Vedolizumab Attenuates Immune-Checkpoint-Therapy-Induced Infliximab-Refractory Colitis
Abstract
:1. Introduction
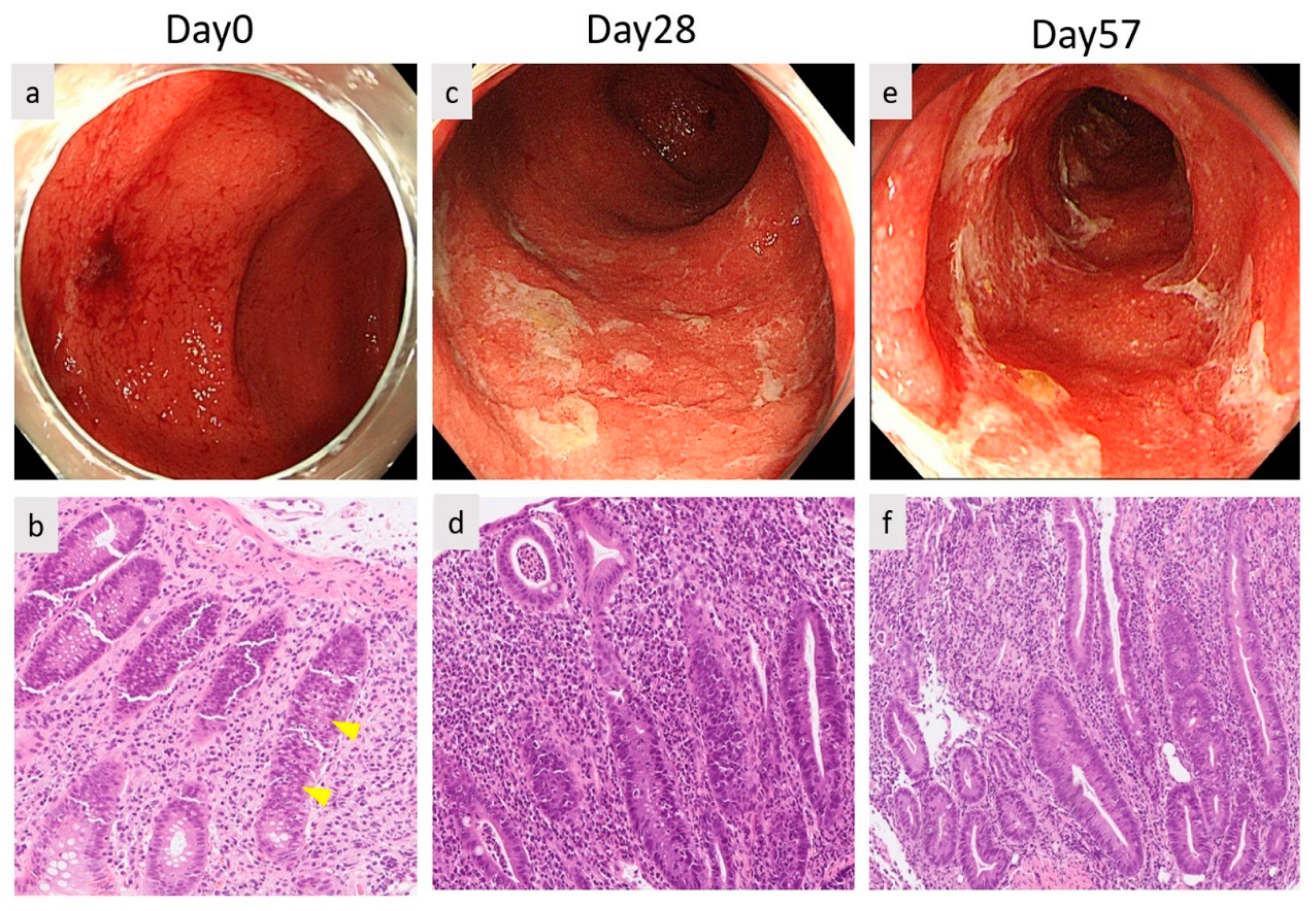
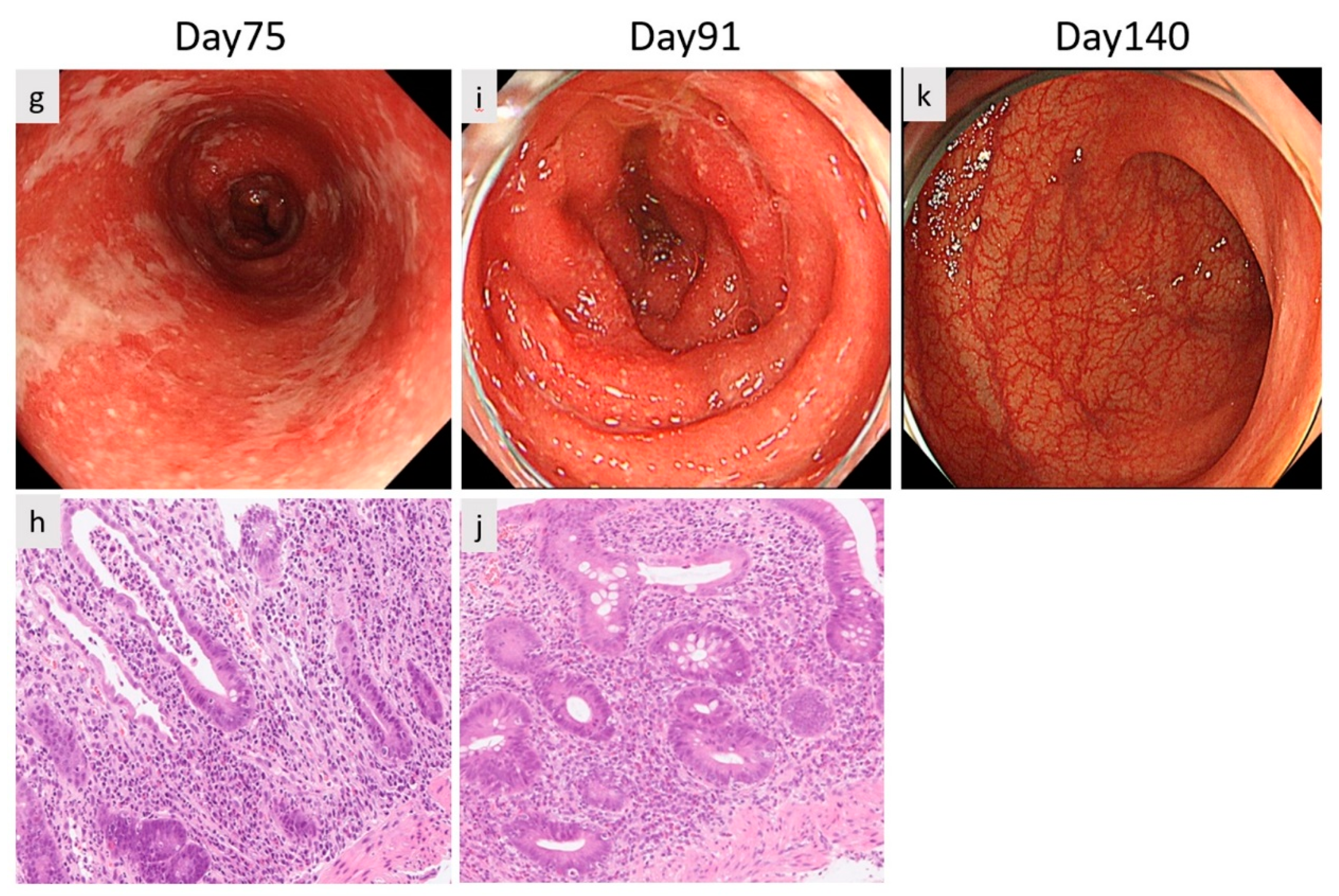
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, P.D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- McLean, L.P.; Shea-Donohue, T.; Cross, R.K. Vedolizumab for the treatment of ulcerative colitis and Crohn’s disease. Immunotherapy 2012, 4, 883–898. [Google Scholar] [CrossRef] [Green Version]
- Beck, K.E.; Blansfield, J.A.; Tran, K.Q.; Feldman, A.; Hughes, M.S.; Royal, R.E.; Kammula, U.S.; Topalian, S.L.; Sherry, R.M.; Kleiner, D.; et al. Enterocolitis in Patients with Cancer after Antibody Blockade of Cytotoxic T-Lymphocyte–Associated Antigen 4. J. Clin. Oncol. 2006, 24, 2283–2289. [Google Scholar] [CrossRef]
- Lord, J.D.; Hackman, R.C.; Moklebust, A.; Thompson, J.A.; Higano, C.S.; Chielens, D.; Steinbach, G.; McDonald, G.B. Refractory colitis following anti-CTLA4 antibody therapy: Analysis of mucosal FOXP3þ T cells. Am. J. Dig. Dis. 2009, 55, 1396–1405. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Wang, Y. Immune Checkpoint Inhibitor–Mediated Diarrhea and Colitis: A Clinical Review. JCO Oncol. Pr. 2020, 16, 453–461. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; Dupont, H.L.; Jiang, Z.-D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.-C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Neyns, B.; Linette, G.; Negrier, S.; Lutzky, J.; Thomas, L.; Waterfield, W.; Schadendorf, D.; Smylie, M.; Guthrie, T.; et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010, 11, 155–164. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef] [Green Version]
- Marthey, L.; Mateus, C.; Mussini, C.; Nachury, M.; Nancey, S.; Grange, F.; Zallot, C.; Peyrin-Biroulet, L.; Rahier, J.F.; De Beauregard, M.B.; et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.S.; Salaria, S.N.; Bohannon, C.D.; Huber, A.R.; Feely, M.M.; Shi, C. PD-1 inhibitor gastroenterocolitis: Case series and appraisal of ‘immunomodulatory gastroenterocolitis’. Histopathology 2016, 70, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Khanna, R.; Feagan, B.G. Vedolizumab for the treatment of inflammatory bowel diseases. Clin. Investig. 2015, 5, 247–255. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.-F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, A.H.-C.; Ferman, M.; Brown, M.; Andrews, J.M. Vedolizumab: A novel treatment for ipilimumab-induced colitis. BMJ Case Rep. 2016, 2016, bcr2016216641. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.; Gaughran, G.; Archer, C.; Pavli, P.; Morey, A.; Ali, S.; Yip, D. Vedolizumab in combined immune checkpoint therapy-induced infliximab-refractory colitis in a patient with metastatic melanoma: A case report. World J. Clin. Oncol. 2019, 10, 350–357. [Google Scholar] [CrossRef]
- Diana, P.; Mankongpaisarnrung, C.; Atkins, M.B.; Zeck, J.C.; Charabaty, A. Emerging Role of Vedolizumab in Managing Refractory Immune Checkpoint Inhibitor-Induced Enteritis. ACG Case Rep. J. 2018, 5, e17. [Google Scholar] [CrossRef] [Green Version]
- Bergqvist, V.; Hertervig, E.; Gedeon, P.; Kopljar, M.; Griph, H.; Kinhult, S.; Carneiro, A.; Marsal, J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol. Immunother. 2017, 66, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Abu-Sbeih, H.; Ali, F.; Alsaadi, D.; Jennings, J.; Luo, W.; Gong, Z.; Richards, D.M.; Charabaty, A.; Wang, Y. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multi-center study. J. Immunother. Cancer 2018, 6, 142. [Google Scholar] [CrossRef] [Green Version]
- Zou, F.; Faleck, D.; Thomas, A.; Harris, J.; Satish, D.; Wang, X.; Charabaty, A.; Ernstoff, M.S.; Oliva, I.C.G.; Hanauer, S.; et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: A two-center observational study. J. Immunother. Cancer 2021, 9, e003277. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Naqash, A.R.; Owen, D.; Patel, S.; Otterson, G.A.; Kendra, K.; Ricciuti, B.; Chiari, R.; De Giglio, A.; et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J. Clin. Oncol. 2019, 37, 2738–2745. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, L.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune check point inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Hamieh, L.; McKay, R.R.; Harshman, L.C.; Brandao, R.; Norton, C.K.; Steinharter, J.A.; Krajewski, K.M.; Gao, X.; Schutz, F.A.; et al. Durable Clinical Benefit in Metastatic Renal Cell Carcinoma Patients Who Discontinue PD-1/PD-L1 Therapy for Immune-Related Adverse Events. Cancer Immunol. Res. 2018, 6, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneoka, A.; Okada, E.; Sugino, H.; Saito-Sasaki, N.; Omoto, D.; Nakamura, M. Vedolizumab Attenuates Immune-Checkpoint-Therapy-Induced Infliximab-Refractory Colitis. Diagnostics 2022, 12, 480. https://doi.org/10.3390/diagnostics12020480
Kaneoka A, Okada E, Sugino H, Saito-Sasaki N, Omoto D, Nakamura M. Vedolizumab Attenuates Immune-Checkpoint-Therapy-Induced Infliximab-Refractory Colitis. Diagnostics. 2022; 12(2):480. https://doi.org/10.3390/diagnostics12020480
Chicago/Turabian StyleKaneoka, Ayaka, Etsuko Okada, Hitomi Sugino, Natsuko Saito-Sasaki, Daisuke Omoto, and Motonobu Nakamura. 2022. "Vedolizumab Attenuates Immune-Checkpoint-Therapy-Induced Infliximab-Refractory Colitis" Diagnostics 12, no. 2: 480. https://doi.org/10.3390/diagnostics12020480
APA StyleKaneoka, A., Okada, E., Sugino, H., Saito-Sasaki, N., Omoto, D., & Nakamura, M. (2022). Vedolizumab Attenuates Immune-Checkpoint-Therapy-Induced Infliximab-Refractory Colitis. Diagnostics, 12(2), 480. https://doi.org/10.3390/diagnostics12020480





