Relationship between Apparent Diffusion Coefficient Distribution and Cancer Grade in Prostate Cancer and Benign Prostatic Hyperplasia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Magnetic Resonance Imaging
2.3. Data Analysis
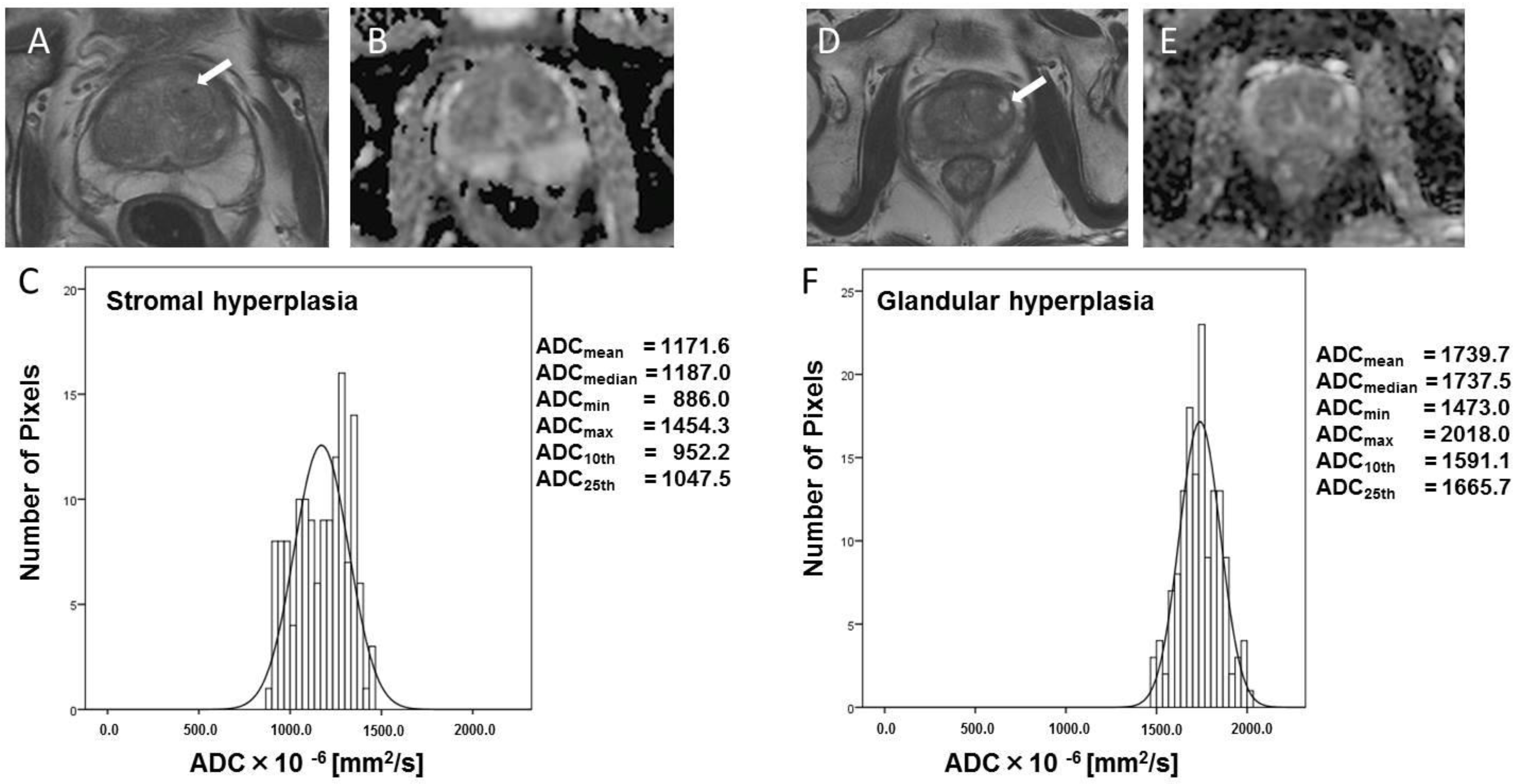
3. Results
3.1. Patients
3.2. ADC Parameters of All Lesions
3.3. Ability of ADC Parameters to Help Differentiate among Tumors with Different GS
3.4. Between-Subject Spearman Correlation Coefficients for Correlation of ADC Parameters with Gleason Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.D.; Kim, J.H.; Ahn, S.H. Transitional Zone Index as a Predictor of the Efficacy of alpha-Blocker and 5alpha-Reductase Inhibitor Combination Therapy in Korean Patients with Benign Prostatic Hyperplasia. Urol. Int. 2016, 96, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Schiebler, M.L.; Tomaszewski, J.E.; Bezzi, M.; Pollack, H.M.; Kressel, H.Y.; Cohen, E.K.; Altman, H.G.; Gefter, W.B.; Wein, A.J.; Axel, L. Prostatic carcinoma and benign prostatic hyperplasia: Correlation of high-resolution MR and histopathologic findings. Radiology 1989, 172, 131–137. [Google Scholar] [CrossRef]
- Ishida, J.; Sugimura, K.; Okizuka, H.; Kaji, Y.; Moriyama, M.; Nagaoka, S.; Mizutani, M.; Ishida, T. Benign prostatic hyperplasia: Value of MR imaging for determining histologic type. Radiology 1994, 190, 329–331. [Google Scholar] [CrossRef]
- Uhl, M.; Altehoefer, C.; Kontny, U.; Il’yasov, K.; Buchert, M.; Langer, M. MRI-diffusion imaging of neuroblastomas: First results and correlation to histology. Eur. Radiol. 2002, 12, 2335–2338. [Google Scholar] [CrossRef]
- Squillaci, E.; Manenti, G.; Cova, M.; Di Roma, M.; Miano, R.; Palmieri, G.; Simonetti, G. Correlation of diffusion-weighted MR imaging with cellularity of renal tumours. Anticancer Res. 2004, 24, 4175–4179. [Google Scholar]
- Yamasaki, F.; Kurisu, K.; Satoh, K.; Arita, K.; Sugiyama, K.; Ohtaki, M.; Takaba, J.; Tominaga, A.; Hanaya, R.; Yoshioka, H.; et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 2005, 235, 985–991. [Google Scholar] [CrossRef]
- Kishimoto, K.; Tajima, S.; Maeda, I.; Takagi, M.; Ueno, T.; Suzuki, N.; Nakajima, Y. Endometrial cancer: Correlation of apparent diffusion coefficient (ADC) with tumor cellularity and tumor grade. Acta Radiol. 2016, 57, 1021–1028. [Google Scholar] [CrossRef]
- Kim, C.K.; Park, B.K.; Lee, H.M.; Kwon, G.Y. Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: Preliminary results. Investig. Radiol. 2007, 42, 842–847. [Google Scholar] [CrossRef]
- Chen, M.; Dang, H.D.; Wang, J.Y.; Zhou, C.; Li, S.Y.; Wang, W.C.; Zhao, W.F.; Yang, Z.H.; Zhong, C.Y.; Li, G.Z. Prostate cancer detection: Comparison of T2-weighted imaging, diffusion-weighted imaging, proton magnetic resonance spectroscopic imaging, and the three techniques combined. Acta Radiol. 2008, 49, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Yagci, A.B.; Ozari, N.; Aybek, Z.; Duzcan, E. The value of diffusion-weighted MRI for prostate cancer detection and localization. Diagn. Interv. Radiol. 2011, 17, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Hegde, J.V.; Mulkern, R.V.; Panych, L.P.; Fennessy, F.M.; Fedorov, A.; Maier, S.E.; Tempany, C.M. Multiparametric MRI of prostate cancer: An update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J. Magn. Reson. Imaging 2013, 37, 1035–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Futterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenkrantz, A.B.; Triolo, M.J.; Melamed, J.; Rusinek, H.; Taneja, S.S.; Deng, F.M. Whole-lesion apparent diffusion coefficient metrics as a marker of percentage Gleason 4 component within Gleason 7 prostate cancer at radical prostatectomy. J. Magn. Reson. Imaging 2015, 41, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quon, J.; Kielar, A.Z.; Jain, R.; Schieda, N. Assessing the utilization of functional imaging in multiparametric prostate MRI in routine clinical practice. Clin. Radiol. 2015, 70, 373–378. [Google Scholar] [CrossRef]
- Hambrock, T.; Somford, D.M.; Huisman, H.J.; van Oort, I.M.; Witjes, J.A.; Hulsbergen-van de Kaa, C.A.; Scheenen, T.; Barentsz, J.O. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011, 259, 453–461. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, Y.; Yang, C.; Brown, J.B.; Antic, T.; Sethi, I.; Schmid-Tannwald, C.; Giger, M.L.; Eggener, S.E.; Oto, A. Quantitative analysis of multiparametric prostate MR images: Differentiation between prostate cancer and normal tissue and correlation with Gleason score—A computer-aided diagnosis development study. Radiology 2013, 267, 787–796. [Google Scholar] [CrossRef]
- Kobus, T.; Vos, P.C.; Hambrock, T.; De Rooij, M.; Hulsbergen-Van de Kaa, C.A.; Barentsz, J.O.; Heerschap, A.; Scheenen, T.W. Prostate cancer aggressiveness: In vivo assessment of MR spectroscopy and diffusion-weighted imaging at 3 T. Radiology 2012, 265, 457–467. [Google Scholar] [CrossRef]
- Noworolski, S.M.; Vigneron, D.B.; Chen, A.P.; Kurhanewicz, J. Dynamic contrast-enhanced MRI and MR diffusion imaging to distinguish between glandular and stromal prostatic tissues. Magn. Reson. Imaging 2008, 26, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Oto, A.; Yang, C.; Kayhan, A.; Tretiakova, M.; Antic, T.; Schmid-Tannwald, C.; Eggener, S.; Karczmar, G.S.; Stadler, W.M. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: Correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am. J. Roentgenol. 2011, 197, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, C.K.; Park, B.K.; Ha, S.Y.; Kwon, G.Y.; Kim, B. Diffusion-tensor MRI at 3 T: Differentiation of central gland prostate cancer from benign prostatic hyperplasia. AJR Am. J. Roentgenol. 2014, 202, W254–W262. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenevert, T.L.; Sundgren, P.C.; Ross, B.D. Diffusion imaging: Insight to cell status and cytoarchitecture. Neuroimaging Clin. N. Am. 2006, 16, 619–632. [Google Scholar] [CrossRef]
- Tamada, T.; Sone, T.; Jo, Y.; Yamamoto, A.; Yamashita, T.; Egashira, N.; Imai, S.; Fukunaga, M. Prostate cancer: Relationships between postbiopsy hemorrhage and tumor detectability at MR diagnosis. Radiology 2008, 248, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Langer, D.L.; van der Kwast, T.H.; Evans, A.J.; Sun, L.; Yaffe, M.J.; Trachtenberg, J.; Haider, M.A. Intermixed normal tissue within prostate cancer: Effect on MR imaging measurements of apparent diffusion coefficient and T2--sparse versus dense cancers. Radiology 2008, 249, 900–908. [Google Scholar] [CrossRef]
- King, C.R.; Long, J.P. Prostate biopsy grading errors: A sampling problem? Int. J. Cancer 2000, 90, 326–330. [Google Scholar] [CrossRef]
- Berglund, R.K.; Masterson, T.A.; Vora, K.C.; Eggener, S.E.; Eastham, J.A.; Guillonneau, B.D. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J. Urol. 2008, 180, 1964–1967; discussion 1967–1968. [Google Scholar] [CrossRef] [Green Version]




| Variable | Value |
|---|---|
| Clinical characteristics | |
| Age (year) | 69.3 ± 6.5 (51–84) |
| PSA (ng/mL) | 17.5 ± 25. 7 (4.2–196.0) |
| Pathologic characteristics | |
| pT2a | 22 (56.4%) |
| pT2b | 1 (2.5%) |
| pT2c | 7 (17.9%) |
| pT3a | 8 (20.5%) |
| pT4 | 1 (2.5%) |
| Gleason grade | |
| GS 3 + 3 | 7 (17.9%) |
| GS 3 + 4 | 17 (43.6%) |
| GS 4 + 3 | 2 (5.1%) |
| GS ≥ 8 | 13 (33.3%) |
| SH vs. GS 6 | p Value | SH vs. GS 7 | p Value | SH vs. GS 8 | p Value | SH vs. GH | p Value | |
|---|---|---|---|---|---|---|---|---|
| Mean | 0.70 | 0.15 | 0.62 | 0.27 | 0.97 | <0.0001 | 1.00 | <0.0001 |
| Median | 0.69 | 0.17 | 0.63 | 0.24 | 0.98 | <0.0001 | 1.00 | <0.0001 |
| Minimum | 0.76 | 0.05 | 0.59 | 0.41 | 0.96 | <0.0001 | 0.98 | <0.0001 |
| Maximum | 0.50 | 0.97 | 0.66 | 0.13 | 0.88 | 0.0003 | 0.98 | <0.0001 |
| 10th | 0.82 | 0.02 | 0.56 | 0.54 | 0.97 | <0.0001 | 1.00 | <0.0001 |
| 25th | 0.73 | 0.08 | 0.59 | 0.41 | 0.97 | <0.0001 | 1.00 | <0.0001 |
| GH vs. GS 6 | p Value | GH vs. GS 7 | p Value | GH vs. GS 8 | p Value | |
|---|---|---|---|---|---|---|
| Mean | 1.00 | <0.0001 | 1.0 | <0.0001 | 1.0 | <0.0001 |
| Median | 0.99 | 0.0002 | 1.0 | <0.0001 | 1.0 | <0.0001 |
| Minimum | 0.91 | 0.002 | 1.0 | <0.0001 | 1.0 | <0.0001 |
| Maximum | 0.94 | 0.001 | 1.0 | <0.0001 | 1.0 | <0.0001 |
| 10th | 1.00 | 0.0002 | 1.0 | <0.0001 | 1.0 | <0.0001 |
| 25th | 1.00 | 0.0002 | 1.0 | <0.0001 | 1.0 | <0.0001 |
| GS 6 vs. GS 7 | p Value | GS 6 vs. GS 8 | p Value | GS 7 vs. GS 8 | p Value | |
|---|---|---|---|---|---|---|
| Mean | 0.78 | 0.04 | 0.98 | 0.0003 | 0.94 | <0.0001 |
| Median | 0.79 | 0.03 | 0.99 | 0.0002 | 0.95 | <0.0001 |
| Minimum | 0.79 | 0.03 | 1.00 | 0.0002 | 0.95 | <0.0001 |
| Maximum | 0.67 | 0.20 | 0.86 | 0.008 | 0.83 | 0.002 |
| 10th | 0.87 | 0.01 | 1.00 | 0.0002 | 0.94 | <0.0001 |
| 25th | 0.79 | 0.03 | 0.99 | 0.0002 | 0.95 | <0.0001 |
| Spearman r | p Value | |
|---|---|---|
| Mean | −0.80 | <0.0001 |
| Median | −0.82 | <0.0001 |
| Minimum | −0.83 | <0.0001 |
| Maximum | −0.59 | <0.0001 |
| 10th | −0.84 | <0.0001 |
| 25th | −0.82 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, S.; Koyama, Y.; Ueda, J.; Hashido, T. Relationship between Apparent Diffusion Coefficient Distribution and Cancer Grade in Prostate Cancer and Benign Prostatic Hyperplasia. Diagnostics 2022, 12, 525. https://doi.org/10.3390/diagnostics12020525
Saito S, Koyama Y, Ueda J, Hashido T. Relationship between Apparent Diffusion Coefficient Distribution and Cancer Grade in Prostate Cancer and Benign Prostatic Hyperplasia. Diagnostics. 2022; 12(2):525. https://doi.org/10.3390/diagnostics12020525
Chicago/Turabian StyleSaito, Shigeyoshi, Yoshihiro Koyama, Junpei Ueda, and Takashi Hashido. 2022. "Relationship between Apparent Diffusion Coefficient Distribution and Cancer Grade in Prostate Cancer and Benign Prostatic Hyperplasia" Diagnostics 12, no. 2: 525. https://doi.org/10.3390/diagnostics12020525
APA StyleSaito, S., Koyama, Y., Ueda, J., & Hashido, T. (2022). Relationship between Apparent Diffusion Coefficient Distribution and Cancer Grade in Prostate Cancer and Benign Prostatic Hyperplasia. Diagnostics, 12(2), 525. https://doi.org/10.3390/diagnostics12020525






