Oral Candida Infection in Psoriatic Patients Treated with IL17A Inhibitors: Report of 3 Cases and a Comprehensive Review of the Literature
Abstract
:1. Introduction
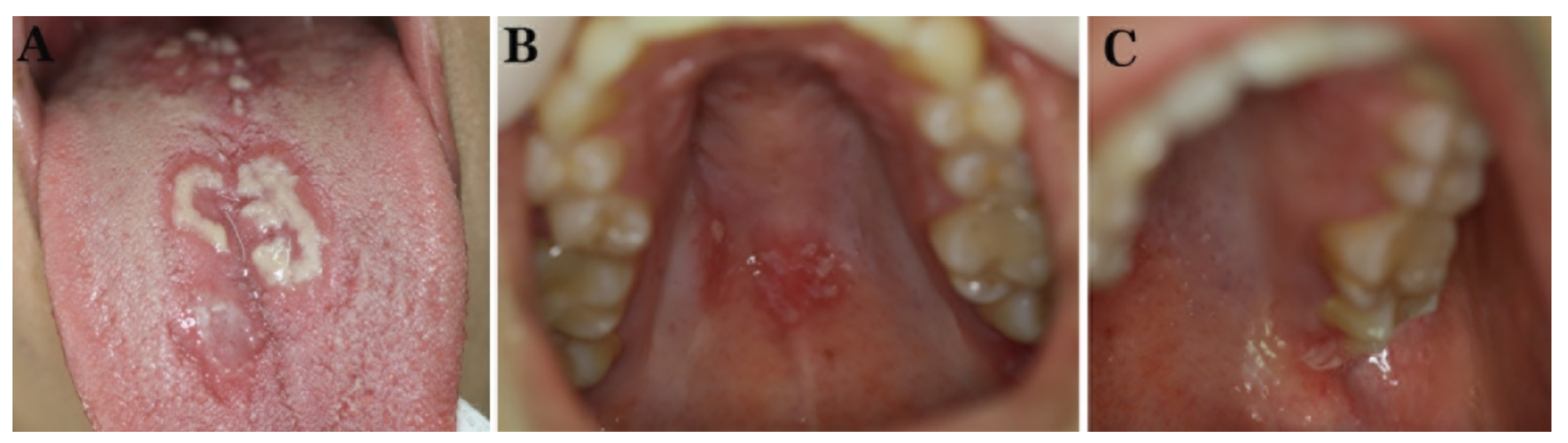
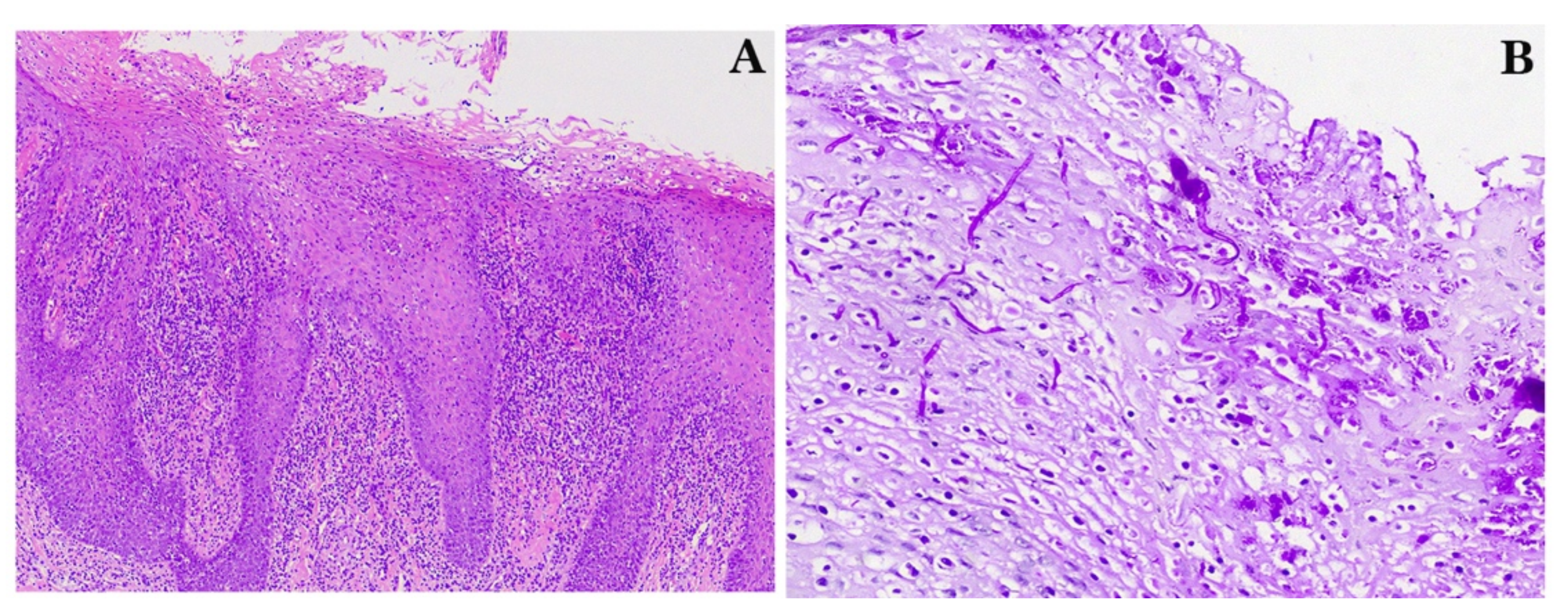
2. Case Reports
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rouvier, E.; Luciani, M.F.; Mattéi, M.G.; Denizot, F.; Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993, 150, 5445–5456. [Google Scholar] [PubMed]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffen, S.L.; Moutsopoulos, N.M. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci Immunol. 2020, 5, eaau4594. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 2017, 18, 612–621. [Google Scholar] [CrossRef]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Towne, J.E.; Kricorian, G.; Klekotka, P.; Gudjonsson, J.E.; Krueger, J.G.; Russell, C.B. The emerging role of IL-17 in the pathogenesis of psoriasis: Preclinical and clinical findings. J. Invest. Dermatol. 2013, 133, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, B.W.; Kavanaugh, A.; Reich, K. Interleukin-17A: A unique pathway in immune-mediated diseases: Psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 2014, 141, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Chiricozzi, A.; Guttman-Yassky, E.; Suárez-Fariñas, M.; Nograles, K.E.; Tian, S.; Cardinale, I.; Chimenti, S.; Krueger, J.G. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 2011, 131, 677–687. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, K.F.; Isaacs, J.D. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum Dis. 2018, 77, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, A.A.; Tyring, S. New and emerging biologic therapies for moderate-to-severe plaque psoriasis: Mechanistic rationales and recent clinical data for IL-17 and IL-23 inhibitors. Dermatol. Ther. 2015, 28, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Saunte, D.M.; Mrowietz, U.; Puig, L.; Zachariae, C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br. J. Dermatol. 2017, 177, 47–62. [Google Scholar] [CrossRef]
- Martínez-Barricarte, R.; Markle, J.G.; Ma, C.S.; Deenick, E.K.; Ramírez-Alejo, N.; Mele, F.; Latorre, D.; Mahdaviani, S.A.; Aytekin, C.; Mansouri, D.; et al. Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 2018, 3, eaau6759. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Markle, J.G.; Deenick, E.K.; Mele, F.; Averbuch, D.; Lagos, M.; Alzahrani, M.; Al-Muhsen, S.; Halwani, R.; Ma, C.S.; et al. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 2015, 349, 606–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Hua, H. Oral manifestation of chronic mucocutaneous candidiasis: Seven case reports. J. Oral Pathol. Med. 2007, 36, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.D.; Brenchley, J.M.; Laurence, A.; Freeman, A.F.; Hill, B.J.; Elias, K.M.; Kanno, Y.; Spalding, C.; Elloumi, H.Z.; Paulson, M.L.; et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008, 452, 773–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisson, B.; Wang, C.; Pedergnana, V.; Wu, L.; Cypowyj, S.; Rybojad, M.; Belkadi, A.; Picard, C.; Abel, L.; Fieschi, C.; et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 2013, 39, 676–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puel, A.; Cypowyj, S.; Bustamante, J.; Wright, J.F.; Liu, L.; Lim, H.K.; Migaud, M.; Israel, L.; Chrabieh, M.; Audry, M.; et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011, 332, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Lévy, R.; Okada, S.; Béziat, V.; Moriya, K.; Liu, C.; Chai, L.Y.; Migaud, M.; Hauck, F.; Al Ali, A.; Cyrus, C.; et al. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc. Natl. Acad. Sci. USA 2016, 113, E8277–E8285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Y.; Cypowyj, S.; Aytekin, C.; Galicchio, M.; Camcioglu, Y.; Nepesov, S.; Ikinciogullari, A.; Dogu, F.; Belkadi, A.; Levy, R.; et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J. Exp. Med. 2015, 212, 619–631. [Google Scholar] [CrossRef]
- Bhattad, S.; Dinakar, C.; Pinnamaraju, H.; Ganapathy, A.; Mannan, A. Chronic Mucocutaneous Candidiasis in an Adolescent Boy Due to a Novel Mutation in TRAF3IP2. J. Clin. Immunol. 2019, 39, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Paiardini, M.; Knox, K.S.; Asher, A.I.; Cervasi, B.; Asher, T.E.; Scheinberg, P.; Price, D.A.; Hage, C.A.; Kholi, L.M.; et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008, 112, 2826–2835. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, M.; Taniguchi, A.; Fujishige, A.; Kaneko, S.; Haemmerle, S.; Porter, B.O.; Kobayashi, S. Efficacy and safety of secukinumab in Japanese patients with active ankylosing spondylitis: 24-week results from an open-label phase 3 study (MEASURE 2-J). Mod. Rheumatol. 2020, 30, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.M.; Cohen, L.M.; Yang, C.S.; Kroumpouzos, G. Severe, ulcerative, lichenoid mucositis associated with secukinumab. JAAD Case Rep. 2016, 2, 384–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capusan, T.M.; Herrero-Moyano, M.; Martínez-Mera, C.R.; Freih-Fraih, A.W.; Dauden, E. Oral lichenoid reaction in a psoriatic patient treated with secukinumab: A drug-related rather than a class-related adverse event? JAAD Case Rep. 2018, 4, 521–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeten, D.; Baraliakos, X.; Braun, J.; Sieper, J.; Emery, P.; van der Heijde, D.; McInnes, I.; van Laar, J.M.; Landewé, R.; Wordsworth, P.; et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: A randomised, double-blind, placebo-controlled trial. Lancet 2013, 382, 1705–1713. [Google Scholar] [CrossRef]
- Wei, J.C.; Baeten, D.; Sieper, J.; Deodhar, A.; Bhosekar, V.; Martin, R.; Porter, B. Efficacy and safety of secukinumab in Asian patients with active ankylosing spondylitis: 52-week pooled results from two phase 3 studies. Int. J. Rheum. Dis. 2017, 20, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Baeten, D.; Sieper, J.; Emery, P.; Readie, A.; Martin, R.; Mpofu, S.; MEASURE 1 Study Group; et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann. Rheum. Dis. 2017, 76, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Tlustochowicz, W.; Rahman, P.; Seriolo, B.; Krammer, G.; Porter, B.; Widmer, A.; Richards, H.B. Efficacy and safety of subcutaneous and intravenous loading dose regimens of secukinumab in patients with active rheumatoid arthritis: Results from a randomized Phase II study. J. Rheumatol. 2016, 43, 495–503. [Google Scholar] [CrossRef]
- Marzo-Ortega, H.; Sieper, J.; Kivitz, A.; Blanco, R.; Cohen, M.; Martin, R.; Readie, A.; Richards, H.B.; Porter, B.; Measure 2 Study Group. Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: Results from a Phase III study. Arthritis Care Res. 2017, 69, 1020–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; van der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; FUTURE 1 Study Group; et al. Secukinumab inhibition of Interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; UNCOVER-1 Study Group; UNCOVER-2 Study Group; UNCOVER-3 Study Group; et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Luger, T.; Thaçi, D.; Toth, D.; Lacombe, A.; Xia, S.; Mazur, R.; Patekar, M.; Charef, P.; Milutinovic, M.; et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J. Eur. Acad. Derm. Venereol. 2018, 32, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Thaçi, D.; Körber, A.; von Kiedrowski, R.; Bachhuber, T.; Melzer, N.; Kasparek, T.; Duetting, E.; Kraehn-Senftleben, G.; Amon, U.; Augustin, M. Secukinumab is effective in treatment of moderate-to-severe plaque psoriasis: Real-life effectiveness and safety from the PROSPECT study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, K.; Leonardi, C.; Menter, A.; Thompson, E.H.; Milmont, C.E.; Kricorian, G.; Nirula, A.; Klekotka, P. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J. Am. Acad. Dermatol. 2014, 71, 1183–1190.e3. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Lacour, J.P.; Tedremets, L.; Kreutzer, K.; Jazayeri, S.; Adams, S.; Guindon, C.; You, R.; Papavassilis, C.; JUNCTURE study group. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: A randomized, controlled trial (JUNCTURE). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1082–1090. [Google Scholar] [CrossRef]
- Blauvelt, A.; Prinz, J.C.; Gottlieb, A.B.; Kingo, K.; Sofen, H.; Ruer-Mulard, M.; Singh, V.; Pathan, R.; Papavassilis, C.; Cooper, S.; et al. Secukinumab administration by pre-filled syringe: Efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br. J. Dermatol. 2015, 172, 484–493. [Google Scholar] [CrossRef]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; MEASURE 1 Study Group; MEASURE 2 Study Group; et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnes, I.B.; Mease, P.J.; Kirkham, B.; Kavanaugh, A.; Ritchlin, C.T.; Rahman, P.; van der Heijde, D.; Landewé, R.; Conaghan, P.G.; FUTURE 2 Study Group; et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, H.; Niiro, H.; Ootaki, K.; Japanese brodalumab study group. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. J. Dermatol. Sci. 2016, 81, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Nakagawa, H.; Kubo, Y.; Ootaki, K.; Japanese Brodalumab Study Group. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: Results from a 52-week, open-label study. Br. J. Dermatol. 2017, 176, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Glatt, S.; Baeten, D.; Baker, T.; Griffiths, M.; Ionescu, L.; Lawson, A.; Maroof, A.; Oliver, R.; Popa, S.; Strimenopoulou, F.; et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: Evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann. Rheum. Dis. 2018, 77, 523–532. [Google Scholar] [CrossRef]
- Blanco, F.J.; Möricke, R.; Dokoupilova, E.; Codding, C.; Neal, J.; Andersson, M.; Rohrer, S.; Richards, H. Secukinumab in Active Rheumatoid Arthritis: A Phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 2017, 69, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Pavelka, K.; Kivitz, A.; Dokoupilova, E.; Blanco, R.; Maradiaga, M.; Tahir, H.; Pricop, L.; Andersson, M.; Readie, A.; Porter, B. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: A randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res. Ther. 2017, 19, 285. [Google Scholar] [CrossRef] [Green Version]
- Kivitz, A.J.; Wagner, U.; Dokoupilova, E.; Supronik, J.; Martin, R.; Talloczy, Z.; Richards, H.B.; Porter, B. Efficacy and Safety of secukinumab 150 mg with and without loading regimen in ankylosing spondylitis: 104-week results from MEASURE 4 Study. Rheumatol. Ther. 2018, 5, 447–462. [Google Scholar] [CrossRef] [Green Version]
- Papp, K.A.; Merola, J.F.; Gottlieb, A.B.; Griffiths, C.E.M.; Cross, N.; Peterson, L.; Cioffi, C.; Blauvelt, A. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: Results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J. Am. Acad. Dermatol. 2018, 79, 277–286.e10. [Google Scholar] [CrossRef] [Green Version]
- Baraliakos, X.; Braun, J.; Deodhar, A.; Poddubnyy, D.; Kivitz, A.; Tahir, H.; Van den Bosch, F.; Delicha, E.M.; Talloczy, Z.; Fierlinger, A. Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study. RMD Open 2019, 5, e001005. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Papp, K.A.; Merola, J.F.; Gottlieb, A.B.; Cross, N.; Madden, C.; Wang, M.; Cioffi, C.; Griffiths, C. Bimekizumab for patients with moderate to severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled, phase 2b extension study. J. Am. Acad. Dermatol. 2020, 83, 1367–1374. [Google Scholar] [CrossRef]
- Huang, F.; Sun, F.; Wan, W.G.; Wu, L.J.; Dong, L.L.; Zhang, X.; Kim, T.H.; Sengupta, R.; Šenolt, L.; Wang, Y.; et al. Secukinumab provided significant and sustained improvement in the signs and symptoms of ankylosing spondylitis: Results from the 52-week, Phase III China-centric study, MEASURE 5. Chin. Med. J. 2020, 133, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, K.; Kivitz, A.J.; Dokoupilova, E.; Blanco, R.; Maradiaga, M.; Tahir, H.; Wang, Y.; Porter, B.O.; Stefanska, A.; MEASURE 3 Study Group; et al. Secukinumab 150/300 mg provides sustained improvements in the signs and symptoms of active ankylosing spondylitis: 3-year results from the phase 3 MEASURE 3 study. ACR Open Rheumatol. 2020, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Kavanaugh, A.; Merola, J.F.; Schett, G.; Scher, J.U.; Warren, R.B.; Gottlieb, A.B.; Assudani, D.; Bedford-Rice, K.; Coarse, J.; et al. Bimekizumab in patients with active psoriatic arthritis: Results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet 2020, 395, 427–440. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Takatsu, N.; Ootaki, K.; Nakagawa, H. Long-term safety of brodalumab in Japanese patients with plaque psoriasis: An open-label extension study. J. Dermatol. 2020, 47, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.; Papp, K.; Puig, L.; Nakagawa, H.; ERASURE Study Group; FIXTURE Study Group; et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrowietz, U.; Leonardi, C.L.; Girolomoni, G.; Toth, D.; Morita, A.; Balki, S.A.; Szepietowski, J.C.; Regnault, P.; Thurston, H.; SCULPTURE Study Group; et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE). J. Am. Acad. Dermatol. 2015, 73, 27–36.e1. [Google Scholar] [CrossRef]
- Phan, C.; Beneton, N.; Delaunay, J.; Reguiai, Z.; Boulard, C.; Fougerousse, A.C.; Cinotti, E.; Romanelli, M.; Mery-Bossard, L.; the Groupe d’Etudes Multicentriques (GEM) RESOPSO; et al. Effectiveness and Safety of Anti-interleukin-17 Therapies in Elderly Patients with Psoriasis. Acta Derm. Venereol. 2020, 100, adv00316. [Google Scholar] [CrossRef]
- Picciani, B.; Dziedzic, A.; Werneck, J.T.; Marinho, M.A.; Dick, T.; Quintanilha, N.R.; Dias, E.P. Atypical oral candidiasis in a psoriatic patient during targeted immunotherapy with an interleukin 17 inhibitor (secukinumab). BMC Oral Health 2021, 21, 292. [Google Scholar] [CrossRef] [PubMed]
- Papini, M.; Natalini, Y. Candida infections in psoriatic patients on anti-IL17 therapy: A case series. J. Dermatolog. Treat 2018, 29, 3–4. [Google Scholar] [CrossRef]
- Farah, C.S. Concurrent chronic hyperplastic candidosis and oral lichenoid lesion as adverse events of secukinumab therapy. Aust. Dent. J. 2021, 66, 340–345. [Google Scholar] [CrossRef]
- Komori, T.; Honda, T.; Endo, Y. Kaku, Y.; Otsuka, A.; Kabashima, K. Oral lichen planus associated with candidiasis during secukinumab treatment. J. Dermatol. 2017, 44, e60–e61. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, I.; Pliquett, R.U.; Kreft, B.; Sunderkötter, C. Severe candidal balanoposthitis on concurrent treatment with secukinumab and the antidiabetic agent empagliflozin (sodium-glucose cotransporter 2 inhibitor-SGLT2-inhibitor). J. Dtsch. Dermatol. Ges. 2019, 17, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.; Ferguson, M.; Rosselli, J.L. Prevention and Management of Genital Mycotic Infections in the Setting of Sodium-Glucose Cotransporter 2 Inhibitors. Ann. Pharmacother. 2021, 55, 543–548. [Google Scholar] [CrossRef]
- Manfredi, M.; Polonelli, L.; Aguirre-Urizar, J.M.; Carrozzo, M.; McCullough, M.J. Urban legends series: Oral candidosis. Oral Dis. 2013, 19, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Castellote, L.; Jiménez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, e279–e286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cuesta, C.; Sarrion-Pérez, M.G.; Bagán, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582. [Google Scholar] [CrossRef]
- Campione, E.; Gaziano, R.; Marino, D.; Orlandi, A. Fungistatic activity of all-trans retinoic acid against Aspergillus fumigatus and Candida albicans. Drug Des. Devel. Ther. 2016, 10, 1551–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majewski, S.; Janik, P.; Langner, A.; Glinska-Ferenz, M.; Swietochowska, B.; Sawicki, I. Decreased levels of vitamin A in serum of patients with psoriasis. Arch. Dermatol. Res. 1989, 280, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Picciani, B.L.; Michalski-Santos, B.; Carneiro, S.; Sampaio, A.L.; Avelleira, J.C.; Azulay, D.R.; Pinto, J.M.; Dias, E.P. Oral candidiasis in patients with psoriasis: Correlation of oral examination and cytopathological evaluation with psoriasis disease severity and treatment. J. Am. Acad. Dermatol. 2013, 68, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J. Pharmacol. Sci. 2020, 144, 52–56. [Google Scholar] [CrossRef] [PubMed]



| Author | Disease | Treatment Regimen | Frequency of Administration | Duration of Trial | # of Patients (per Treatment Group) | Dosage (per Treatment Group) | % and # of Patients with OC per Treatment Group |
|---|---|---|---|---|---|---|---|
| Papp et al., 2014 [37] | Psoriasis | Brodalumab | Every 2 weeks | 120 weeks | 148 | 210 mg | 3.38% (5/148) |
| Paul et al., 2014 [38] | Psoriasis | Secukinumab | 1/weekly to week 4, then every 4 weeks | 12 weeks | 60 61 | 300 mg 150 mg | 0% (0/60) 0% (0/61) |
| Blauvelt et al., 2015 [39] | Psoriasis | Secukinumab | 1/weekly for the 1st month and then week 8 | 12 weeks | 59 59 | 300 mg 150 mg | 1.79% (1/59) 0% (0/59) |
| Baeten et al., 2015 [40] | Ankylosing Spondylitis | Secukinumab | Every 2 weeks for the 1st month and then every 4 weeks | 52 weeks | 276 285 | 150 mg 75 mg | 0.36% (1/276) 0.7% (2/285) |
| Mease et al., 2015 [33] | Psoriatic Arthritis | Secukinumab | Every 4 weeks | 52 weeks | 295 292 | 150 mg 75 mg | 1.36% (4/295) 1.37% (4/292) |
| McInnes et al., 2015 [41] | Psoriatic Arthritis | Secukinumab | 1/weekly for the 1st month and then every 4 weeks | 52 weeks | 133 127 75 | 300 mg 150 mg 75 mg | 1.5% (2/133) 2.36% (3/127) 1.33% (1/75) |
| Nakagawa et al., 2016 [42] | Psoriasis/ Psoriatic Arthritis | Brodalumab | 1/weekly for the 1st month and then every 2 weeks until week 10 | 12 weeks | 37 37 37 | 210 mg 140 mg 70 mg | 0.9% (1/111) |
| Yamasaki et al., 2016 [43] | Psoriasis/ Psoriatic Erythroderma | Brodalumab | 1/weekly for the 1st 3 weeks and then every 2 weeks until week 52 | 52 weeks | 30 | 140 mg | 3.33% (1/30) |
| Gordon et al., 2016 [34] | Psoriasis | Ixekizumab | Every 2 or 4 weeks | 60 weeks | 3736 | 80 mg | 1.7% (63/3736) |
| Braun et al., 2017 [30] | Ankylosing Spondylitis | Secukinumab | Every 4 weeks | 104 weeks | 181 179 | 150 mg 75 mg | 0.55% (1/181) 0.56% (1/179) |
| Margo-Ortega et al., 2017 [32] | Ankylosing Spondylitis | Secukinumab | 1/weekly for the 1st 3 weeks and then every 4 weeks | 104 weeks | 106 105 | 150 mg 75 mg | 0.94% (1/106) 0.95% (1/105) |
| Glatt et al., 2017 [44] | Psoriatic Arthritis | Bimekizumab | Baseline, week 3, 6 | 20 weeks | 20 6 6 6 | 240/160/160 mg 80/40/40 mg 160/80/80 mg 560/320/320 mg | 0% (0/20) 0% (0/6) 0% (0/6) 0% (0/6) |
| Blanco et al., 2017 [45] | Rheumatoid Arthritis | Secukinumab | Every 2 weeks for the 1st month and then every 4 weeks | 52 weeks | 215 218 | 150 mg 75 mg | 0% (0/215) 0% (0/218) |
| Pavelka et al., 2017 [46] | Ankylosing Spondylitis | Secukinumab | Every 2 weeks for the 1st month and then every 4 weeks | 52 weeks | 113 110 | 300 mg 150 mg | 0.88% (1/113) 0.9% (1/110) |
| Kivitz et al., 2018 [47] | Ankylosing Spondylitis | Secukinumab | 1/weekly for the 1st month and then every 4 weeks | 104 weeks | 116 117 | 150 mg 150 mg (no load) | 1.73% (2/116) 0.85% (1/117) |
| Bissonnette et al., 2018 [35] | Psoriasis | Secukinumab | Every 4 weeks | 2 or 4 years | 168 | 300 mg | 1.19% (2/168) |
| Papp et al., 2018 [48] | Psoriasis | Bimekizumab | Every 4 weeks until week 8 | 12 weeks | 43 43 40 43 39 | 480 mg 320 mg 320/160 mg 160 mg 64 mg | 0% (0/43) 7% (3/43) 2.5% (1/40) 0% (0/43) 0% (0/39) |
| Baraliakos et al., 2019 [49] | Ankylosing Spondylitis | Secukinumab | Every 2 weeks for the 1st month and then every 4 weeks | 260 weeks | 210 64 | 150 mg 75 mg | 1.1% (3/274) |
| Thaci et al., 2019 [36] | Psoriasis | Secukinumab | Every 4 weeks until week 36 | 48 weeks | 20 | 300 mg | 5% (1/20) |
| Kishimoto et al., 2019 [25] | Ankylosing Spondylitis | Secukinumab | 1/weekly for the 1st month and then every 4 weeks | 52 weeks | 30 | 150 mg | 0% (0/30) |
| Blauvelt et al., 2020 [50] | Psoriasis | Bimekizumab | Every 4 weeks | 48 weeks | 91 111 15 | 320 mg 160 mg 64 mg | 16.5% (15/91) 11.7% (13/111) 6.7% (1/15) |
| Huang et al., 2020 [51] | Ankylosing Spondylitis | Secukinumab | 1/weekly for the 1st month and then every 4 weeks until week 48 | 52 weeks | 453 | 150 mg | 0.44% (2/453) |
| Pavelka et al., 2020 [52] | Ankylosing Spondylitis | Secukinumab | Every 2 weeks for the 1st month and then every 4 weeks | 156 weeks | 113 110 | 300 mg 150 mg | 0.45% (1/223) |
| Ritchlin et al., 2020 [53] | Psoriatic Arthritis | Bimekizumab | Every 4 weeks | 48 weeks | 80 126 | 320 mg 160 mg | 5% (4/80) 4.8% (6/126) |
| Yamaguchi et al., 2020 [54] | Psoriasis | Brodalumab | Every 2 or 4 weeks | 108 weeks | 129 | 210 mg 140 mg | 7% (9/129) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettas, E.; Savva, V.; Theofilou, V.I.; Georgaki, M.; Nikitakis, N.G. Oral Candida Infection in Psoriatic Patients Treated with IL17A Inhibitors: Report of 3 Cases and a Comprehensive Review of the Literature. Diagnostics 2022, 12, 3. https://doi.org/10.3390/diagnostics12010003
Pettas E, Savva V, Theofilou VI, Georgaki M, Nikitakis NG. Oral Candida Infection in Psoriatic Patients Treated with IL17A Inhibitors: Report of 3 Cases and a Comprehensive Review of the Literature. Diagnostics. 2022; 12(1):3. https://doi.org/10.3390/diagnostics12010003
Chicago/Turabian StylePettas, Efstathios, Vasiliki Savva, Vasileios Ionas Theofilou, Maria Georgaki, and Nikolaos G. Nikitakis. 2022. "Oral Candida Infection in Psoriatic Patients Treated with IL17A Inhibitors: Report of 3 Cases and a Comprehensive Review of the Literature" Diagnostics 12, no. 1: 3. https://doi.org/10.3390/diagnostics12010003
APA StylePettas, E., Savva, V., Theofilou, V. I., Georgaki, M., & Nikitakis, N. G. (2022). Oral Candida Infection in Psoriatic Patients Treated with IL17A Inhibitors: Report of 3 Cases and a Comprehensive Review of the Literature. Diagnostics, 12(1), 3. https://doi.org/10.3390/diagnostics12010003






