Anatomical-MRI Correlations in Adults and Children with Hypertrophic Cardiomyopathy
Abstract
1. Introduction
2. An Anatomical and Histopathological Review of Hypertrophic Cardiomyopathy
3. Cardiac MR for HCM
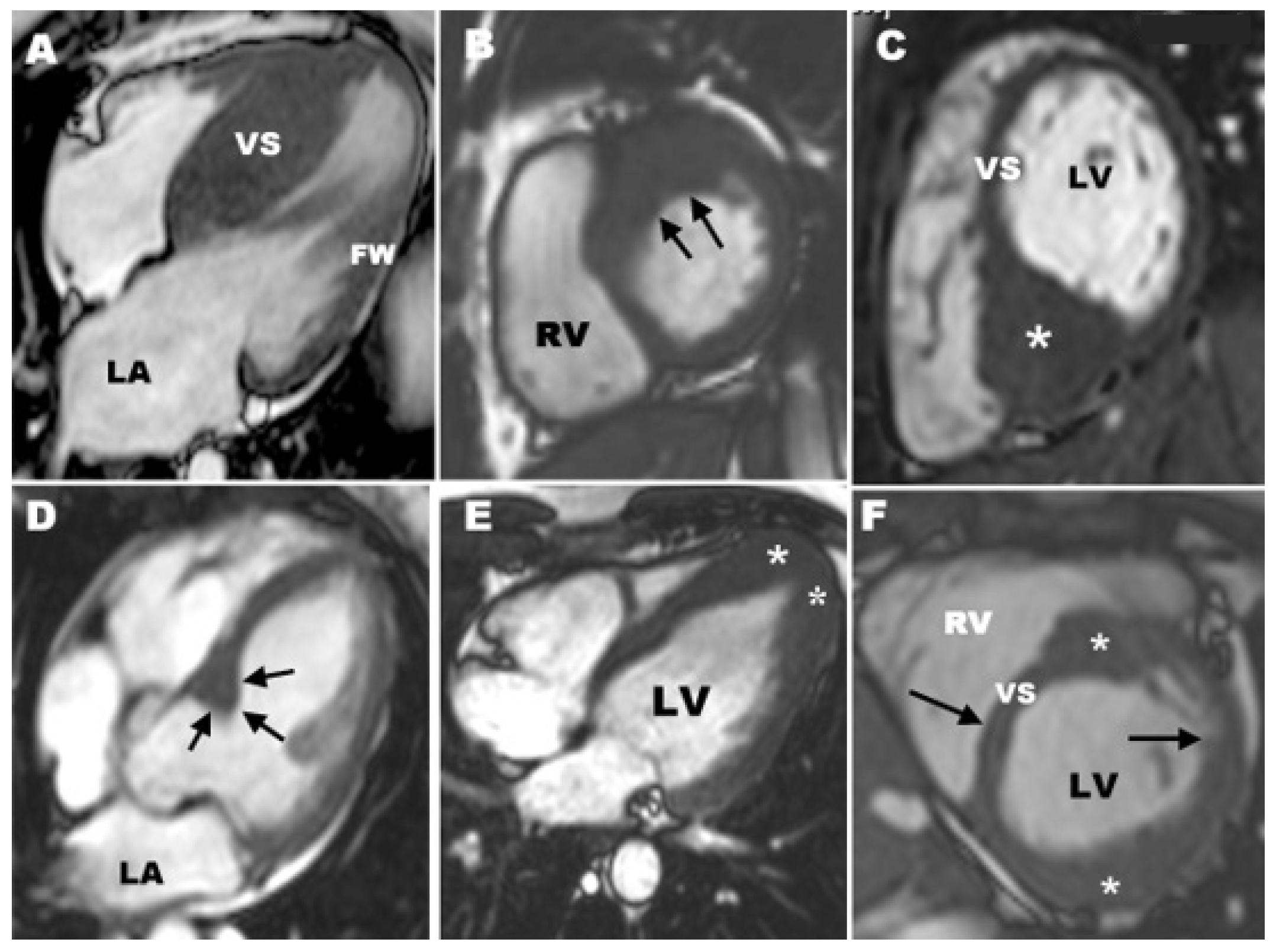
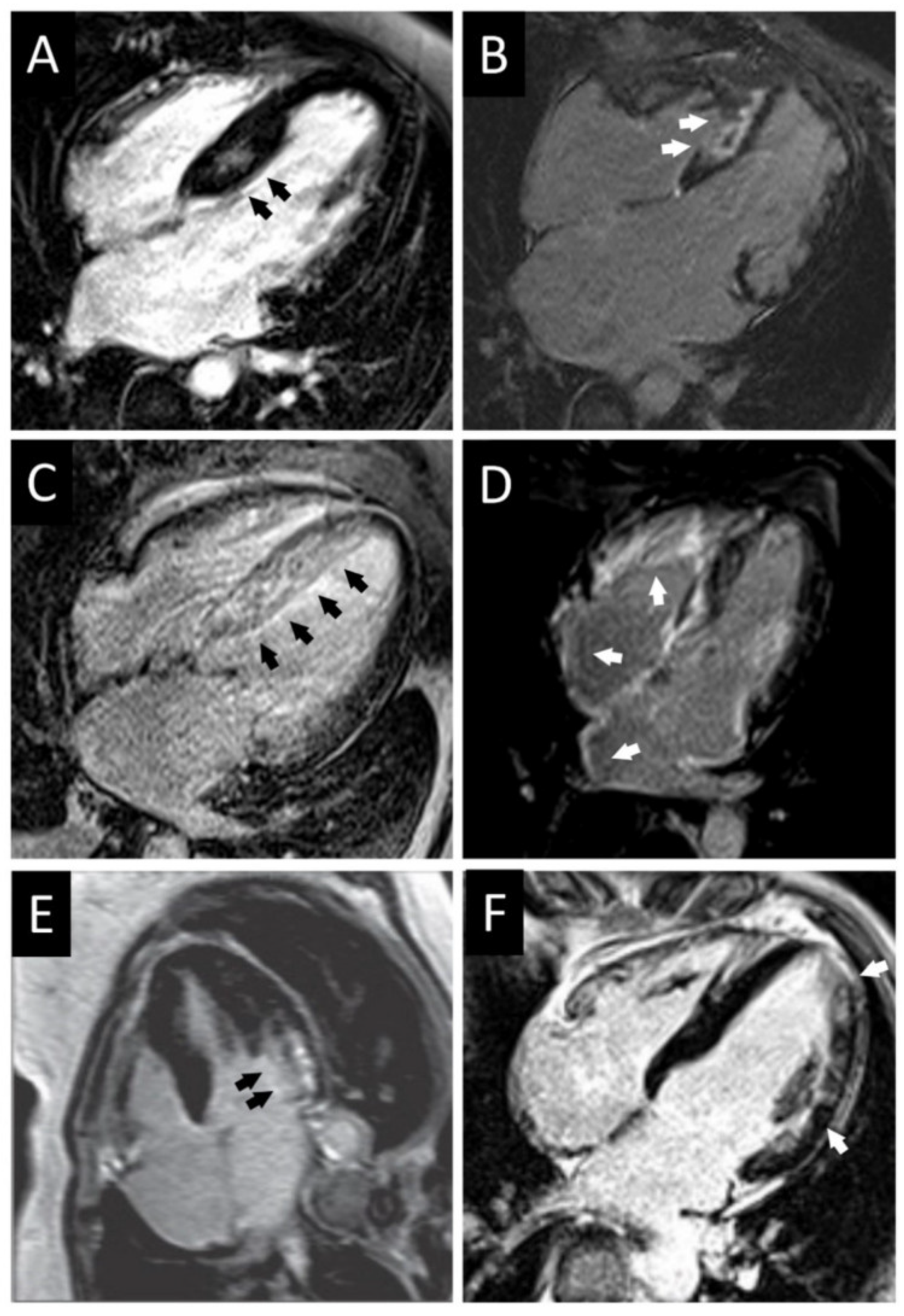
3.1. CMR Evaluation of Apical Hypertrophic Cardiomyopathy
3.2. CMR Evaluation of Focal Hypertrophic Cardiomyopathy
3.3. CMR Evaluation of Midventricular Obstruction
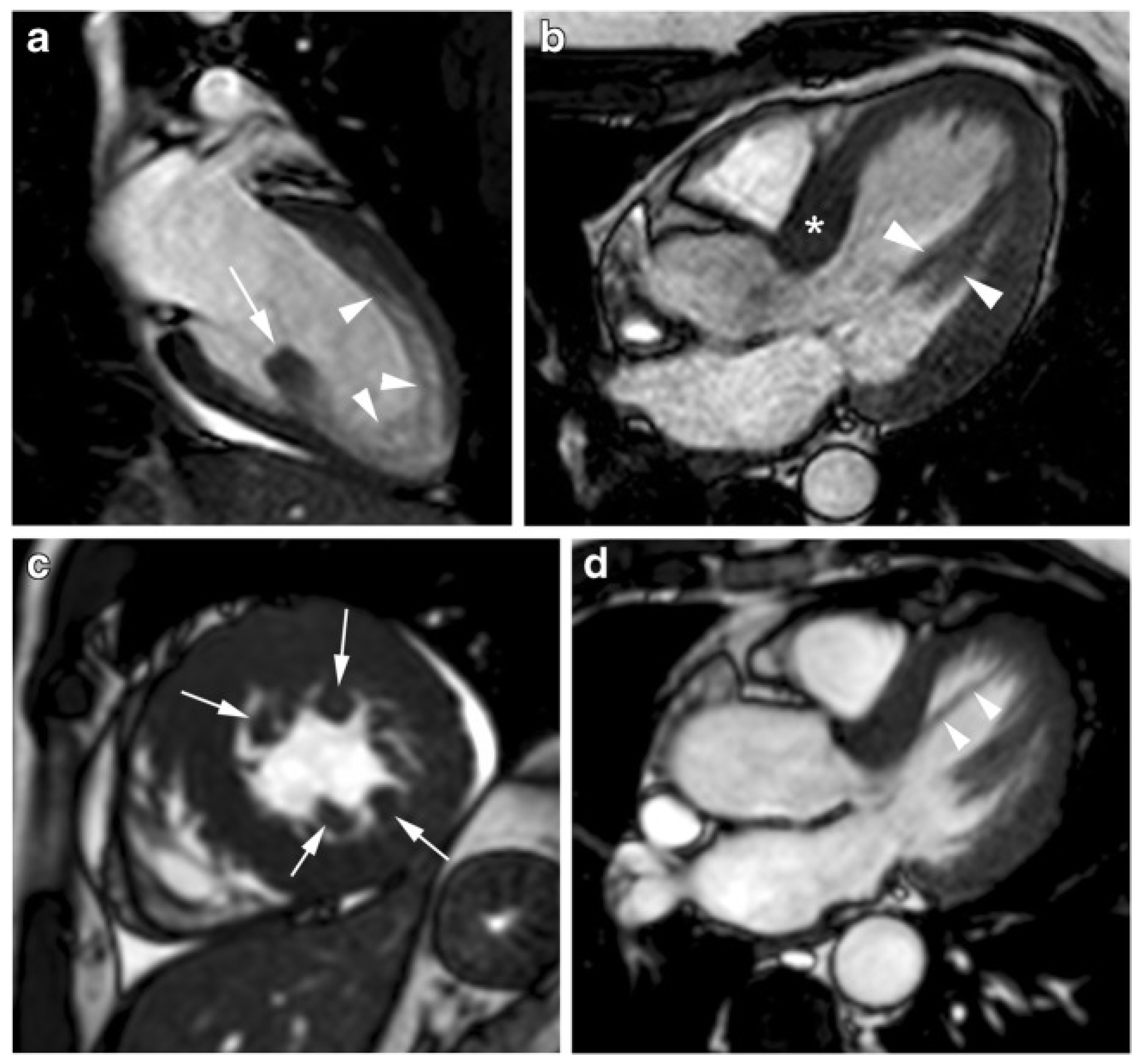
3.4. CMR Evaluation of Apical Aneurysm
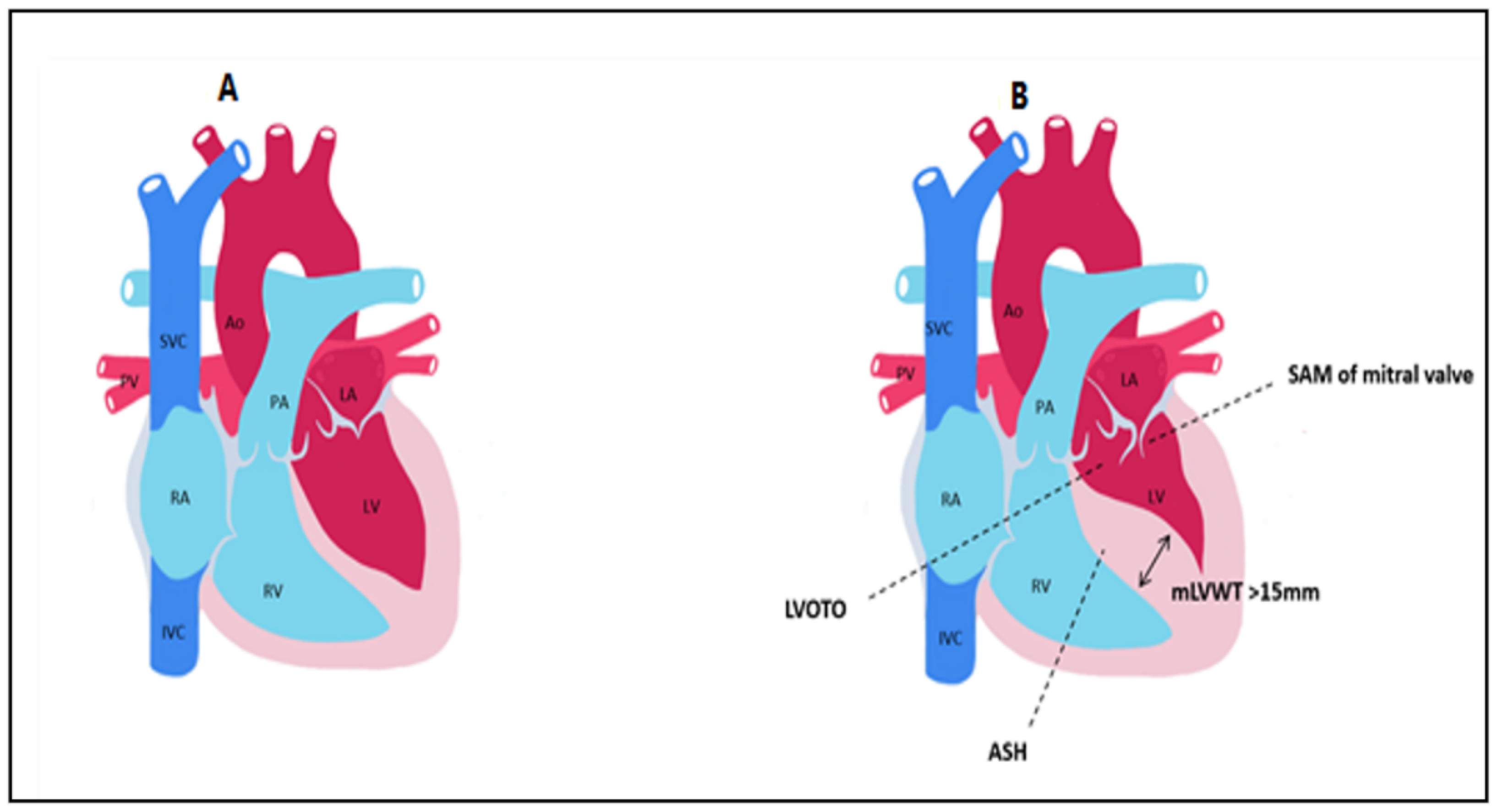
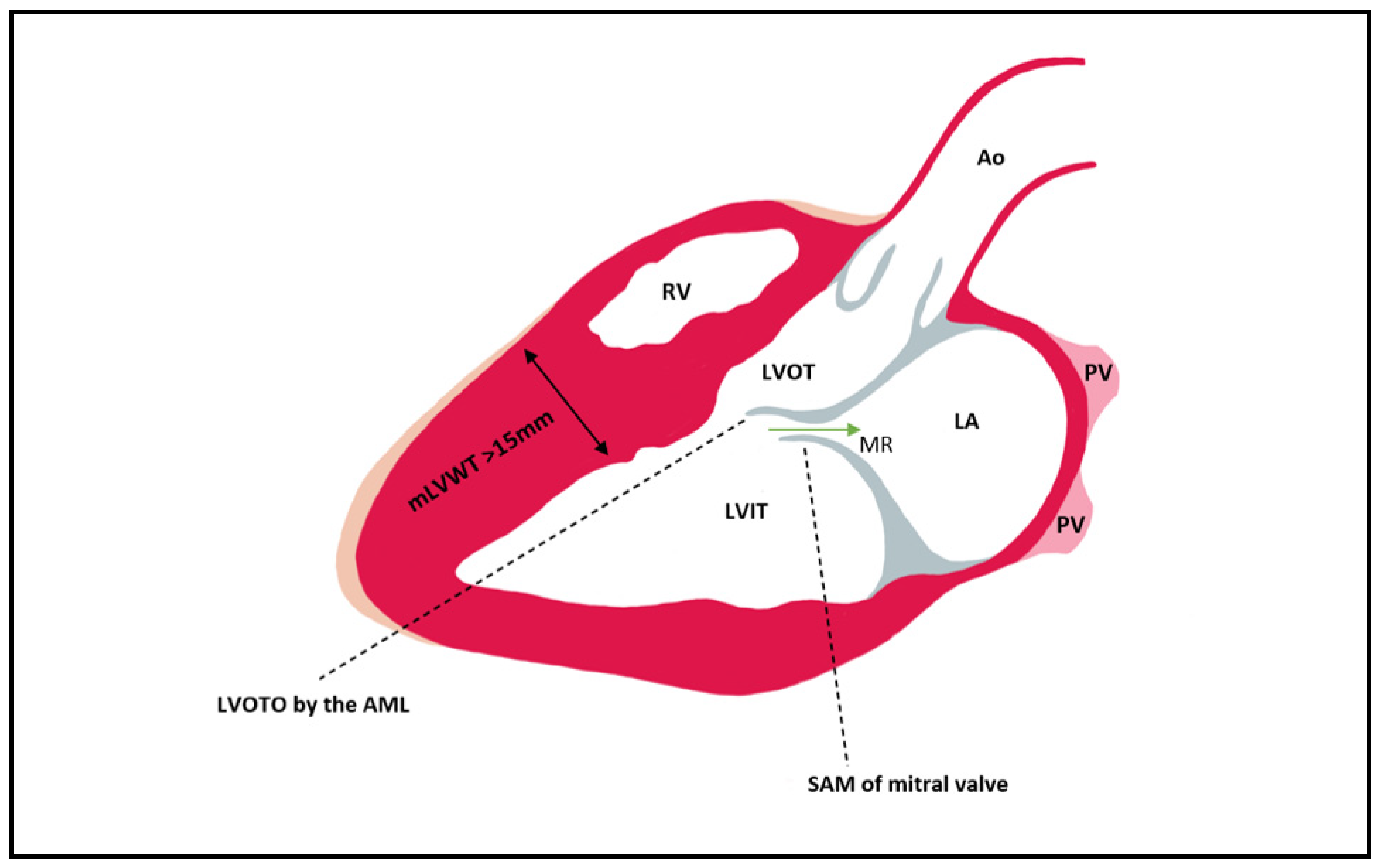
3.5. CMR Evaluation of LVOT Obstruction
3.6. CMR Evaluation of Mitral Valve and Papillary Muscles
3.7. CMR Evaluation of Left Atrial Dimensions and Function
4. CMR Differential Diagnosis of Thickened Myocardium
4.1. Differential Diagnosis with Athlete’s Heart
4.2. Differential Diagnosis with Hypertensive Heart Disease
4.3. Differential Diagnosis with Infiltrative Cardiomyopathies
5. CMR for Risk Stratification in Adults with HCM
6. Special Considerations in Children
7. Risk Stratification in Children with HCM
8. Conclusions
9. Teaching Points
- CMR allows accurate thickness measurements of the LV walls.
- Localized or diffuse myocardial fibrosis inside the left ventricle can be identified with CMR.
- CMR can be used to describe the various phenotypic manifestations of sarcomeric gene mutations.
- CMR can identify other issues associated with HCM, such as apical aneurysm, mitral valve and papillary muscle anomalies, LV outflow tract obstruction, midventricular obstruction, and left atrial enlargement.
- CMR with late gadolinium enhancement can be used to differentiate HCM from hypertensive heart disease, athlete’s heart and infiltrative cardiomyopathies.
- In children, late gadolinium enhancement might be used to improve risk stratification, as LV outflow tract gradient and family history of SCD are not associated with an elevated risk of SCD, and there is a non-linear relationship between LVH and the risk of SCD.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prinz, C.; Farr, M.; Hering, D.; Horstkotte, D.; Faber, L. The Diagnosis and Treatment of Hypertrophic Cardiomyopathy. Dtsch. Ärzteblatt Int. 2011, 108, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Motevali, M.; Siahi, Z.; Mohammadzadeh, A.; Sangi, A. Cardiac Magnetic Resonance Imaging (MRI) Findings in Arrhythmogenic Right Ventricular Dysplasia (ARVD) Compared with Echocardiography. Med. Sci. 2018, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.; Rahman, M.S.; Elliott, P.M. A systematic review and meta-analysis of genotype–phenotype associations in patients with hypertrophic cardiomyopathy caused by sarcomeric protein mutations. Heart 2013, 99, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Elliott, P. The genetics of hypertrophic cardiomyopathy. Glob. Cardiol. Sci. Pr. 2018, 2018, 36. [Google Scholar] [CrossRef]
- Richard, P.; Charron, P.; Carrier, L. Hypertrophic cardiomyopathy. Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. ACC Curr. J. Rev. 2003, 12, 60. [Google Scholar] [CrossRef]
- Gruner, C.; Ivanov, J.; Care, M.; Williams, L.; Moravsky, G.; Yang, H.; Laczay, B.; Siminovitch, K.; Woo, A.; Rakowski, H. Toronto Hypertrophic Cardiomyopathy Genotype Score for Prediction of a Positive Genotype in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Genet. 2013, 6, 19–26. [Google Scholar] [CrossRef]
- Hindieh, W.; Chan, R.; Rakowski, H. Complementary Role of Echocardiography and Cardiac Magnetic Resonance in Hypertrophic Cardiomyopathy. Curr. Cardiol. Rep. 2017, 19, 81. [Google Scholar] [CrossRef]
- Reichek, N. Imaging cardiac morphology in hypertrophic cardiomyopathy: Recent advances. Curr. Opin. Cardiol. 2015, 30, 461–467. [Google Scholar] [CrossRef]
- Varma, P.; Neema, P.; Pk, V. Hypertrophic cardiomyopathy: Part 1—Introduction, pathology and pathophysiology. Ann. Card. Anaesth. 2014, 17, 118. [Google Scholar] [CrossRef]
- Makavos, G.; Κairis, C.; Tselegkidi, M.-E.; Karamitsos, T.; Rigopoulos, A.G.; Noutsias, M.; Ikonomidis, I. Hypertrophic cardiomyopathy: An updated review on diagnosis, prognosis, and treatment. Heart Fail. Rev. 2019, 24, 439–459. [Google Scholar] [CrossRef]
- Habib, M.; Hoss, S.; Rakowski, H. Evaluation of Hypertrophic Cardiomyopathy: Newer Echo and MRI Approaches. Curr. Cardiol. Rep. 2019, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Hensley, N.; Dietrich, J.; Nyhan, D.; Mitter, N.; Yee, M.S.; Brady, M. Hypertrophic cardiomyopathy: A review. Anesth. Analg. 2015, 120, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Teare, D. Asymmetrical hypertrophy of the heart in young adults. Br. Heart J. 1958, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.G.S.J.; Lie, J.T.; Anderson, K.R.; O’Brien, P.C.; Frye, R.L. Histopathological specificity of hypertrophic obstructive cardiomyopathy. Myocardial fibre disarray and myocardial fibrosis. Heart 1980, 44, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Mackey-Bojack, S.; Facile, E.; Duncanson, E.; Rowin, E.J.; Maron, M.S. Hypertrophic Cardiomyopathy and Sudden Death Initially Identified at Autopsy. Am. J. Cardiol. 2020, 127, 139–141. [Google Scholar] [CrossRef]
- McLeod, C.J.; Ommen, S.R.; Ackerman, M.J.; Weivoda, P.L.; Shen, W.K.; Dearani, J.A.; Schaff, H.; Tajik, A.J.; Gersh, B.J. Surgical septal myectomy decreases the risk for appropriate implantable cardioverter defibrillator discharge in obstructive hypertrophic cardiomyopathy. Eur. Heart J. 2007, 28, 2583–2588. [Google Scholar] [CrossRef]
- Kocovski, L.; Fernandes, J.; Cardiac, S. Death: A Modern Pathology Approach to Hypertrophic Cardiomyopathy. Arch. Pathol. Lab. Med. 2015, 139, 413–416. [Google Scholar] [CrossRef]
- Efthimiadis, G.K.; Pagourelias, E.D.; Parcharidou, D.; Gossios, T.; Kamperidis, V.; Theofilogiannakos, E.K.; Pappa, Z.; Meditskou, S.; Hadjimiltiades, S.; Pliakos, C.; et al. Clinical Characteristics and Natural History of Hypertrophic Cardiomyopathy With Midventricular Obstruction. Circ. J. 2013, 77, 2366–2374. [Google Scholar] [CrossRef]
- Lamke, G.T.; Allen, R.D.; Edwards, W.D.; Tazelaar, H.D.; Danielson, G.K. Surgical pathology of subaortic septal myectomy associated with hypertrophic cardiomyopathy. Cardiovasc. Pathol. 2003, 12, 149–158. [Google Scholar] [CrossRef]
- Galati, G.; Leone, O.; Pasquale, F.; Olivotto, I.; Biagini, E.; Grigioni, F.; Pilato, E.; Lorenzini, M.; Corti, B.; Foà, A.; et al. Histological and Histometric Characterization of Myocardial Fibrosis in End-Stage Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2016, 9, e003090. [Google Scholar] [CrossRef]
- Shapiro, L.M.; McKenna, W.J. Distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy: A two-dimensional echocardiographic study. J. Am. Coll. Cardiol. 1983, 2, 437–444. [Google Scholar] [CrossRef]
- To, A.C.; Dhillon, A.; Desai, M.Y. Cardiac magnetic resonance in hypertrophic cardiomyopathy. JACC Cardiovasc. Imaging 2011, 4, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Rowin, E.J.; Maron, M.S. The Role of Cardiac MRI in the Diagnosis and Risk Stratification of Hypertrophic Cardiomyopathy. Arrhythmia Electrophysiol. Rev. 2016, 5, 197–202. [Google Scholar] [CrossRef]
- Ariga, R.; Tunnicliffe, E.M.; Manohar, S.; Mahmod, M.; Raman, B.; Piechnik, S.K.; Francis, J.M.; Robson, M.D.; Neubauer, S.; Watkins, H. Identification of Myocardial Disarray in Patients With Hypertrophic Cardiomyopathy and Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2019, 73, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.A.; Stevens, G.R. Hypertrophic Cardiomyopathy: A Review. Clin. Med. Insights: Cardiol. 2014, 8, CMC.S15717–65. [Google Scholar] [CrossRef] [PubMed]
- Popa-Fotea, N.M.; Micheu, M.M.; Bataila, V.; Scafa-Udriste, A.; Dorobantu, L.; Scarlatescu, A.I.; Zamfir, D.; Stoian, M.; Onciul, S.; Dorobantu, M. Exploring the Continuum of Hypertrophic Cardiomyopathy—From DNA to Clinical Expression. Medicina 2019, 55, 299. [Google Scholar] [CrossRef]
- Maron, M.S. Clinical Utility of Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. J. Cardiovasc. Magn. Reson. 2012, 14, 13. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nishiyama, S.; Nakanishi, S.; Nishimura, S. Electrocardiographic, echocardiographic and ventriculographic characterization of hypertrophic non-obstructive cardiomyopathy. Eur. Heart J. 1983, 4, 105–119. [Google Scholar] [CrossRef]
- Keren, G.; Belhassen, B.; Sherez, J.; I Miller, H.; Megidish, R.; Berenfeld, D.; Laniado, S. Apical hypertrophic cardiomyopathy: Evaluation by noninvasive and invasive techniques in 23 patients. Circulkation 1985, 71, 45–56. [Google Scholar] [CrossRef]
- Eriksson, M.J.; Sonnenberg, B.; Woo, A.; Rakowski, P.; Parker, T.G.; Wigle, E.; Rakowski, H. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 638–645. [Google Scholar] [CrossRef]
- Pons-Lladó, G.; Carreras, F.; Borrás, X.; Palmer, J.; Llauger, J.; de Luna, A.B. Comparison of Morphologic Assessment of Hypertrophic Cardiomyopathy by Magnetic Resonance Versus Echocardiographic Imaging. Am. J. Cardiol. 1997, 79, 1651–1656. [Google Scholar] [CrossRef]
- Yamada, M.; Teraoka, K.; Kawade, M.; Hirano, M.; Yamashina, A. Frequency and distribution of late gadolinium enhancement in magnetic resonance imaging of patients with apical hypertrophic cardiomyopathy and patients with asymmetrical hypertrophic cardiomyopathy: A comparative study. Int. J. Cardiovasc. Imaging 2009, 25, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, L.; Mahrholdt, H.; Wagner, A.; Choi, K.M.; Elliott, M.D.; Klocke, F.J.; Bonow, R.O.; Judd, R.M.; Kim, R.J. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 2156–2164. [Google Scholar] [CrossRef]
- Neubauer, S.; Kolm, P.; Ho, C.Y.; Kwong, R.Y.; Desai, M.Y.; Dolman, S.F.; Appelbaum, E.; Desvigne-Nickens, P.; DiMarco, J.P.; Friedrich, M.G.; et al. Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM Registry. J. Am. Coll. Cardiol. 2019, 74, 2333–2345. [Google Scholar] [CrossRef]
- Hughes, R.K.; Knott, K.D.; Malcolmson, J.; Augusto, J.B.; Mohiddin, S.A.; Kellman, P.; Moon, J.C.; Captur, G. Apical Hypertrophic Cardiomyopathy: The Variant Less Known. J. Am. Heart Assoc. 2020, 9, e015294. [Google Scholar] [CrossRef]
- Maron, M.S.; Maron, B.J.; Harrigan, C.; Buros, J.; Gibson, C.M.; Olivotto, I.; Biller, L.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2009, 54, 220–228. [Google Scholar] [CrossRef]
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines., American Association for Thoracic Surgery., American Society of Echocardiography., American Society of Nuclear Cardiology., Heart Failure Society of America., Heart Rhythm Society., Society for Cardiovascular Angiography and Interventions., Society of Thoracic Surgeons. Circulation 2011, 124, e783–e831. [Google Scholar]
- Caselli, S.; Maron, M.S.; Urbano-Moral, J.A.; Pandian, N.G.; Maron, B.J.; Pelliccia, A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2014, 114, 1383–1389. [Google Scholar] [CrossRef]
- Bergey, P.D.; Axel, L. Focal hypertrophic cardiomyopathy simulating a mass: MR tagging for correct diagnosis. AJR Am. J. Roentgenol. 2000, 174, 242–244. [Google Scholar] [CrossRef]
- Hansen, M.W.; Merchant, N. MRI of Hypertrophic Cardiomyopathy: Part I, MRI Appearances. Am. J. Roentgenol. 2007, 189, 1335–1343. [Google Scholar] [CrossRef]
- Duncan, K.; Shah, A.; Chaudhry, F.; Sherrid, M.V. Hypertrophic cardiomyopathy with massive midventricular hypertrophy, midventricular obstruction and an akinetic apical chamber. Anadolu Kardiyol. Derg. 2006, 6, 279–282. [Google Scholar] [PubMed]
- Cooke, J.C.; Cotton, J.; Monaghan, M.J. Mid-ventricular HOCM with apical asynergy. Heart 2000, 83, 517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maron, M.S.; Finley, J.J.; Bos, J.M.; Hauser, T.H.; Manning, W.J.; Haas, T.S.; Lesser, J.R.; Udelson, J.E.; Ackerman, M.J.; Maron, B.J. Prevalence, Clinical Significance, and Natural History of Left Ventricular Apical Aneurysms in Hypertrophic Cardiomyopathy. Circulation 2008, 118, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Nakamura, T.; Kuribayashi, T.; Azuma, A.; Nakagawa, M. Sustained cavity obliteration and apical aneurysm formation in apical hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2003, 42, 288–295. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Hirasaki, S.; Kuribayashi, T.; Matsuo, A.; Matsui, H.; Sawada, T.; Nakamura, T.; Azuma, A.; Sugihara, H.; Matsubara, H. Systolic Outward Motion of the Left Ventricular Apical Wall as Detected by Magnetic Resonance Tagging in Patients with Apical Hypertrophic Cardiomyopathy. J. Cardiovasc. Magn. Reson. 2006, 8, 453–460. [Google Scholar] [CrossRef]
- Fan, K.; Chau, E.; Chiu, C.S.W. Hypertrophic cardiomyopathy with mid ventricular obstruction, apical infarction and aneurysm formation. Heart 2005, 91, e42. [Google Scholar] [CrossRef]
- Lin, C.-S.; Chen, C.-H.; Ding, P.Y.-A. Apical hypertrophic cardiomyopathy mimicking acute myocardial infarction. Int. J. Cardiol. 1998, 64, 305–307. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsubara, K.; Furukawa, K.; Azuma, A.; Sugihara, H.; Katsume, H.; Nakagawa, M. Diastolic paradoxic jet flow in patients with hypertrophic cardiomyopathy: Evidence of concealed apical asynergy with cavity obliteration. J. Am. Coll. Cardiol. 1992, 19, 516–524. [Google Scholar] [CrossRef]
- Moravsky, G.; Ofek, E.; Rakowski, H.; Butany, J.; Williams, L.; Ralph-Edwards, A.; Wintersperger, P.J.; Crean, A. Myocardial fibrosis in hypertrophic cardiomyopathy: Accurate reflection of histopathological findings by CMR. JACC Cardiovasc. Imaging 2013, 6, 587–596. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Chokshi, A.; Maron, M.S. Left ventricular apical aneurysm in hypertrophic cardiomyopathy as a risk factor for sudden death at any age. Pacing Clin. Electrophysiol. 2018, 41, 1031–1033. [Google Scholar] [CrossRef]
- Yang, K.; Song, Y.-Y.; Chen, X.-Y.; Wang, J.-X.; Li, L.; Yin, G.; Zheng, Y.-C.; Wei, M.-D.; Lu, M.-J.; Zhao, S.-H. Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: Prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Rowin, E.J.; Maron, B.J.; Haas, T.S.; Garberich, R.F.; Wang, W.J.; Link, M.S.; Maron, M.S. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: Implications for risk stratification and management. J. Am. Coll. Cardiol. 2017, 69, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Ommen, S.R.; Semsarian, C.; Spirito, P.; Olivotto, I.; Maron, M.S. Hypertrophic cardiomyopathy: Present and future, with translation into contemporary cardiovascular medicine. J. Am. Coll. Cardiol. 2014, 64, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Fulton, N.L.; Bolen, M. Magnetic resonance imaging of the papillary muscles of the left ventricle: Normal anatomy, variants, and abnormalities. Insights Imaging 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Harrigan, C.J.; Appelbaum, E.; Maron, B.J.; Buros, J.; Gibson, C.M.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; Maron, M.S. Significance of Papillary Muscle Abnormalities Identified by Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2008, 101, 668–673. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Harrigan, C.; Appelbaum, E.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Captur, G.; Lopes, L.R.; Mohun, T.J.; Patel, V.; Li, C.; Bassett, P.; Finocchiaro, G.; Ferreira, V.M.; Esteban, M.T.; Muthurangu, V.; et al. Prediction of sarcomere mutations in subclinical hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 863–871. [Google Scholar] [CrossRef]
- Klues, H.G.; Proschan, M.A.; Dollar, A.L.; Spirito, P.; Roberts, W.C.; Maron, B.J. Echocardiographic assessment of mitral valve size in obstructive hypertrophic cardiomyopathy. Anatomic validation from mitral valve specimen. Circulation 1993, 88, 548–555. [Google Scholar] [CrossRef]
- Balaram, S.K.; Sherrid, M.; Derose, J.J.; Hillel, Z.; Winson, G.; Swistel, D. Beyond Extended Myectomy for Hypertrophic Cardiomyopathy: The Resection-Plication-Release (RPR) Repair. Ann. Thorac. Surg. 2005, 80, 217–223. [Google Scholar] [CrossRef]
- Kaple, R.K.; Murphy, R.T.; DiPaola, L.M.; Houghtaling, P.L.; Lever, H.M.; Lytle, B.W.; Blackstone, E.H.; Smedira, N.G. Mitral Valve Abnormalities in Hypertrophic Cardiomyopathy: Echocardiographic Features and Surgical Outcomes. Ann. Thorac. Surg. 2008, 85, 1527–1535.e2. [Google Scholar] [CrossRef]
- Teraoka, K.; Hirano, M.; Ookubo, H.; Sasaki, K.; Katsuyama, H.; Amino, M.; Abe, Y.; Yamashina, A. Delayed contrast enhancement of MRI in hypertrophic cardiomyopathy. Magn. Reson. Imaging 2004, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Distinguishing hypertrophic cardiomyopathy from athlete’s heart: A clinical problem of increasing magnitude and significance. Heart 2005, 91, 1380–1382. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Dhillon, A.; Popovic, Z.B.; Smedira, N.G.; Rizzo, J.; Thamilarasan, M.; Agler, D.; Lytle, B.W.; Lever, H.M.; Desai, M.Y. Left Ventricular Outflow Tract Obstruction in Hypertrophic Cardiomyopathy Patients Without Severe Septal Hypertrophy: Implications of Mitral Valve and Papillary Muscle Abnormalities Assessed Using Cardiac Magnetic Resonance and Echocardiography. Circ. Cardiovasc. Imaging 2015, 8, e003132. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Méndez, C.; Rodríguez, E.; Barriales-Villa, R.; Ochoa, J.P.; Monserrat, L. Phenotypes of hypertrophic cardiomyopathy. An illustrative review of MRI findings. Insights Imaging 2018, 9, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Kuchynka, P.; Podzimkova, J.; Mašek, M.; Lambert, L.; Černý, V.; Danek, B.; Palecek, T. The Role of Magnetic Resonance Imaging and Cardiac Computed Tomography in the Assessment of Left Atrial Anatomy, Size, and Function. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, S.; Elliott, P.; Whyte, G.; Mahon, N.; Virdee, M.S.; Mist, B.; McKenna, W.J. Utility of metabolic exercise testing in distinguishing hypertrophic cardiomyopathy from physiologic left ventricular hypertrophy in athletes. J. Am. Coll. Cardiol. 2000, 36, 864–870. [Google Scholar] [CrossRef]
- Luijkx, T.; Cramer, M.J.; Buckens, C.F.; Zaidi, A.; Rienks, R.; Mosterd, A.; Prakken, N.; Dijkman, B.; Mali, W.P.T.M.; Velthuis, B.K. Unravelling the grey zone: Cardiac MRI volume to wall mass ratio to differentiate hypertrophic cardiomyopathy and the athlete’s heart. Br. J. Sports Med. 2013, 49, 1404–1409. [Google Scholar] [CrossRef]
- Neisius, U.; El-Rewaidy, H.; Nakamori, S.; Rodriguez, J.; Manning, W.J.; Nezafat, R. Radiomic Analysis of Myocardial Native T1 Imaging Discriminates Between Hypertensive Heart Disease and Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2019, 12, 1946–1954. [Google Scholar] [CrossRef]
- Hinojar, R.; Varma, N.; Child, N.; Goodman, B.; Jabbour, A.; Yu, C.-Y.; Gebker, R.; Doltra, A.; Kelle, S.; Khan, S.; et al. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2015, 8, e003285. [Google Scholar] [CrossRef]
- Takeda, M.; Amano, Y.; Tachi, M.; Tani, H.; Mizuno, K.; Kumita, S. MRI differentiation of cardiomyopathy showing left ventricular hypertrophy and heart failure: Differentiation between cardiac amyloidosis, hypertrophic cardiomyopathy, and hypertensive heart disease. Jpn. J. Radiol. 2013, 31, 693–700. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Pennell, D.J. Cardiovascular Magnetic Resonance in the Evaluation of Hypertrophic and Infiltrative Cardiomyopathies. Heart Fail. Clin. 2009, 5, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.-D.; Kim, S.M.; Na Jung, H.; Kim, Y.; Choe, Y.H. Comparison of quantitative imaging parameters using cardiovascular magnetic resonance between cardiac amyloidosis and hypertrophic cardiomyopathy: Inversion time scout versus T1 mapping. Int. J. Cardiovasc. Imaging 2018, 34, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Vio, R.; Angelini, A.; Basso, C.; Cipriani, A.; Zorzi, A.; Melacini, P.; Thiene, G.; Rampazzo, A.; Corrado, D.; Calore, C. Hypertrophic Cardiomyopathy and Primary Restrictive Cardiomyopathy: Similarities, Differences and Phenocopies. J. Clin. Med. 2021, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur. Heart J. 2013, 35, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2011, 58, 2703–2738. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Sleeper, L.A.; Towbin, J.A.; Lowe, A.M.; Orav, E.J.; Cox, G.F.; Lurie, P.R.; McCoy, K.L.; McDonald, M.A.; Messere, J.E.; et al. The Incidence of Pediatric Cardiomyopathy in Two Regions of the United States. N. Engl. J. Med. 2003, 348, 1647–1655. [Google Scholar] [CrossRef]
- Colan, S.D.; Lipshultz, S.E.; Lowe, A.M.; Sleeper, L.A.; Messere, J.; Cox, G.F.; Lurie, P.R.; Orav, E.J.; Towbin, J.A. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: Findings from the Pediatric Cardiomyopathy Registry. Circulation 2007, 115, 773–781. [Google Scholar] [CrossRef]
- Chaowu, Y.; Shihua, Z.; Jian, L.; Li, L.; Wei, F. Cardiovascular Magnetic Resonance Characteristics in Children with Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2013, 6, 1013–1020. [Google Scholar] [CrossRef]
- Kindel, S.J.; Miller, E.M.; Gupta, R.; Cripe, L.H.; Hinton, R.B.; Spicer, R.L.; Towbin, J.A.; Ware, S.M. Pediatric Cardiomyopathy: Importance of Genetic and Metabolic Evaluation. J. Card. Fail. 2012, 18, 396–403. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic value of LGE-CMR in HCM: A meta-analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.A.; Rossano, J.W.; Smith, E.O.; Cannon, B.; Clunie, S.K.; Gates, C.; Jefferies, J.L.; Kim, J.J.; Price, J.F.; Dreyer, W.J.; et al. Risk Factors and Mode of Death in Isolated Hypertrophic Cardiomyopathy in Children. J. Am. Coll. Cardiol. 2009, 54, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Norrish, G.; Cantarutti, N.; Pissaridou, E.; A Ridout, D.; Limongelli, G.; Elliott, P.; Kaski, J.P. Risk factors for sudden cardiac death in childhood hypertrophic cardiomyopathy: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1220–1230. [Google Scholar] [CrossRef] [PubMed]




| Implanted ICDs * |
| implanted pacemakers * |
| brain ferromagnetic clips |
| cochlear implants |
| metal foreign body (bullet fragments, metallic splinter in the eye) |
| claustrophobia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosu, R.O.; Lupsor, A.; Necula, A.; Cismaru, G.; Cainap, S.S.; Iacob, D.; Lazea, C.; Cismaru, A.; Negru, A.G.; Pop, D.; et al. Anatomical-MRI Correlations in Adults and Children with Hypertrophic Cardiomyopathy. Diagnostics 2022, 12, 489. https://doi.org/10.3390/diagnostics12020489
Rosu RO, Lupsor A, Necula A, Cismaru G, Cainap SS, Iacob D, Lazea C, Cismaru A, Negru AG, Pop D, et al. Anatomical-MRI Correlations in Adults and Children with Hypertrophic Cardiomyopathy. Diagnostics. 2022; 12(2):489. https://doi.org/10.3390/diagnostics12020489
Chicago/Turabian StyleRosu, Radu Ovidiu, Ana Lupsor, Alexandru Necula, Gabriel Cismaru, Simona Sorana Cainap, Daniela Iacob, Cecilia Lazea, Andrei Cismaru, Alina Gabriela Negru, Dana Pop, and et al. 2022. "Anatomical-MRI Correlations in Adults and Children with Hypertrophic Cardiomyopathy" Diagnostics 12, no. 2: 489. https://doi.org/10.3390/diagnostics12020489
APA StyleRosu, R. O., Lupsor, A., Necula, A., Cismaru, G., Cainap, S. S., Iacob, D., Lazea, C., Cismaru, A., Negru, A. G., Pop, D., & Gusetu, G. (2022). Anatomical-MRI Correlations in Adults and Children with Hypertrophic Cardiomyopathy. Diagnostics, 12(2), 489. https://doi.org/10.3390/diagnostics12020489









