EVAR Follow-Up with Ultrasound Superb Microvascular Imaging (SMI) Compared to CEUS and CT Angiography for Detection of Type II Endoleak
Abstract
:1. Introduction
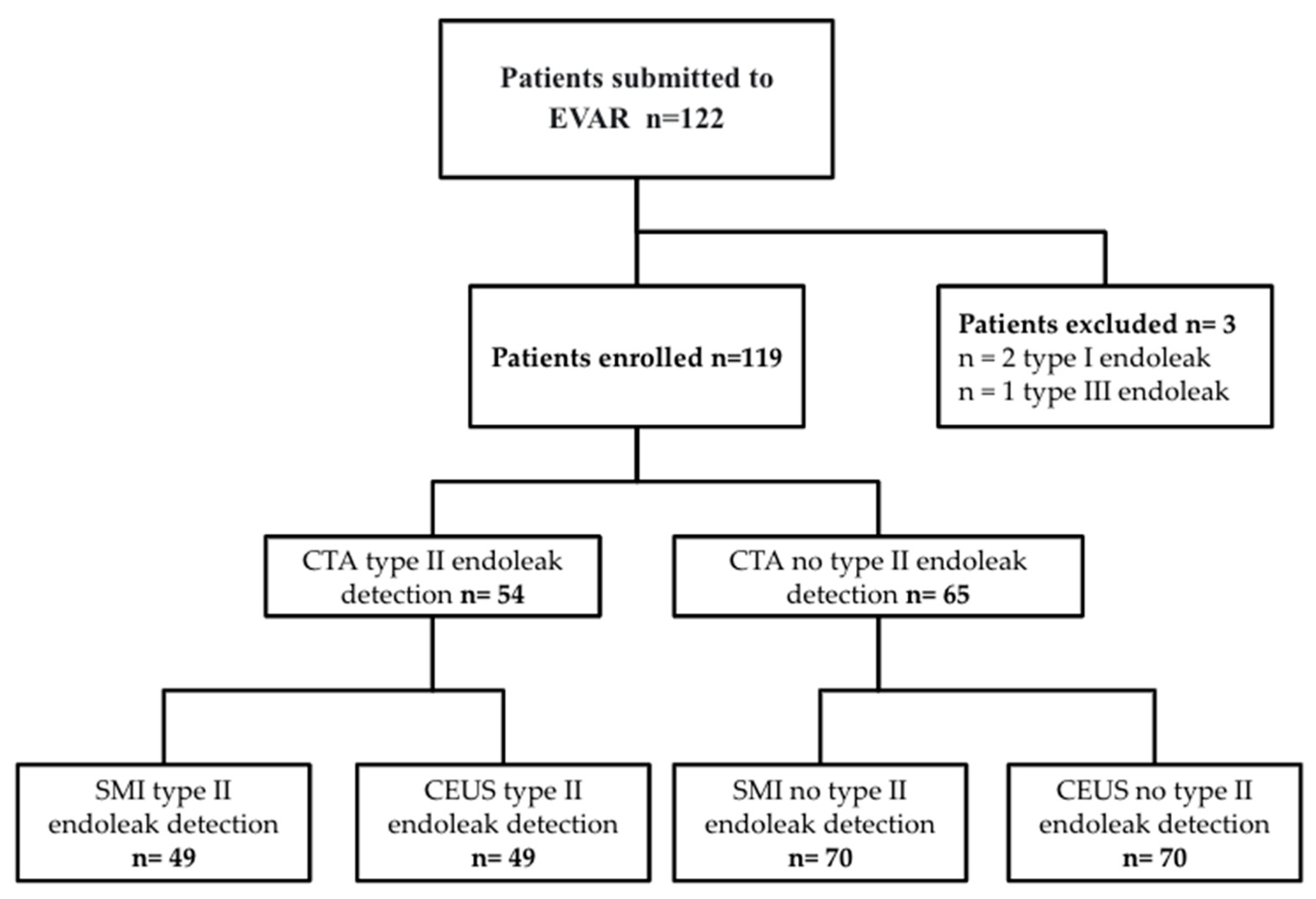
2. Materials and Methods
2.1. Outcome Measures
2.2. Statistical Analysis
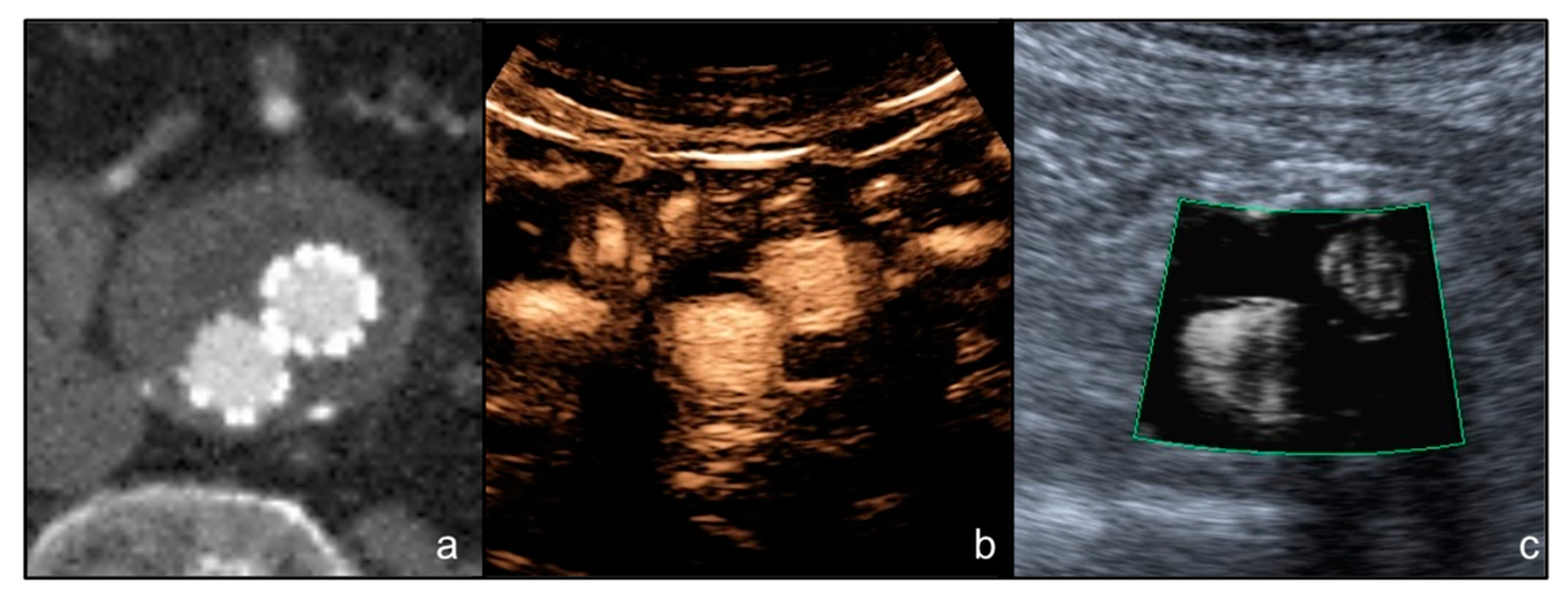
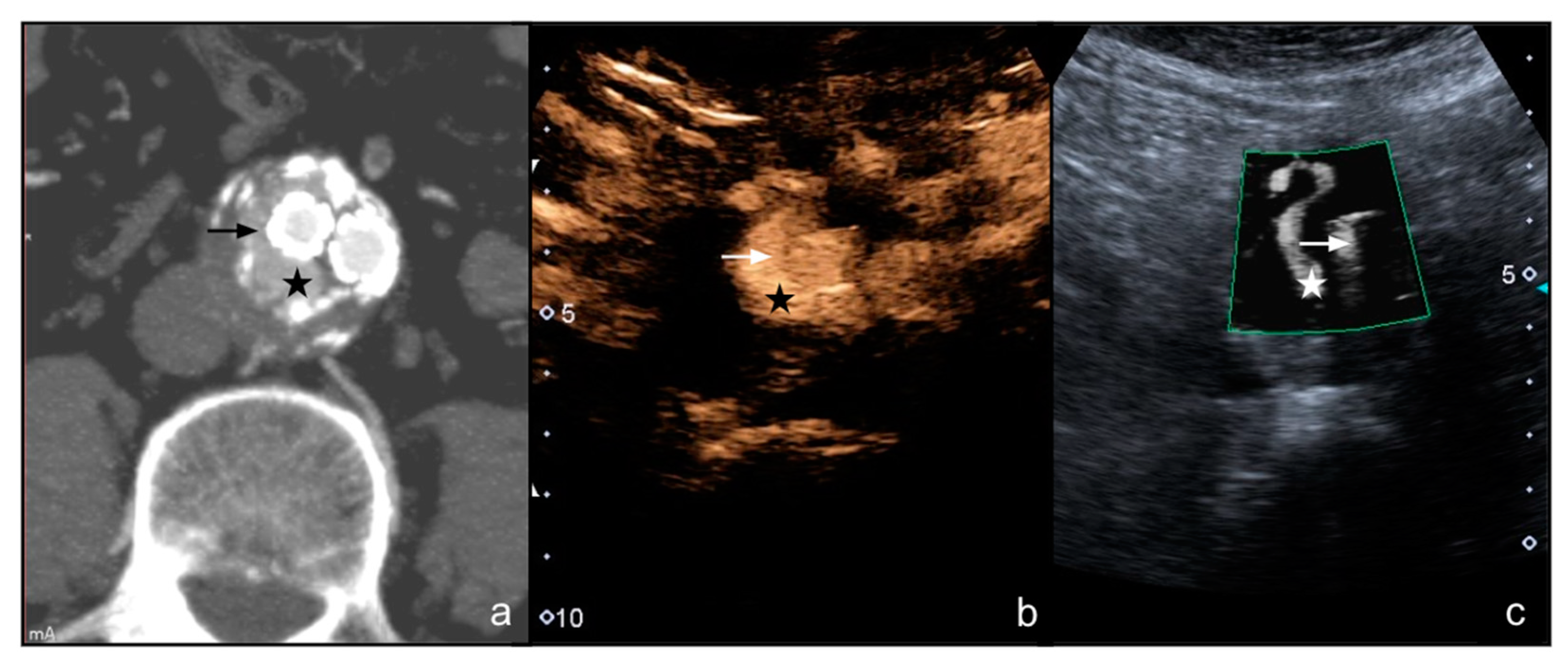
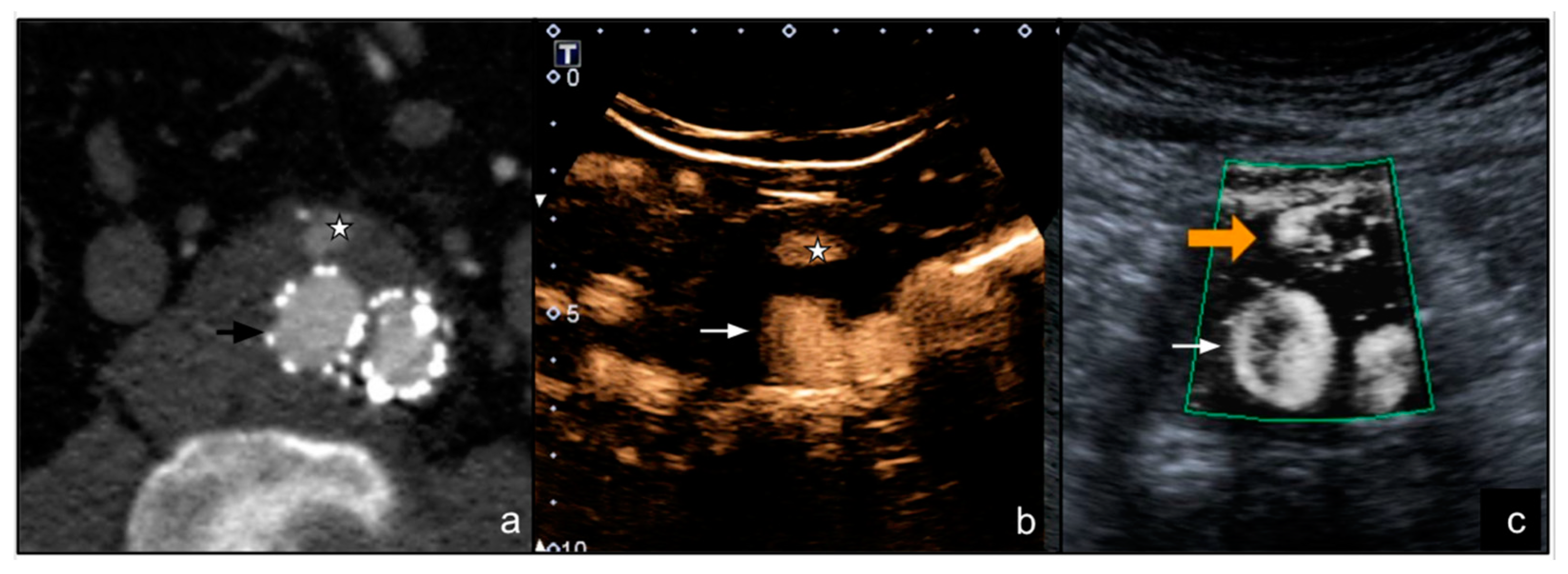
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nargesi, S.; Abutorabi, A.; Alipour, V.; Tajdini, M.; Salimi, J. Cost-Effectiveness of Endovascular Versus Open Repair of Abdominal Aortic Aneurysm: A Systematic Review. Cardiovasc. Drugs Ther. 2021, 35, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.C.; Nguyen, C.H.; Bartlett, A.S.; Thomas, R.H.; Merry, A.F. Reporting of Clinical Outcomes After Endovascular Aortic Aneurysm Repair: A Systematic Review. Ann. Vasc. Surg. 2021, 77, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Laczynski, D.J.; Caputo, F.J. Systematic Review and Meta-Analysis of Endovascular Abdominal Aortic Repair in Large Diameter Infrarenal Necks. J. Vasc. Surg. 2021, 74, 309–315.e2. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Galeazzi, E.; Veraldi, G.F.; Garriboli, L.; Saccà, S.; Farneti, F.; Mezzetto, L.; Mastrorilli, D.; Lepidi, S.; ITA-ENDOBOOT Registry Collaborators. Impact of Proximal Neck Anatomy on Short-Term and Mid-Term Outcomes after Treatment of Abdominal Aortic Aneurysms with New-Generation Low-Profile Endografts. Results from the Multicentric “ITAlian North-East Registry of ENDOvascular Aortic Repair with the BOltOn Treo Endograft (ITA-ENDOBOOT)”. Ann. Vasc. Surg. 2021. [Google Scholar] [CrossRef]
- Li, C.; Teter, K.; Rockman, C.; Garg, K.; Cayne, N.; Sadek, M.; Jacobowitz, G.; Silvestro, M.; Ramkhelawon, B.; Maldonado, T.S. Abdominal Aortic Aneurysm Neck Dilatation and Sac Remodeling in Fenestrated Compared to Standard Endovascular Aortic Repair. Vascular 2021. [Google Scholar] [CrossRef]
- Rossi, M.; Rebonato, A.; Greco, L.; Citone, M.; David, V. Endovascular Exclusion of Visceral Artery Aneurysms with Stent-Grafts: Technique and Long-Term Follow-Up. Cardiovasc. Intervent. Radiol. 2008, 31, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Marra, P.; Colombo, M.; Alparone, M.; Agostini, G.; Bertoglio, L.; Sallemi, C.; Salvioni, M.; Gusmini, S.; Balzano, G.; et al. Endovascular Treatment of Visceral Artery Aneurysms and Pseudoaneurysms in 100 Patients: Covered Stenting vs Transcatheter Embolization. J. Endovasc. Ther. 2017, 24, 709–717. [Google Scholar] [CrossRef]
- Venturini, M.; Marra, P.; Colombo, M.; Panzeri, M.; Gusmini, S.; Sallemi, C.; Salvioni, M.; Lanza, C.; Agostini, G.; Balzano, G.; et al. Endovascular Repair of 40 Visceral Artery Aneurysms and Pseudoaneurysms with the Viabahn Stent-Graft: Technical Aspects, Clinical Outcome and Mid-Term Patency. Cardiovasc. Intervent. Radiol. 2018, 41, 385–397. [Google Scholar] [CrossRef]
- Sommer, W.H.; Becker, C.R.; Haack, M.; Rubin, G.D.; Weidenhagen, R.; Schwarz, F.; Nikolaou, K.; Reiser, M.F.; Johnson, T.R.; Clevert, D.A. Time-Resolved CT Angiography for the Detection and Classification of Endoleaks. Radiology 2012, 263, 917–926. [Google Scholar] [CrossRef]
- Bashir, M.R.; Ferral, H.; Jacobs, C.; McCarthy, W.; Goldin, M. Endoleaks after Endovascular Abdominal Aortic Aneurysm Repair: Management Strategies According to CT Findings. AJR Am. J. Roentgenol. 2009, 192, W178–W186. [Google Scholar] [CrossRef]
- Böning, G.; Rotzinger, R.A.; Kahn, J.F.; Freyhardt, P.; Renz, D.M.; Maurer, M.; Streitparth, F. Tailored CT Angiography in Follow-up after Endovascular Aneurysm Repair (EVAR): Combined Dose Reduction Techniques. Acta Radiol. 2018, 59, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Apfaltrer, G.; Lavra, F.; Schoepf, U.J.; Scarabello, M.; Yamada, R.; van Assen, M.; Varga-Szemes, A.; Jacobs, B.E.; Bauer, M.J.; Greenberg, W.T.; et al. Quantitative Analysis of Dynamic Computed Tomography Angiography for the Detection of Endoleaks after Abdominal Aorta Aneurysm Endovascular Repair: A Feasibility Study. PLoS ONE 2021, 16, e0245134. [Google Scholar] [CrossRef] [PubMed]
- Meurer, F.; Kopp, F.; Renz, M.; Harder, F.N.; Leonhardt, Y.; Bippus, R.; Noël, P.B.; Makowski, M.R.; Sauter, A.P. Sparse-Sampling Computed Tomography for Detection of Endoleak after Endovascular Aortic Repair (EVAR). Eur. J. Radiol. 2021, 142, 109843. [Google Scholar] [CrossRef] [PubMed]
- Iscan, H.Z.; Unal, E.U.; Akkaya, B.; Daglı, M.; Karahan, M.; Civelek, I.; Ozbek, M.H.; Okten, R.S. Color Doppler Ultrasound for Surveillance Following EVAR as the Primary Tool. J. Card. Surg. 2021, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kapetanios, D.; Kontopodis, N.; Mavridis, D.; McWilliams, R.G.; Giannoukas, A.D.; Antoniou, G.A. Meta-Analysis of the Accuracy of Contrast-Enhanced Ultrasound for the Detection of Endoleak after Endovascular Aneurysm Repair. J. Vasc. Surg. 2019, 69, 280–294.e6. [Google Scholar] [CrossRef]
- Harky, A.; Zywicka, E.; Santoro, G.; Jullian, L.; Joshi, M.; Dimitri, S. Is Contrast-Enhanced Ultrasound (CEUS) Superior to Computed Tomography Angiography (CTA) in Detection of Endoleaks in Post-EVAR Patients? A Systematic Review and Meta-Analysis. J. Ultrasound 2019, 22, 65–75. [Google Scholar] [CrossRef]
- Froelich, M.F.; Kunz, W.G.; Kim, S.H.; Sommer, W.H.; Clevert, D.-A.; Rübenthaler, J. Cost-Effectiveness of Contrast-Enhanced Ultrasound for the Detection of Endovascular Aneurysm Repair-Related Endoleaks Requiring Treatment. J. Vasc. Surg. 2021, 73, 232–239.e2. [Google Scholar] [CrossRef]
- Durmaz, M.S.; Sivri, M. Comparison of Superb Micro-Vascular Imaging (SMI) and Conventional Doppler Imaging Techniques for Evaluating Testicular Blood Flow. J. Med. Ultrason. 2018, 45, 443–452. [Google Scholar] [CrossRef]
- Tomee, S.M.; Meijer, C.A.; Kies, D.A.; le Cessie, S.; Wasser, M.N.J.M.; Golledge, J.; Hamming, J.F.; Lindeman, J.H.N. Systematic Approach towards Reliable Estimation of Abdominal Aortic Aneurysm Size by Ultrasound Imaging and CT. BJS Open 2021, 5, zraa041. [Google Scholar] [CrossRef]
- Buck, D.B.; van Herwaarden, J.A.; Schermerhorn, M.L.; Moll, F.L. Endovascular Treatment of Abdominal Aortic Aneurysms. Nat. Rev. Cardiol. 2014, 11, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Greenhalgh, R.M.; Brown, L.C.; Kwong, G.P.S.; Powell, J.T.; Thompson, S.G.; EVAR Trial Participants. Comparison of Endovascular Aneurysm Repair with Open Repair in Patients with Abdominal Aortic Aneurysm (EVAR Trial 1), 30-Day Operative Mortality Results: Randomised Controlled Trial. Lancet 2004, 364, 843–848. [Google Scholar] [CrossRef]
- Hallett, R.L.; Ullery, B.W.; Fleischmann, D. Abdominal Aortic Aneurysms: Pre- and Post-Procedural Imaging. Abdom. Radiol. N. Y. 2018, 43, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Cassagnes, L.; Pérignon, R.; Amokrane, F.; Petermann, A.; Bécaud, T.; Saint-Lebes, B.; Chabrot, P.; Rousseau, H.; Boyer, L. Aortic Stent-Grafts: Endoleak Surveillance. Diagn. Interv. Imaging 2016, 97, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantisani, V.; Grazhdani, H.; Clevert, D.-A.; Iezzi, R.; Aiani, L.; Martegani, A.; Fanelli, F.; Di Marzo, L.; Wlderk, A.; Cirelli, C.; et al. EVAR: Benefits of CEUS for Monitoring Stent-Graft Status. Eur. J. Radiol. 2015, 84, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Partovi, S.; Trischman, T.; Rafailidis, V.; Ganguli, S.; Rengier, F.; Goerne, H.; Rajiah, P.; Staub, D.; Patel, I.J.; Oliveira, G.; et al. Multimodality Imaging Assessment of Endoleaks Post-Endovascular Aortic Repair. Br. J. Radiol. 2018, 91, 20180013. [Google Scholar] [CrossRef]
- Gabriel, M.; Tomczak, J.; Snoch-Ziółkiewicz, M.; Dzieciuchowicz, Ł.; Strauss, E.; Pawlaczyk, K.; Wojtusik, D.; Oszkinis, G. Superb Micro-Vascular Imaging (SMI): A Doppler Ultrasound Technique with Potential to Identify, Classify, and Follow up Endoleaks in Patients after Endovascular Aneurysm Repair (EVAR). Abdom. Radiol. N. Y. 2018, 43, 3479–3486. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.B.; Williams, Z.B. Imaging Modalities for Endoleak Surveillance. J. Med. Radiat. Sci. 2021, 68, 446–452. [Google Scholar] [CrossRef]
- Habets, J.; Zandvoort, H.J.A.; Reitsma, J.B.; Bartels, L.W.; Moll, F.L.; Leiner, T.; van Herwaarden, J.A. Magnetic Resonance Imaging Is More Sensitive than Computed Tomography Angiography for the Detection of Endoleaks after Endovascular Abdominal Aortic Aneurysm Repair: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Kawada, H.; Goshima, S.; Sakurai, K.; Noda, Y.; Kajita, K.; Tanahashi, Y.; Kawai, N.; Ishida, N.; Shimabukuro, K.; Doi, K.; et al. Utility of Noncontrast Magnetic Resonance Angiography for Aneurysm Follow-Up and Detection of Endoleaks after Endovascular Aortic Repair. Korean J. Radiol. 2021, 22, 513–524. [Google Scholar] [CrossRef]
- Piacentino, F.; Fontana, F.; Micieli, C.; Angeretti, M.G.; Cardim, L.N.; Coppola, A.; Molinelli, V.; Piffaretti, G.; Novario, R.; Fugazzola, C. Nonenhanced MRI Planning for Endovascular Repair of Abdominal Aortic Aneurysms: Comparison With Contrast-Enhanced CT Angiography. Vasc. Endovasc. Surg. 2018, 52, 39–45. [Google Scholar] [CrossRef]
- Mirza, T.A.; Karthikesalingam, A.; Jackson, D.; Walsh, S.R.; Holt, P.J.; Hayes, P.D.; Boyle, J.R. Duplex Ultrasound and Contrast-Enhanced Ultrasound versus Computed Tomography for the Detection of Endoleak after EVAR: Systematic Review and Bivariate Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantisani, V.; David, E.; Ferrari, D.; Fanelli, F.; Di Marzo, L.; Catalano, C.; Benedetto, F.; Spinelli, D.; Katsargyris, A.; Blandino, A.; et al. Color Doppler Ultrasound with Superb Microvascular Imaging Compared to Contrast-Enhanced Ultrasound and Computed Tomography Angiography to Identify and Classify Endoleaks in Patients Undergoing EVAR. Ann. Vasc. Surg. 2017, 40, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Perini, P.; Sediri, I.; Midulla, M.; Delsart, P.; Gautier, C.; Haulon, S. Contrast-Enhanced Ultrasound vs. CT Angiography in Fenestrated EVAR Surveillance: A Single-Center Comparison. J. Endovasc. Ther. 2012, 19, 648–655. [Google Scholar] [CrossRef]
- Millen, A.; Canavati, R.; Harrison, G.; McWilliams, R.G.; Wallace, S.; Vallabhaneni, S.R.; Fisher, R.K. Defining a Role for Contrast-Enhanced Ultrasound in Endovascular Aneurysm Repair Surveillance. J. Vasc. Surg. 2013, 58, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikesalingam, A.; Al-Jundi, W.; Jackson, D.; Boyle, J.R.; Beard, J.D.; Holt, P.J.E.; Thompson, M.M. Systematic Review and Meta-Analysis of Duplex Ultrasonography, Contrast-Enhanced Ultrasonography or Computed Tomography for Surveillance after Endovascular Aneurysm Repair. Br. J. Surg. 2012, 99, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Tomczak, J.; Snoch-Ziółkiewicz, M.; Dzieciuchowicz, Ł.; Strauss, E.; Oszkinis, G. Comparison of Superb Micro-Vascular Ultrasound Imaging (SMI) and Contrast-Enhanced Ultrasound (CEUS) for Detection of Endoleaks After Endovascular Aneurysm Repair (EVAR). Am. J. Case Rep. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantisani, V.; Ricci, P.; Grazhdani, H.; Napoli, A.; Fanelli, F.; Catalano, C.; Galati, G.; D’Andrea, V.; Biancari, F.; Passariello, R. Prospective Comparative Analysis of Colour-Doppler Ultrasound, Contrast-Enhanced Ultrasound, Computed Tomography and Magnetic Resonance in Detecting Endoleak after Endovascular Abdominal Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 186–192. [Google Scholar] [CrossRef] [Green Version]
| Variable | |
| Demographic data | |
| M:F | 110:12 |
| Age (years, mean) | Males 76.72 years Females 76.83 years |
| Comorbidities | |
| Hypertension | 95 (84.82%) |
| Dyslipidemia | 83 (74.10%) |
| Diabetes | 60 (53.57%) |
| BMI (average) | 28.9 |
| Previous CV surgery | 17 (15.17%) 12 Carotid 5 valve surgery |
| CKD, (eGFR<30 mL/min) | 28 (25%) |
| No Endoleak Detection | Endoleak Detection | |
|---|---|---|
| SMI | 70 | 49 |
| CEUS | 70 | 49 |
| CTA | 65 | 54 |
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Accuracy | |
|---|---|---|---|---|---|
| SMI | 91.53% | 100.00% | 100.00% | 92.86% | 95.97% |
| CEUS | 91.53% | 100.00% | 100.00% | 92.86% | 95.97% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curti, M.; Piacentino, F.; Fontana, F.; Ossola, C.; Coppola, A.; Marra, P.; Basile, A.; Ierardi, A.M.; Carrafiello, G.; Carcano, G.; et al. EVAR Follow-Up with Ultrasound Superb Microvascular Imaging (SMI) Compared to CEUS and CT Angiography for Detection of Type II Endoleak. Diagnostics 2022, 12, 526. https://doi.org/10.3390/diagnostics12020526
Curti M, Piacentino F, Fontana F, Ossola C, Coppola A, Marra P, Basile A, Ierardi AM, Carrafiello G, Carcano G, et al. EVAR Follow-Up with Ultrasound Superb Microvascular Imaging (SMI) Compared to CEUS and CT Angiography for Detection of Type II Endoleak. Diagnostics. 2022; 12(2):526. https://doi.org/10.3390/diagnostics12020526
Chicago/Turabian StyleCurti, Marco, Filippo Piacentino, Federico Fontana, Christian Ossola, Andrea Coppola, Paolo Marra, Antonio Basile, Anna Maria Ierardi, Gianpaolo Carrafiello, Giulio Carcano, and et al. 2022. "EVAR Follow-Up with Ultrasound Superb Microvascular Imaging (SMI) Compared to CEUS and CT Angiography for Detection of Type II Endoleak" Diagnostics 12, no. 2: 526. https://doi.org/10.3390/diagnostics12020526
APA StyleCurti, M., Piacentino, F., Fontana, F., Ossola, C., Coppola, A., Marra, P., Basile, A., Ierardi, A. M., Carrafiello, G., Carcano, G., Tozzi, M., Piffaretti, G., & Venturini, M. (2022). EVAR Follow-Up with Ultrasound Superb Microvascular Imaging (SMI) Compared to CEUS and CT Angiography for Detection of Type II Endoleak. Diagnostics, 12(2), 526. https://doi.org/10.3390/diagnostics12020526







