Non-Contrast-Enhanced and Contrast-Enhanced Magnetic Resonance Angiography in Living Donor Liver Vascular Anatomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Candidates
2.2. CTA Examination
2.3. MRI Examination
2.4. Image Interpretation
2.5. Imaging Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| bSSFP | Balanced steady-state free precession |
| CMRA | Contrast-enhanced MR angiography |
| CNR | Contrast-to-noise ratio |
| CTA | Computed tomography angiography |
| FASE | Fast advanced spin echo |
| IFIR | Inflow-sensitive inversion recovery |
| IRHV | Inferior right hepatic vein |
| LDLT | Living donor liver transplantation |
| LHA | Left hepatic artery |
| LHV | Left hepatic vein |
| LPV | Left portal vein |
| MHV | Middle hepatic vein |
| MIP | Maximum intensity projection |
| MPV | Main portal vein |
| MRCP | Magnetic resonance cholangiopancreatography |
| non-CE MRA | Non-contrast-enhanced MR angiography |
| PHA | Proper hepatic artery |
| RHA | Right hepatic artery |
| RHV | Right hepatic vein |
| ROI | Region-of-interest |
| RPV | Right portal vein |
| SMA | Superior mesenteric artery |
| S4HA | Segment IV hepatic artery |
| S8HV | Segment VIII hepatic vein |
| SNR | Signal-to-noise ratio |
| SSFP | Steady-state free precession |
| TI | Inversion time |
References
- Pillai, V.G.; Chen, C.L. Living donor liver transplantation in Taiwan-challenges beyond surgery. Hepatobiliary Surg. Nutr. 2016, 5, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Yan, L.; Wang, W. Donor safety in living donor liver transplantation: A single-center analysis of cases. PLoS ONE 2013, 8, e61769. [Google Scholar] [CrossRef]
- Hecht, E.M.; Kambadakone, A.; Griesemer, A.D.; Fowler, K.J.; Wang, Z.J.; Heimbach, J.K.; Fidler, J.L. Living Donor Liver Transplantation: Overview, Imaging Technique, and Diagnostic Considerations. AJR Am. J. Roentgenol. 2019, 213, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Torres, A.; Fernandez-Cuadrado, J.; Pinilla, I.; Parron, M.; de Vicente, E.; Lopez-Santamaria, M. Multidetector CT in the evaluation of potential living donors for liver transplantation. Radiographics 2005, 25, 1017–1030. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Park, E.A.; Han, J.K.; Kim, Y.J.; Shin, K.S.; Suh, K.S.; Choi, B.I. Preoperative evaluation of the hepatic vascular anatomy in living liver donors: Comparison of CT angiography and MR angiography. J. Magn. Reson. Imaging 2006, 24, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Yeh, B.M.; Breiman, R.S.; Taouli, B.; Qayyum, A.; Roberts, J.P.; Coakley, F.V. Biliary tract depiction in living potential liver donors: Comparison of conventional MR, mangafodipir trisodium-enhanced excretory MR, and multi-detector row CT cholangiography--initial experience. Radiology 2004, 230, 645–651. [Google Scholar] [CrossRef]
- Kinner, S.; Steinweg, V.; Maderwald, S.; Radtke, A.; Sotiropoulos, G.; Forsting, M.; Schroeder, T. Bile duct evaluation of potential living liver donors with Gd-EOB-DTPA enhanced MR cholangiography: Single-dose, double dose or half-dose contrast enhanced imaging. Eur. J. Radiol. 2014, 83, 763–767. [Google Scholar] [CrossRef]
- Cha, M.J.; Kang, D.Y.; Lee, W.; Yoon, S.H.; Choi, Y.H.; Byun, J.S.; Lee, J.; Kim, Y.H.; Choo, K.S.; Cho, B.S.; et al. Hypersensitivity Reactions to Iodinated Contrast Media: A Multicenter Study of 196 081 Patients. Radiology 2019, 293, 117–124. [Google Scholar] [CrossRef]
- Jung, J.W.; Kang, H.R.; Kim, M.H.; Lee, W.; Min, K.U.; Han, M.H.; Cho, S.H. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 2012, 264, 414–422. [Google Scholar] [CrossRef]
- Bae, S.; Lee, H.J.; Han, K.; Park, Y.W.; Choi, Y.S.; Ahn, S.S.; Kim, J.; Lee, S.K. Gadolinium deposition in the brain: Association with various GBCAs using a generalized additive model. Eur. Radiol. 2017, 27, 3353–3361. [Google Scholar] [CrossRef]
- Xu, J.L.; Shi, D.P.; Li, Y.L.; Zhang, J.L.; Zhu, S.C.; Shen, H. Non-enhanced MR angiography of renal artery using inflow-sensitive inversion recovery pulse sequence: A prospective comparison with enhanced CT angiography. Eur. J. Radiol. 2011, 80, e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Masaryk, T.J.; Laub, G.A.; Modic, M.T.; Ross, J.S.; Haacke, E.M. Carotid-CNS MR flow imaging. Magn. Reson. Med. 1990, 14, 308–314. [Google Scholar] [CrossRef]
- Laub, G.A. Time-of-flight method of MR angiography. Magn. Reson. Imaging Clin. N. Am. 1995, 3, 391–398. [Google Scholar] [CrossRef]
- Miyazaki, M.; Isoda, H. Non-contrast-enhanced MR angiography of the abdomen. Eur. J. Radiol. 2011, 80, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Isoda, H.; Okada, T.; Kamae, T.; Maetani, Y.; Arizono, S.; Hirokawa, Y.; Shibata, T.; Togashi, K. Non-contrast-enhanced MR angiography for selective visualization of the hepatic vein and inferior vena cava with true steady-state free-precession sequence and time-spatial labeling inversion pulses: Preliminary results. J. Magn. Reson. Imaging 2009, 29, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Liang, J.L.; Chen, M.H.; Liao, C.C.; Huang, T.L.; Chen, T.Y.; Tsang, L.L.; Ou, H.Y.; Hsu, H.W.; Lazo, M.Z.; et al. Noncontrast Magnetic Resonance Angiography Using Inflow Sensitive Inversion Recovery Technique for Vascular Evaluation in Pre-liver Transplantation Recipients. Transpl. Proc. 2016, 48, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Nadalin, S.; Stattaus, J.; Debatin, J.F.; Malago, M.; Ruehm, S.G. Potential living liver donors: Evaluation with an all-in-one protocol with multi-detector row CT. Radiology 2002, 224, 586–591. [Google Scholar] [CrossRef]
- Yu, P.Y.; Chen, M.H.; Ou, H.Y.; Huang, T.L.; Yu, C.Y.; Chen, C.L.; Cheng, Y.F. Magnetic resonance angiographic inflow-sensitive inversion recovery technique for vascular evaluation before liver transplantation. Transpl. Proc. 2014, 46, 682–685. [Google Scholar] [CrossRef]
- Shimada, K.; Isoda, H.; Okada, T.; Kamae, T.; Arizono, S.; Hirokawa, Y.; Shibata, T.; Togashi, K. Non-contrast-enhanced MR portography with time-spatial labeling inversion pulses: Comparison of imaging with three-dimensional half-fourier fast spin-echo and true steady-state free-precession sequences. J. Magn. Reson. Imaging 2009, 29, 1140–1146. [Google Scholar] [CrossRef]
- Streitparth, F.; Pech, M.; Figolska, S.; Denecke, T.; Grieser, C.; Pascher, A.; Jonas, S.; Langrehr, J.; Ricke, J.; Neuhaus, P.; et al. Living related liver transplantation: Preoperative magnetic resonance imaging for assessment of hepatic vasculature of donor candidates. Acta Radiol. 2007, 48, 20–26. [Google Scholar] [CrossRef]
- Mitchell, G. Blood Supply and Anatomy of the Upper Abdominal Organs. J. Anat. 1956, 90, 308. [Google Scholar]
- Wu, T.C.; Lee, R.C.; Chau, G.Y.; Chiang, J.H.; Chang, C.Y. Reappraisal of right portal segmental ramification based on 3-dimensional volume rendering of computed tomography during arterial portography. J. Comput. Assist. Tomogr. 2007, 31, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Magnotta, V.A.; Friedman, L.; First, B. Measurement of Signal-to-Noise and Contrast-to-Noise in the fBIRN Multicenter Imaging Study. J. Digit. Imaging 2006, 19, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.J.; Hsu, H.W.; Chen, P.C.; Chiang, H.W.; Huang, T.L.; Chen, T.Y.; Chen, C.L.; Cheng, Y.F. Magnetic resonance cholangiography in living donor liver transplantation: Comparison of preenhanced and post-gadolinium-enhanced methods. Transpl. Proc. 2012, 44, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.Y.; Yu, H.C.; Lim, H.S.; Moon, J.I.; Lee, J.H.; Chung, J.W.; Cho, B.H. Anatomical variations of the origin of the segment hepatic artery and their clinical implications. Liver Transpl. 2008, 14, 1180–1184. [Google Scholar] [CrossRef]
- Shimada, K.; Isoda, H.; Okada, T.; Kamae, T.; Arizono, S.; Hirokawa, Y.; Shibata, T.; Togashi, K. Non-contrast-enhanced hepatic MR angiography: Do two-dimensional parallel imaging and short tau inversion recovery methods shorten acquisition time without image quality deterioration? Eur. J. Radiol 2011, 77, 137–142. [Google Scholar] [CrossRef]
- Huang, C.M.; Chen, R.F.; Chen, Q.Y.; Wei, J.; Zheng, C.H.; Li, P.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Lu, J.; et al. Application Value of a 6-Type Classification System for Common Hepatic Artery Absence During Laparoscopic Radical Resections for Gastric Cancer: A Large-Scale Single-Center Study. Medicine 2015, 94, e1280. [Google Scholar] [CrossRef]
- Cirocchi, R.; D’Andrea, V.; Lauro, A.; Renzi, C.; Henry, B.M.; Tomaszewski, K.A.; Rende, M.; Lancia, M.; Carlini, L.; Gioia, S.; et al. The absence of the common hepatic artery and its implications for surgical practice: Results of a systematic review and meta-analysis. Surgeon 2019, 17, 172–185. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nitta, H.; Takahara, T.; Katagiri, H.; Kanno, S.; Sasaki, A. Pure laparoscopic living donor hepatectomy using the Glissonean pedicle approach (with video). Surg. Endosc. 2019, 33, 2704–2709. [Google Scholar] [CrossRef]
- Dokmak, S.; Cauchy, F.; Sepulveda, A.; Choinier, P.M.; Dondero, F.; Aussilhou, B.; Hego, C.; Chopinet, S.; Infantes, P.; Weiss, E.; et al. Laparoscopic Liver Transplantation: Dream or Reality? The First Step With Laparoscopic Explant Hepatectomy. Ann. Surg. 2020, 272, 889–893. [Google Scholar] [CrossRef]
- Peng, Y.; Li, B.; Xu, H.; Chen, K.; Wei, Y.; Liu, F. Pure Laparoscopic Versus Open Approach for Living Donor Right Hepatectomy: A Systematic Review and Meta-Analysis. J. Laparoendosc. Adv. Surg. Tech. A, 2021; Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Kalra, V.B.; Gilbert, J.W.; Krishnamoorthy, S.; Cornfeld, D. Value of non-contrast sequences in magnetic resonance angiography of hepatic arterial vasculature. Eur. J. Radiol. 2014, 83, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.R.; Yan, L.N.; Du, C.Y. Donor safety and remnant liver volume in living donor liver transplantation. World J. Gastroenterol. 2012, 18, 7327–7332. [Google Scholar] [CrossRef] [PubMed]
- Gotra, A.; Sivakumaran, L.; Chartrand, G.; Vu, K.N.; Vandenbroucke-Menu, F.; Kauffmann, C.; Kadoury, S.; Gallix, B.; de Guise, J.A.; Tang, A. Liver segmentation: Indications, techniques and future directions. Insights Imaging 2017, 8, 377–392. [Google Scholar] [CrossRef]
- Grieser, C.; Denecke, T.; Rothe, J.H.; Geisel, D.; Stelter, L.; Cannon Walter, T.; Seehofer, D.; Steffen, I.G. Gd-EOB enhanced MRI T1-weighted 3D-GRE with and without elevated flip angle modulation for threshold-based liver volume segmentation. Acta Radiol. 2015, 56, 1419–1427. [Google Scholar] [CrossRef]
- Reiner, C.S.; Karlo, C.; Petrowsky, H.; Marincek, B.; Weishaupt, D.; Frauenfelder, T. Preoperative liver volumetry: How does the slice thickness influence the multidetector computed tomography- and magnetic resonance-liver volume measurements? J Comput. Assist. Tomogr. 2009, 33, 390–397. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.W.; Kim, S.Y.; Kim, B.; Lee, S.J.; Kim, H.J.; Lee, J.S.; Lee, M.G.; Song, G.W.; Hwang, S.; et al. Feasibility of semiautomated MR volumetry using gadoxetic acid-enhanced MRI at hepatobiliary phase for living liver donors. Magn. Reson. Med. 2014, 72, 640–645. [Google Scholar] [CrossRef]
- Shimada, K.; Isoda, H.; Okada, T.; Kamae, T.; Arizono, S.; Hirokawa, Y.; Togashi, K. Unenhanced MR portography with a half-Fourier fast spin-echo sequence and time-space labeling inversion pulses: Preliminary results. AJR Am. J. Roentgenol. 2009, 193, 106–112. [Google Scholar] [CrossRef]
- Yamashita, R.; Isoda, H.; Arizono, S.; Ono, A.; Onishi, N.; Furuta, A.; Togashi, K. Non-contrast-enhanced magnetic resonance venography using magnetization-prepared rapid gradient-echo (MPRAGE) in the preoperative evaluation of living liver donor candidates: Comparison with conventional computed tomography venography. Eur. J. Radiol. 2017, 90, 89–96. [Google Scholar] [CrossRef][Green Version]
- Mu, X.; Wang, H.; Ma, Q.; Wu, C.; Ma, L. Contrast-enhanced magnetic resonance angiography for the preoperative evaluation of hepatic vascular anatomy in living liver donors: A meta-analysis. Acad. Radiol. 2014, 21, 743–749. [Google Scholar] [CrossRef]
- Wu, B.; Sun, J.; Wang, C.; Xia, C.; Li, C. Non-contrast-Enhanced MR angiography for selective evaluation of the hepatic portal vein. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2011, 28, 670–675. [Google Scholar] [PubMed]
- Huang, P.H.; Liao, C.C.; Chen, M.H.; Huang, T.L.; Chen, C.L.; Ou, H.Y.; Cheng, Y.F. Noncontrast Magnetic Resonance Angiography Clinical Application in Pre-Liver Transplant Recipients with Impaired Renal Function. Liver Transpl. 2020, 26, 196–202. [Google Scholar] [CrossRef] [PubMed]

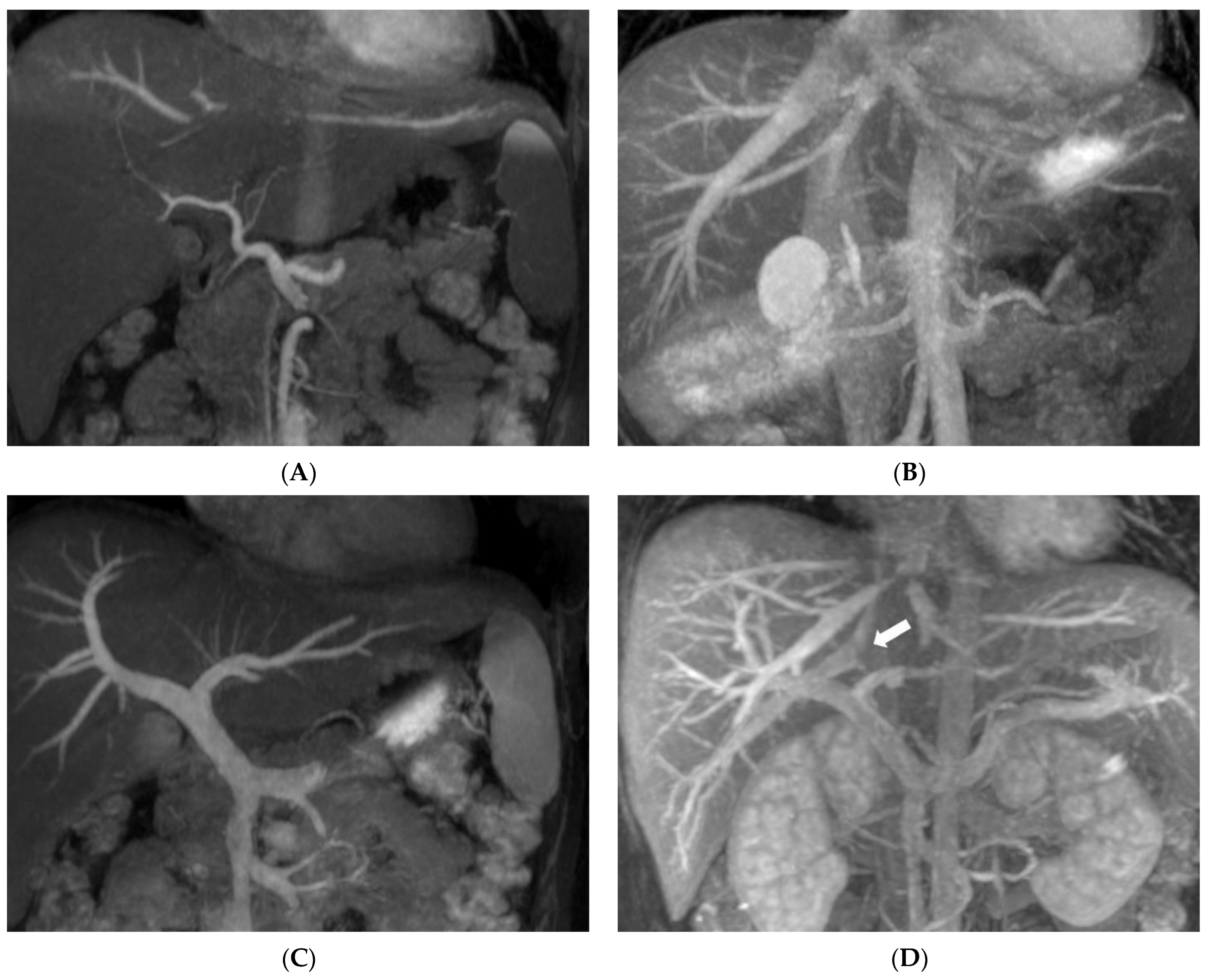
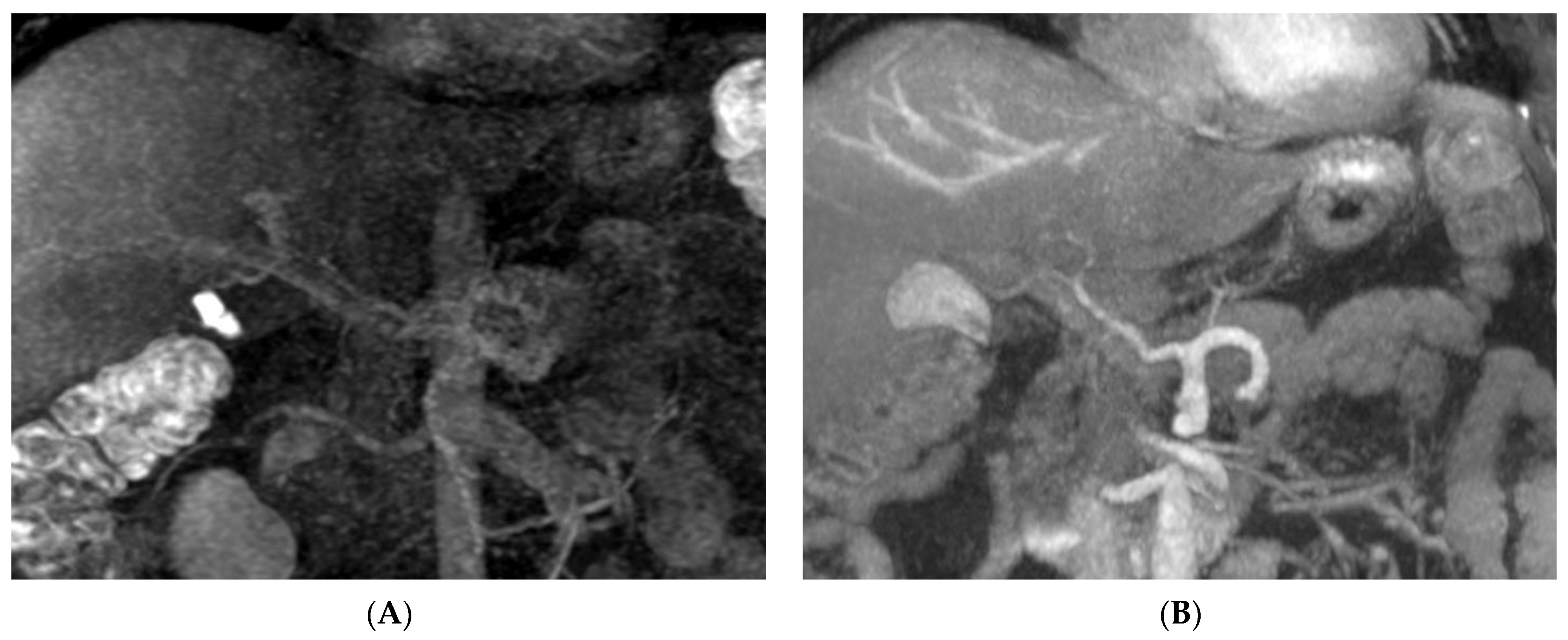
| Visualized Score | Score (Mean ± SD) | Kappa Value |
|---|---|---|
| MPV | 3.5 ± 0.7 | 0.8 |
| RPV | 3.5 ± 0.7 | 0.9 |
| LPV | 3.5 ± 0.7 | 0.8 |
| PHA | 3.5 ± 0.7 | 0.8 |
| RHA | 3.2 ± 1.0 | 0.7 |
| RHA-2 | 2.3 ± 1.2 | 0.5 |
| RHA-3 | 1.5 ± 0.8 | 0.6 |
| LHA | 3.0 ± 1.0 | 0.9 |
| LHA-2 | 1.6 ± 0.9 | 0.8 |
| LHA-3 | 1.2 ± 0.6 | 0.4 |
| MHV | 3.4 ± 0.8 | 0.7 |
| RHV | 3.5 ± 0.6 | 0.9 |
| LHV | 3.3 ± 0.9 | 0.7 |
| S4 HA | 1.7 ± 0.9 | 0.7 |
| S8 HV | 3.3 ± 0.8 | 0.7 |
| IRHV | 3.1 ± 1.2 | 0.7 |
| Michel’s Classification of Hepatic Arteries | CTA | Non-CE MRA | CMRA | CMRA + Non-CE MRA | Pearson Chi-Square p-Value |
|---|---|---|---|---|---|
| I | 35 | 29/35 (83%) | 34/35 (97%) | 35/35 (100%) | |
| III | 1 | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | |
| V | 5 | 4/5 (80%) | 5/5 (100%) | 5/5 (100%) | |
| VI | 1 | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | |
| VIII | 1 | 1/1 (100%) | 0/1 (0%) | 1/1 (100%) | |
| Total | 43 | 36/43 (84%) | 41/43 (95%) | 43/43 (100%) | <0.01 |
| Portal vein ramification | |||||
| Type I | 40 | 39/40 (99%) | 39/40 (99%) | 39/40 (99%) | |
| Type II | 1 | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | |
| Type III | 2 | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) | |
| Total | 43 | 42/43 (98%) | 42/43 (98%) | 42/43 (98%) | |
| Hepatic vein tributary | |||||
| Common trunk of MHV and LHV | 43 | 41/43 (95%) | 41/43 (95%) | 43/43 (100%) | |
| Single RHV | 43 | 43/43(100%) | 43/43 (100%) | 43/43(100%) | |
| Total | 46 | 84/86 (98%) | 84/86 (98%) | 86/86 (100%) | |
| Essential vascular anatomy | |||||
| S4HA from either RHA or LHA | 43 | 22/43 (51%) | 37/43 (86%) | 40/43 (93%) | <0.01 |
| IRHV | 20 | 16/20 (80%) | 15/20 (75%) | 19/20 (95%) | <0.01 |
| S8HV | 43 | 43/43 (100%) | 40/43 (93%) | 43/43 (100%) | |
| Total | 106 | 81/106 (75%) | 92/106 (87%) | 102/106 (96%) | |
| Group | Mean ± SD | p-Value |
|---|---|---|
| Non-CE MPV-CNR | 11.00 ± 7.12 | <0.01 |
| C-MPV-CNR | 3.02 ± 2.42 | |
| Non-CE RPV-CNR | 14.27 ± 14.04 | <0.01 |
| C-RPV-CNR | 3.61 ± 2.53 | |
| Non-CE LPV-CNR | 10.87 ± 6.51 | <0.01 |
| C-LPV-CNR | 3.75 ± 2.49 | |
| Non-CE PHA-CNR | 9.50 ± 5.43 | <0.01 |
| C-PHA-CNR | 12.76 ± 6.43 | |
| Non-CE RHA-CNR | 8.66 ± 15.21 | 0.90 |
| C-RHA-CNR | 8.99 ± 6.97 | |
| Non-CE LHA-CNR | 5.25 ± 4.44 | 0.13 |
| C-LHA-CNR | 6.75 ± 4.94 | |
| Non-CE MHV-CNR | 11.28 ± 5.81 | <0.01 |
| C-MHV-CNR | 2.90 ± 2.24 | |
| Non-CE RHV-CNR | 11.94 ± 6.23 | <0.01 |
| C-RHV-CNR | 2.66 ± 2.12 | |
| Non-CE LHV-CNR | 9.35 ± 5.03 | <0.01 |
| C-LHV-CNR | 2.01 ± 2.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-C.; Chen, M.-H.; Yu, C.-Y.; Tsang, L.-C.L.; Chen, C.-L.; Hsu, H.-W.; Lim, W.-X.; Chuang, Y.-H.; Huang, P.-H.; Cheng, Y.-F.; et al. Non-Contrast-Enhanced and Contrast-Enhanced Magnetic Resonance Angiography in Living Donor Liver Vascular Anatomy. Diagnostics 2022, 12, 498. https://doi.org/10.3390/diagnostics12020498
Liao C-C, Chen M-H, Yu C-Y, Tsang L-CL, Chen C-L, Hsu H-W, Lim W-X, Chuang Y-H, Huang P-H, Cheng Y-F, et al. Non-Contrast-Enhanced and Contrast-Enhanced Magnetic Resonance Angiography in Living Donor Liver Vascular Anatomy. Diagnostics. 2022; 12(2):498. https://doi.org/10.3390/diagnostics12020498
Chicago/Turabian StyleLiao, Chien-Chang, Meng-Hsiang Chen, Chun-Yen Yu, Leung-Chit Leo Tsang, Chao-Long Chen, Hsien-Wen Hsu, Wei-Xiong Lim, Yi-Hsuan Chuang, Po-Hsun Huang, Yu-Fan Cheng, and et al. 2022. "Non-Contrast-Enhanced and Contrast-Enhanced Magnetic Resonance Angiography in Living Donor Liver Vascular Anatomy" Diagnostics 12, no. 2: 498. https://doi.org/10.3390/diagnostics12020498
APA StyleLiao, C.-C., Chen, M.-H., Yu, C.-Y., Tsang, L.-C. L., Chen, C.-L., Hsu, H.-W., Lim, W.-X., Chuang, Y.-H., Huang, P.-H., Cheng, Y.-F., & Ou, H.-Y. (2022). Non-Contrast-Enhanced and Contrast-Enhanced Magnetic Resonance Angiography in Living Donor Liver Vascular Anatomy. Diagnostics, 12(2), 498. https://doi.org/10.3390/diagnostics12020498






