Pugliese, N.; Rimassa, L.; Finocchiaro, G.; Di Tommaso, L.; Lancellotti, C.; Aghemo, A.; Lleo, A. Pembrolizumab-Induced Vanishing Bile Duct Syndrome: A Case Report. SN Compr. Clin. Med. 2021, 3, 906–908. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Pignon, J.C.; Jegede, O.; Shukla, S.A.; Braun, D.A.; Horak, C.E.; Wind-Rotolo, M.; Ishii, Y.; Catalano, P.J.; Grosha, J.; Flaifel, A.; et al. irRECIST for the Evaluation of Candidate Biomarkers of Response to Nivolumab in Metastatic Clear Cell Renal Cell Carcinoma: Analysis of a Phase II Prospective Clinical Trial. Clin. Cancer Res. 2019, 25, 2174–2184. [Google Scholar] [CrossRef]
- Subrahmanyam, P.B.; Dong, Z.; Gusenleitner, D.; Giobbie-Hurder, A.; Severgnini, M.; Zhou, J.; Manos, M.; Eastman, L.M.; Maecker, H.T.; Hodi, F.S. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J. Immunother. Cancer 2018, 6, 18. [Google Scholar] [CrossRef]
| Reference | Neoplasia | Suspected Causal Therapy | Time-to-Onset | Peak c- Bilirubin/ALAT | Signs of Hepatic Failure | Therapy | Issue |
|---|---|---|---|---|---|---|---|
| Doherty, 2017 [7] | Melanoma | Pembrolizumab | 1 cycle (8 d) | 23 mg/dL 1536 IU/L | Absent | CS 1 m/kg, MMF, UDCA | Normalized c-bili under CS and UDCA therapy |
| Thorsteinsdottir, 2020 [18] | Melanoma | Pembrolizumab | 12 cycles | 32 mg/dL 450 IU/L | Absent | CS 2 mg/kg MMF, UDCA, plasmapheresis | Death (d+26 after cholestasis onset) |
| Zhong, 2020 [13] | mRCC | Pembrolizumab (previously) Pazopanib (ongoing) | 5 cycles (25 d) | 22 mg/dL 800 IU/L | Absent | CS 2 mg/kg, UDCA | Death (d+30 after cholestasis onset) |
| Masetti, 2021 [19] | NSCLC | Pembrolizumab | 1 cycle (21 d) | 24 mg/dL 818 IU/L | Absent | None (patient refusal) | Normalized c-bili after 16 weeks |
| Present case | mRCC | Nivolumab, Cabozantinib (both ongoing) | 6 cycles (28 d) | 28 mg/dL 1137 IU/L | Absent | CS 2 mg/kg, MMF, UDCA | Normalized c-bili after 28 weeks under UDCA therapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gourari, K.; Catherine, J.; Garaud, S.; Kerger, J.; Lepida, A.; Georgala, A.; Lebrun, F.; Gomez Galdon, M.; Gil, T.; Willard-Gallo, K.; et al. A Rare Case of Hepatic Vanishing Bile Duct Syndrome Occurring after Combination Therapy with Nivolumab and Cabozantinib in a Patient with Renal Carcinoma. Diagnostics 2022, 12, 539. https://doi.org/10.3390/diagnostics12020539
Gourari K, Catherine J, Garaud S, Kerger J, Lepida A, Georgala A, Lebrun F, Gomez Galdon M, Gil T, Willard-Gallo K, et al. A Rare Case of Hepatic Vanishing Bile Duct Syndrome Occurring after Combination Therapy with Nivolumab and Cabozantinib in a Patient with Renal Carcinoma. Diagnostics. 2022; 12(2):539. https://doi.org/10.3390/diagnostics12020539
Chicago/Turabian StyleGourari, Karim, Julien Catherine, Soizic Garaud, Joseph Kerger, Antonia Lepida, Aspasia Georgala, Fabienne Lebrun, Maria Gomez Galdon, Thierry Gil, Karen Willard-Gallo, and et al. 2022. "A Rare Case of Hepatic Vanishing Bile Duct Syndrome Occurring after Combination Therapy with Nivolumab and Cabozantinib in a Patient with Renal Carcinoma" Diagnostics 12, no. 2: 539. https://doi.org/10.3390/diagnostics12020539
APA StyleGourari, K., Catherine, J., Garaud, S., Kerger, J., Lepida, A., Georgala, A., Lebrun, F., Gomez Galdon, M., Gil, T., Willard-Gallo, K., & Langouo Fontsa, M. (2022). A Rare Case of Hepatic Vanishing Bile Duct Syndrome Occurring after Combination Therapy with Nivolumab and Cabozantinib in a Patient with Renal Carcinoma. Diagnostics, 12(2), 539. https://doi.org/10.3390/diagnostics12020539






