Radiomics Analysis of [18F]FDG PET/CT Thyroid Incidentalomas: How Can It Improve Patients’ Clinical Management? A Systematic Review from the Literature
Abstract
:1. Introduction
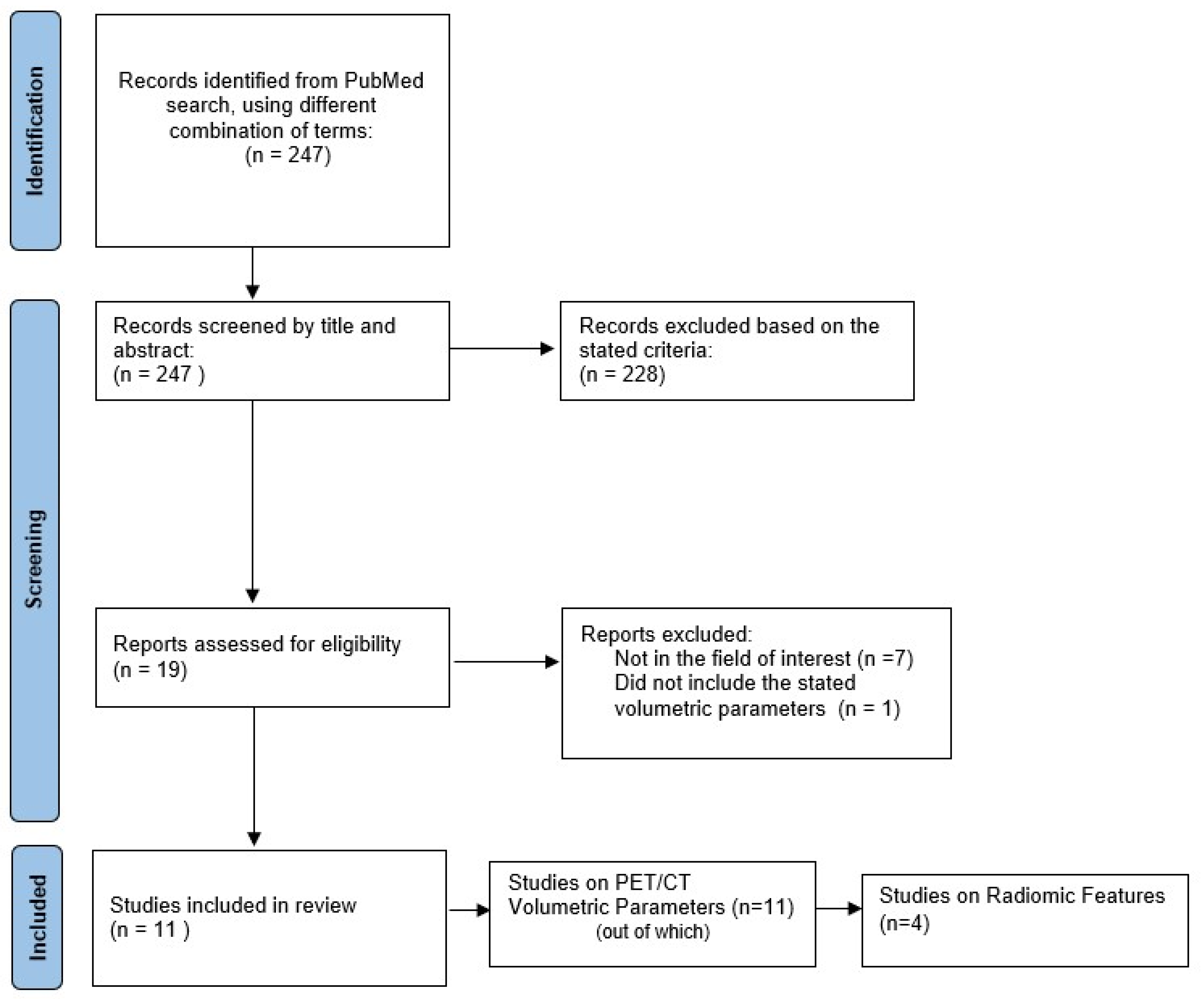
2. Materials and Methods
2.1. Literature Search
2.2. Data Synthesis
3. Results
3.1. Diagnostic Value of PET/CT Volumetric Parameters for Characterization of FDG-Avid Thyroid Incidentaloma
3.2. The Potential Value of Radiomics as a Diagnostic Tool in [18F]FDG-Avid Thyroid Incidentalomas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russ, G.; Leboulleux, S.; Leenhardt, L.; Hegedüs, L. Thyroid Incidentalomas: Epidemiology, Risk Stratification with Ultrasound and Workup. Eur. Thyroid J. 2014, 3, 154–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertagna, F.; Treglia, G.; Piccardo, A.; Giubbini, R. Diagnostic and Clinical Significance of F-18-FDG-PET/CT Thyroid Incidentalomas. J. Clin. Endocrinol. Metab. 2012, 97, 3866–3875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scappaticcio, L.; Piccardo, A.; Treglia, G.; Poller, D.N.; Trimboli, P. The Dilemma of A18F-FDG PET/CT Thyroid Incidentaloma: What We Should Expect from FNA. A Systematic Review and Meta-Analysis. Endocrine 2021, 73, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Roddy, S.; Biggans, T.; Raofi, A.K.; Kanodia, A.; Sudarshan, T.; Guntur Ramkumar, P. Prevalence of Incidental Thyroid Ma-lignancy on Routine 18F-Fluorodeoxyglucose PET-CT in a Large Teaching Hospital. Eur. J. Hybrid Imaging 2020, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.A.; Bozin, M.; Gorelik, A.; Hofman, M.S.; Hicks, R.J.; Skandarajah, A. 18F-FDG-Avid Thyroid Incidentalomas: The Importance of Contextual Interpretation. J. Nucl. Med. 2018, 59, 749–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larg, M.-I.; Apostu, D.; Peștean, C.; Gabora, K.; Bădulescu, I.C.; Olariu, E.; Piciu, D. Evaluation of Malignancy Risk in 18F-FDG PET/CT Thyroid Incidentalomas. Diagnostics 2019, 9, E92. [Google Scholar] [CrossRef] [Green Version]
- Hagenimana, N.; Dallaire, J.; Vallée, É.; Belzile, M. Thyroid Incidentalomas on 18FDG-PET/CT: A Metabolico-Pathological Correlation. J. Otolaryngol. Head Neck Surg. 2017, 46, 22. [Google Scholar] [CrossRef] [Green Version]
- Pagano, L.; Samà, M.T.; Morani, F.; Prodam, F.; Rudoni, M.; Boldorini, R.; Valente, G.; Marzullo, P.; Baldelli, R.; Appetecchia, M.; et al. Thyroid Incidentaloma Identified by 18F-Fluorodeoxyglucose Positron Emission Tomography with CT (FDG-PET/CT): Clinical and Pathological Relevance. Clin. Endocrinol. 2011, 75, 528–534. [Google Scholar] [CrossRef]
- Soelberg, K.K.; Bonnema, S.J.; Brix, T.H.; Hegedüs, L. Risk of Malignancy in Thyroid Incidentalomas Detected by 18F-Fluorodeoxyglucose Positron Emission Tomography: A Systematic Review. Thyroid 2012, 22, 918–925. [Google Scholar] [CrossRef]
- Treglia, G.; Giovanella, L.; Bertagna, F.; Di Franco, D.; Salvatori, M. Prevalence and Risk of Malignancy of Thyroid Inciden-talomas Detected by 18F-Fluorodeoxyglucose Positron-Emission Tomography. Thyroid 2013, 23, 124–126. [Google Scholar] [CrossRef]
- Presotto, L.; Bettinardi, V.; De Bernardi, E.; Belli, M.L.; Cattaneo, G.M.; Broggi, S.; Fiorino, C. PET Textural Features Stability and Pattern Discrimination Power for Radiomics Analysis: An “Ad-Hoc” Phantoms Study. Phys. Med. 2018, 50, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tixier, F. Radiomics. In Advances in PET/CT Imaging: A Technologists’ Guide; European Association of Nuclear Medicine (EANM): Vienna, Austria, 2021; pp. 122–131. [Google Scholar]
- Sollini, M.; Cozzi, L.; Pepe, G.; Antunovic, L.; Lania, A.; Di Tommaso, L.; Magnoni, P.; Erba, P.A.; Kirienko, M. [18F]FDG-PET/CT Texture Analysis in Thyroid Incidentalomas: Preliminary Results. Eur. J. Hybrid Imaging 2017, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlhac, F.; Soussan, M.; Maisonobe, J.-A.; Garcia, C.A.; Vanderlinden, B.; Buvat, I. Tumor Texture Analysis in 18F-FDG PET: Relationships between Texture Parameters, Histogram Indices, Standardized Uptake Values, Metabolic Volumes, and Total Lesion Glycolysis. J. Nucl. Med. 2014, 55, 414–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollini, M.; Cozzi, L.; Chiti, A.; Kirienko, M. Texture Analysis and Machine Learning to Characterize Suspected Thyroid Nodules and Differentiated Thyroid Cancer: Where Do We Stand? Eur. J. Radiol. 2018, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McBee, M.P.; Awan, O.A.; Colucci, A.T.; Ghobadi, C.W.; Kadom, N.; Kansagra, A.P.; Tridandapani, S.; Auffermann, W.F. Deep Learning in Radiology. Acad. Radiol. 2018, 25, 1472–1480. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A.; DEPRESsion Screening Data (DEPRESSD) Collaboration. Esti-mating the Sample Mean and Standard Deviation from Commonly Reported Quantiles in Meta-Analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef] [Green Version]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, S.-J.; Kim, H.; Jeon, Y.K.; Kim, S.S.; Kim, I.J.; Kim, Y.K. Diagnostic Value of Metabolic Tumor Volume Assessed by 18F-FDG PET/CT Added to SUVmax for Characterization of Thyroid 18F-FDG Incidentaloma. Nucl. Med. Commun. 2013, 34, 868–876. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, S.-J.; Kim, K.; Kim, H.; Kim, S.J.; Kim, W.J.; Jeon, Y.K.; Kim, S.S.; Kim, Y.K.; Kim, I.J. High Metabolic Tumor Volume and Total Lesion Glycolysis Are Associated with Lateral Lymph Node Metastasis in Patients with Incidentally Detected Thyroid Carcinoma. Ann. Nucl. Med. 2015, 29, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Chang, S. Predictive Value of Intratumoral Heterogeneity of F-18 FDG Uptake for Characterization of Thyroid Nodules According to Bethesda Categories of Fine Needle Aspiration Biopsy Results. Endocrine 2015, 50, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yuan, Z.; Yuan, Z.; Yang, C.; Zhang, J.; Shou, Y.; Zhang, W.; Ping, Z.; Gao, X.; Liu, S. Diagnostic Value of Volume-Based Fluorine-18-Fluorodeoxyglucose PET/CT Parameters for Characterizing Thyroid Incidentaloma. Korean J. Radiol. 2018, 19, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Yuan, Z.; Yang, C.; Zhang, J.; Liu, C.; Sun, J.; Ye, X. Role of Multi-Modality Functional Imaging in Differentiation between Benign and Malignant Thyroid 18F-Fluorodeoxyglucose Incidentaloma. Clin. Transl. Oncol. 2019, 21, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, P.; Bourhis, D.; Roudaut, N.; Crouzeix, G.; Alavi, Z.; Schick, U.; Robin, P.; Kerlan, V.; Salaun, P.-Y.; Abgral, R. Di-agnostic Value of FDG PET-CT Quantitative Parameters and Deauville-Like 5 Point-Scale in Predicting Malignancy of Focal Thyroid Incidentaloma. Front. Med. 2019, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Erdoğan, M.; Korkmaz, H.; Torus, B.; Avcı, M.; Boylubay, Ş.M.; Çiriş, M.; Yıldız, M.; Şengül, S.S. The Role of Metabolic Vol-umetric Parameters in Predicting Malignancy in Incidental Thyroid Nodules Detected in 18F-FDG PET/CT Scans. Mol. Imaging Radionucl. Ther. 2021, 30, 86–92. [Google Scholar] [CrossRef]
- Ceriani, L.; Milan, L.; Virili, C.; Cascione, L.; Paone, G.; Trimboli, P.; Giovanella, L. Radiomics Analysis of [18F]-Fluorodeoxyglucose-Avid Thyroid Incidentalomas Improves Risk Stratification and Selection for Clinical Assessment. Thyroid 2021, 31, 88–95. [Google Scholar] [CrossRef]
- Aksu, A.; Karahan Şen, N.P.; Acar, E.; Çapa Kaya, G. Evaluating Focal 18F-FDG Uptake in Thyroid Gland with Radiomics. Nucl. Med. Mol. Imaging 2020, 54, 241–248. [Google Scholar] [CrossRef]
- Giovanella, L.; Milan, L.; Piccardo, A.; Bottoni, G.; Cuzzocrea, M.; Paone, G.; Ceriani, L. Radiomics Analysis Improves 18FDG PET/CT-Based Risk Stratification of Cytologically Indeterminate Thyroid Nodules. Endocrine 2022, 75, 202–210. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Hoang, J.K.; Langer, J.E.; Middleton, W.D.; Wu, C.C.; Hammers, L.W.; Cronan, J.J.; Tessler, F.N.; Grant, E.G.; Berland, L.L. Managing Incidental Thyroid Nodules Detected on Imaging: White Paper of the ACR Incidental Thyroid Findings Committee. J. Am. Coll. Radiol. 2015, 12, 143–150. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubaki, F.; Kurata, S.; Tani, J.; Sumi, A.; Fujimoto, K.; Abe, T. Clinical Significance of Patterns of Increased [18F]-FDG Uptake in the Thyroid Gland: A Pictorial Review. Jpn. J. Radiol. 2018, 36, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Fonti, R.; Pellegrino, S.; Catalano, L.; Pane, F.; Del Vecchio, S.; Pace, L. Visual and Volumetric Parameters by 18F-FDG-PET/CT: A Head to Head Comparison for the Prediction of Outcome in Patients with Multiple Myeloma. Ann. Hematol. 2020, 99, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Yoon, Y.H.; Yoon, Y.H.; Kim, S.M.; Koo, B.S. Characteristics of Primary Papillary Thyroid Carcinoma with False-Negative Findings on Initial (18)F-FDG PET/CT. Ann. Surg. Oncol. 2011, 18, 1306–1311. [Google Scholar] [CrossRef]
- Dibble, E.H.; Alvarez, A.C.L.; Truong, M.-T.; Mercier, G.; Cook, E.F.; Subramaniam, R.M. 18F-FDG Metabolic Tumor Volume and Total Glycolytic Activity of Oral Cavity and Oropharyngeal Squamous Cell Cancer: Adding Value to Clinical Staging. J. Nucl. Med. 2012, 53, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Nakajo, M.; Jinguji, M.; Shinaji, T.; Tani, A.; Nakabeppu, Y.; Nakajo, M.; Nakajo, A.; Natsugoe, S.; Yoshiura, T. 18F-FDG-PET/CT Features of Primary Tumours for Predicting the Risk of Recurrence in Thyroid Cancer after Total Thyroid-ectomy: Potential Usefulness of Combination of the SUV-Related, Volumetric, and Heterogeneous Texture Parameters. Br. J. Radiol. 2019, 92, 20180620. [Google Scholar] [CrossRef]
- Shie, P.; Cardarelli, R.; Sprawls, K.; Fulda, K.G.; Taur, A. Systematic Review: Prevalence of Malignant Incidental Thyroid Nodules Identified on Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography. Nucl. Med. Commun. 2009, 30, 742–748. [Google Scholar] [CrossRef]
- Qu, N.; Zhang, L.; Lu, Z.; Wei, W.; Zhang, Y.; Ji, Q. Risk of Malignancy in Focal Thyroid Lesions Identified by 18F-Fluorodeoxyglucose Positron Emission Tomography or Positron Emission Tomography/Computed Tomography: Evidence from a Large Series of Studies. Tumor Biol. 2014, 35, 6139–6147. [Google Scholar] [CrossRef]
- Bertagna, F.; Treglia, G.; Piccardo, A.; Giovannini, E.; Bosio, G.; Biasiotto, G.; Bahij, E.K.; Maroldi, R.; Giubbini, R. F18-FDG-PET/CT Thyroid Incidentalomas: A Wide Retrospective Analysis in Three Italian Centres on the Significance of Focal Uptake and SUV Value. Endocrine 2013, 43, 678–685. [Google Scholar] [CrossRef]
- Moon, S.H.; Hyun, S.H.; Choi, J.Y. Prognostic Significance of Volume-Based PET Parameters in Cancer Patients. Korean J. Radiol. 2013, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in Medical Imaging—“How-to” Guide and Critical Reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.-P.; Samala, R.K.; Hadjiiski, L.M. CAD and AI for Breast Cancer-Recent Development and Challenges. Br. J. Radiol. 2020, 93, 20190580. [Google Scholar] [CrossRef] [PubMed]
- Ziyad, S.R.; Radha, V.; Vayyapuri, T. Overview of Computer Aided Detection and Computer Aided Diagnosis Systems for Lung Nodule Detection in Computed Tomography. Curr. Med. Imaging Rev. 2020, 16, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kikushima, S.; Hanawa, N.; Kotake, F. Diagnostic Performance of Bone Scintigraphy Analyzed by Three Artificial Neural Network Systems. Ann. Nucl. Med. 2015, 29, 125–131. [Google Scholar] [CrossRef]
- Apostolopoulos, I.D.; Apostolopoulos, D.I.; Spyridonidis, T.I.; Papathanasiou, N.D.; Panayiotakis, G.S. Multi-Input Deep Learning Approach for Cardiovascular Disease Diagnosis Using Myocardial Perfusion Imaging and Clinical Data. Phys. Med. 2021, 84, 168–177. [Google Scholar] [CrossRef]

| Reference | Malignancy Rate |
Mean SUVmax (Mean ± SD) |
Mean MTV (Mean ± SD) |
Mean TLG (Mean ± SD) |
|---|---|---|---|---|
| Kim B.H. et al. [20] | 20.9% | 8.27 ± 11.09 (AUC: 0.601) | 0.27 ± 0.39 (AUC: 0.613) | NA |
| Kim B.H. et al. [21] | 21.7% | 10.52 ± 15.28 (AUC: 0.716) | 4.18 ± 7.68 (AUC: 0.839) | 49.33 ± 599.39 (AUC: 0.815) |
| Kim S.J. et al. [22] | NA | 5.96 ± 2.61 (AUC: 0.586) | 5.76 ± 2.0 (AUC: 0.566) | 16.01 ± 6.9 (AUC: 0.562) |
| Shi et al. [23] | 64.6% | 11.30 ± 8.40 (AUC: 0.866) | 2.7 ± 4.0 (AUC: 0.872) | 30.0 ± 75.5 (AUC: 0.895) |
| Shi et al. [24] | 59.8% | 11.90 ± 8.90 (AUC: 0.872) | 3.06 ± 4.30 (AUC: 0.895) | 35.2 ± 83.0 (AUC: 0.916) |
| Thuillier et al. [25] | NA | 9.27 ± 3.28 (AUC: 0.550) | 5.46 ± 12.18 (AUC: 0.530) | 22.66 ± 38.41 (AUC: 0.610) |
| Erdogan et al. [26] | 9.8% | 5.33 ± 2.93 (AUC: 0.827) | 5.76 ± 9.78 (AUC: 0.668) | 21.14 ± 37.51 (AUC: 0.726) |
| Sollini et al. [13] | 36% | 9 ± 8.70 (AUC: 0.600) | 27 ± 94.5 (AUC: 0.660) | 309.5 ± 1881.7 (AUC: 0.660) |
| Ceriani et al. [27] | 28% | 10.91 ± 2.04 (AUC: 0.652) | 12.60 ± 7.46 (AUC: 0.733) | 45.33 ± 13.84 (AUC: 0.756) |
| Aksu et al. [28] | 44.7% | 16.11 ± 38.99 (AUC: 0.758) | NA | 107.59 ± 213.16 (AUC: 0.822) |
| PET/CT Parameter | Mean SUVmax (Mean ± SD) | Mean MTV (Mean ± SD) | Mean TLG (Mean ± SD) |
|---|---|---|---|
| Obtained Pooled Value | 9.85 ± 3.09 | 7.42 ± 8.08 | 70.82 ± 93.62 |
| p-value | p = 0.994 | p = 1 | p = 1 |
| Reference | No. Patients/Lesions | ROI Segmentation | Software | No. Radiomics Features | Model Construction | Components of Predictive Model | Validation Method | AUC |
|---|---|---|---|---|---|---|---|---|
| Sollini et al. [13] | 50 | Fixed threshold (SUVmax > 40%) | LifeX package | 43 | NA | NA | NA | NA |
| Ceriani et al. [27] | 107 nodules (104 patients) | Fixed threshold | PyRadiomics Version 2.2.0 | 107 | Univariate Logistic Regression of Dichotomized Data Logistic Stepwise Regression Function | SUVmax TLG Shape_Sphericity | 1000-resampled bootstrapping CV | 0.830 |
| Aksu et al. [28] | 60 (42 train set, 18 test set) | Fixed threshold (SUVmax > 40%) | LifeX package | 46 | RF SVM DT NB k nearest neighbour Logistic regression for binary risk classification | SUVmax GLRLMRLNU | Tenfold CV + EV | 0.849 |
| Giovanella et al. [29] | 78 | Fixed threshold algorithm (mean SUV of the contralateral lobe) | PyRadiomics Version 2.2.0 | 107 | LASSO (with tenfold CV) | Shape_Sphericity GLCM_autocorrelation | 1000-resampled bootstrapping CV | 0.733 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherghe, M.; Lazar, A.M.; Mutuleanu, M.-D.; Stanciu, A.E.; Martin, S. Radiomics Analysis of [18F]FDG PET/CT Thyroid Incidentalomas: How Can It Improve Patients’ Clinical Management? A Systematic Review from the Literature. Diagnostics 2022, 12, 471. https://doi.org/10.3390/diagnostics12020471
Gherghe M, Lazar AM, Mutuleanu M-D, Stanciu AE, Martin S. Radiomics Analysis of [18F]FDG PET/CT Thyroid Incidentalomas: How Can It Improve Patients’ Clinical Management? A Systematic Review from the Literature. Diagnostics. 2022; 12(2):471. https://doi.org/10.3390/diagnostics12020471
Chicago/Turabian StyleGherghe, Mirela, Alexandra Maria Lazar, Mario-Demian Mutuleanu, Adina Elena Stanciu, and Sorina Martin. 2022. "Radiomics Analysis of [18F]FDG PET/CT Thyroid Incidentalomas: How Can It Improve Patients’ Clinical Management? A Systematic Review from the Literature" Diagnostics 12, no. 2: 471. https://doi.org/10.3390/diagnostics12020471
APA StyleGherghe, M., Lazar, A. M., Mutuleanu, M.-D., Stanciu, A. E., & Martin, S. (2022). Radiomics Analysis of [18F]FDG PET/CT Thyroid Incidentalomas: How Can It Improve Patients’ Clinical Management? A Systematic Review from the Literature. Diagnostics, 12(2), 471. https://doi.org/10.3390/diagnostics12020471






