Application of Mass Multivariate Analysis on Neuroimaging Data Sets for Precision Diagnostics of Depression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Image Acquisition
2.3. FMRI Task
2.4. MRI Data Analysis
2.4.1. Structural Data Analysis—Voxel-Based Morphometry
2.4.2. Task-Related Functional Data Analysis
2.4.3. Resting State Data Analysis
2.5. Mass Multivariate Analysis
2.5.1. Defining the Regions of Interest and Individual Multivariate Features
2.5.2. Statistical Analysis and Model Estimation
2.5.3. Statistical Analysis and Model Testing
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Mass Multivariate Analysis Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zachar, P.; Stoyanov, D.S.; Aragona, M.; Jablensky, A. (Eds.) Alternative Perspectives on Psychiatric Validation. In Alternative Perspectives Psychiatric on Validation; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Stoyanov, D.; Maes, M.H. How to Construct Neuroscience-Informed Psychiatric Classification? Towards Nomothetic Networks Psychiatry. World J. Psychiatry 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Guze, S.B. Nature of Psychiatric Illness: Why Psychiatry Is a Branch of Medicine. Compr. Psychiatry 1978, 19, 295–307. [Google Scholar] [CrossRef]
- Jollans, L.; Whelan, R. Neuromarkers for Mental Disorders: Harnessing Population Neuroscience. Front. Psychiatry 2018, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Yahata, N.; Kasai, K.; Kawato, M. Computational Neuroscience Approach to Biomarkers and Treatments for Mental Disorders. Psychiatry Clin. Neurosci. 2017, 71, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.H.; Costafreda, S.G. Neuroimaging-Based Biomarkers in Psychiatry: Clinical Opportunities of a Paradigm Shift. Can. J. Psychiatry 2013, 58, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, D.S. Psychiatry and Neurolaw an Essay on the Mind-Brain Problem and Legal Proof. Balk. J. Philos. 2018, 10, 27–36. [Google Scholar] [CrossRef]
- Ahmed, A.O.; Buckley, P.F.; Hanna, M. Neuroimaging Schizophrenia: A Picture Is Worth a Thousand Words, but Is It Saying Anything Important? Curr. Psychiatry Rep. 2013, 15, 345. [Google Scholar] [CrossRef]
- Kandilarova, S.; Stoyanov, D.; Sirakov, N.; Maes, M.; Specht, K. Reduced Grey Matter Volume in Frontal and Temporal Areas in Depression: Contributions from Voxel-Based Morphometry Study. Acta Neuropsychiatr. 2019, 31, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Kandilarova, S.; Stoyanov, D.; Kostianev, S.; Specht, K. Altered Resting State Effective Connectivity of Anterior Insula in Depression. Front. Psychiatry 2018, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, D.; Kandilarova, S.; Borgwardt, S.; Stieglitz, R.D.; Hugdahl, K.; Kostianev, S. Psychopathology Assessment Methods Revisited: On Translational Cross-Validation of Clinical Self-Evaluation Scale and FMRI. Front. Psychiatry 2018, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Serra-Blasco, M.; Radua, J.; Soriano-Mas, C.; Gómez-Benlloch, A.; Porta-Casteràs, D.; Carulla-Roig, M.; Albajes-Eizagirre, A.; Arnone, D.; Klauser, P.; Canales-Rodríguez, E.J.; et al. Structural Brain Correlates in Major Depression, Anxiety Disorders and Post-Traumatic Stress Disorder: A Voxel-Based Morphometry Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 129, 269–281. [Google Scholar] [CrossRef]
- Krynicki, C.R.; Upthegrove, R.; Deakin, J.F.W.; Barnes, T.R.E. The Relationship between Negative Symptoms and Depression in Schizophrenia: A Systematic Review. Acta Psychiatr. Scand. 2018, 137, 380–390. [Google Scholar] [CrossRef]
- Stoyanov, D.; Kandilarova, S.; Arabadzhiev, Z.; Paunova, R.; Schmidt, A.; Borgwardt, S. Cross-Validation of Paranoid-Depressive Scale and Functional MRI: New Paradigm for Neuroscience Informed Clinical Psychopathology. Front. Psychiatry 2019, 10, 711. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, D.; Kandilarova, S.; Paunova, R.; Barranco Garcia, J.; Latypova, A.; Kherif, F. Cross-Validation of Functional MRI and Paranoid-Depressive Scale: Results From Multivariate Analysis. Front. Psychiatry 2019, 10, 869. [Google Scholar] [CrossRef]
- Kherif, F.; Poline, J.B.; Flandin, G.; Benali, H.; Simon, O.; Dehaene, S.; Worsley, K.J. Multivariate Model Specification for FMRI Data. Neuroimage 2002, 16, 1068–1083. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Suzuki, M.; Kherif, F.; Takahashi, T.; Zhou, S.Y.; Nakamura, K.; Matsui, M.; Sumiyoshi, T.; Seto, H.; Kurachi, M. Multivariate Voxel-Based Morphometry Successfully Differentiates Schizophrenia Patients from Healthy Controls. Neuroimage 2007, 34, 235–242. [Google Scholar] [CrossRef]
- Worsley, K.J.; Poline, J.B.; Friston, K.J.; Evans, A.C. Characterizing the Response of PET and FMRI Data Using Multivariate Linear Models. Neuroimage 1997, 6, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, D.; Kandilarova, S.; Aryutova, K.; Paunova, R.; Todeva-Radneva, A.; Latypova, A.; Kherif, F. Multivariate Analysis of Structural and Functional Neuroimaging Can Inform Psychiatric Differential Diagnosis. Diagnostics 2021, 11, 19. [Google Scholar] [CrossRef]
- Schrantee, A.; Ferguson, B.; Stoffers, D.; Booij, J.; Rombouts, S.; Reneman, L. Effects of Dexamphetamine-Induced Dopamine Release on Resting-State Network Connectivity in Recreational Amphetamine Users and Healthy Controls. Brain Imaging Behav. 2016, 10, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol. Psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Graziano, M.S.A. The Temporoparietal Junction and Awareness. Neurosci. Conscious. 2018, 2018, niy005. [Google Scholar] [CrossRef] [Green Version]
- Hoff, P. The Kraepelinian Tradition. Dialogues Clin. Neurosci. 2015, 17, 31. [Google Scholar] [CrossRef]
- Upthegrove, R.; Marwaha, S.; Birchwood, M. Depression and Schizophrenia: Cause, Consequence, or Trans-Diagnostic Issue? Schizophr. Bull. 2017, 43, 240–244. [Google Scholar] [CrossRef] [Green Version]
- The Mini-International Neuropsychiatric Interview (M.I.N.I): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10.—PsycNET. Available online: https://psycnet.apa.org/record/1998-03251-004 (accessed on 10 December 2021).
- Busner, J.; Targum, S.D. The Clinical Global Impressions Scale: Applying a Research Tool in Clinical Practice. Psychiatry 2007, 4, 28. [Google Scholar]
- Montgomery, S.A.; Asberg, M. A New Depression Scale Designed to Be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Aryutova, K.; Paunova, R.; Kandilarova, S.; Todeva-Radneva, A.; Stoyanov, D. Implications from Translational Cross-Validation of Clinical Assessment Tools for Diagnosis and Treatment in Psychiatry. World J. Psychiatry 2021, 11, 169. [Google Scholar] [CrossRef]
- Gyger, L.; Ramponi, C.; Mall, J.F.; Swierkocz-Lenart, K.; Stoyanov, D.; Lutti, A.; von Gunten, A.; Kherif, F.; Draganski, B. Temporal Trajectory of Brain Tissue Property Changes Induced by Electroconvulsive Therapy. Neuroimage 2021, 232, 117895. [Google Scholar] [CrossRef]
- Dobrushina, O.R.; Arina, G.A.; Dobrynina, L.A.; Novikova, E.S.; Gubanova, M.V.; Belopasova, A.V.; Vorobeva, V.P.; Suslina, A.D.; Pechenkova, E.V.; Perepelkina, O.S.; et al. Sensory Integration in Interoception: Interplay between Top-down and Bottom-up Processing. Cortex 2021, 144, 185–197. [Google Scholar] [CrossRef]
- Leminen, A.; Verwoert, M.; Moisala, M.; Salmela, V.; Wikman, P.; Alho, K. Modulation of Brain Activity by Selective Attention to Audiovisual Dialogues. Front. Neurosci. 2020, 14, 436. [Google Scholar] [CrossRef]
- Bora, E.; Fornito, A.; Radua, J.; Walterfang, M.; Seal, M.; Wood, S.J.; Yücel, M.; Velakoulis, D.; Pantelis, C. Neuroanatomical Abnormalities in Schizophrenia: A Multimodal Voxelwise Meta-Analysis and Meta-Regression Analysis. Schizophr. Res. 2011, 127, 46–57. [Google Scholar] [CrossRef]
- Honea, R.; Crow, T.J.; Passingham, D.; Mackay, C.E. Regional Deficits in Brain Volume in Schizophrenia: A Meta-Analysis of Voxel-Based Morphometry Studies. Am. J. Psychiatry 2005, 162, 2233–2245. [Google Scholar] [CrossRef]
- Klaus, C. Das Problem Der “Nosologischen Einheit” in Der Psychiatrie. Nervenarzt 1959, 202, 487. [Google Scholar] [CrossRef]
- Li, X.B.; Wang, L.B.; Xiong, Y.B.; Bo, Q.J.; He, F.; Li, F.; Hou, W.P.; Wen, Y.J.; Wang, X.Q.; Yang, N.B.; et al. Altered Resting-State Functional Connectivity of the Insula in Individuals with Clinical High-Risk and Patients with First-Episode Schizophrenia. Psychiatry Res. 2019, 282, 112608. [Google Scholar] [CrossRef]
- Shi, W.X. The Auditory Cortex in Schizophrenia. Biol. Psychiatry 2007, 61, 829. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Wood, S.J.; Yung, A.R.; Soulsby, B.; McGorry, P.D.; Suzuki, M.; Kawasaki, Y.; Phillips, L.J.; Velakoulis, D.; Pantelis, C. Progressive Gray Matter Reduction of the Superior Temporal Gyrus during Transition to Psychosis. Arch. Gen. Psychiatry 2009, 66, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.H.; Chang, W.C.; Hsu, J.W.; Huang, K.L.; Tu, P.C.; Su, T.P.; Li, C.T.; Lin, W.C.; Bai, Y.M. Correlation of Proinflammatory Cytokines Levels and Reduced Gray Matter Volumes between Patients with Bipolar Disorder and Unipolar Depression. J. Affect. Disord. 2019, 245, 8–15. [Google Scholar] [CrossRef]
- Rössler, J.; Rössler, W.; Seifritz, E.; Unterrassner, L.; Wyss, T.; Haker, H.; Wotruba, D. Dopamine-Induced Dysconnectivity between Salience Network and Auditory Cortex in Subjects with Psychotic-like Experiences: A Randomized Double-Blind Placebo-Controlled Study. Schizophr. Bull. 2020, 46, 732–740. [Google Scholar] [CrossRef]
- Bonner-Jackson, A.; Haut, K.; Csernansky, J.G.; Barch, D.M. The Influence of Encoding Strategy on Episodic Memory and Cortical Activity in Schizophrenia. Biol. Psychiatry 2005, 58, 47. [Google Scholar] [CrossRef] [Green Version]
- Horn, H.; Jann, K.; Federspiel, A.; Walther, S.; Wiest, R.; Müller, T.; Strik, W. Semantic Network Disconnection in Formal Thought Disorder. Neuropsychobiology 2012, 66, 14–23. [Google Scholar] [CrossRef]
- Buchy, L.; Ad-Dab’bagh, Y.; Lepage, C.; Malla, A.; Joober, R.; Evans, A.; Lepage, M. Symptom Attribution in First Episode Psychosis: A Cortical Thickness Study. Psychiatry Res. 2012, 203, 6–13. [Google Scholar] [CrossRef]
- Burke, L.; Androutsos, C.; Jogia, J.; Byrne, P.; Frangou, S. The Maudsley Early Onset Schizophrenia Study: The Effect of Age of Onset and Illness Duration on Fronto-Parietal Gray Matter. Eur. Psychiatry 2008, 23, 233–236. [Google Scholar] [CrossRef]
- Cheng, W.; Rolls, E.T.; Qiu, J.; Liu, W.; Tang, Y.; Huang, C.C.; Wang, X.F.; Zhang, J.; Lin, W.; Zheng, L.; et al. Medial Reward and Lateral Non-Reward Orbitofrontal Cortex Circuits Change in Opposite Directions in Depression. Brain 2016, 139, 3296–3309. [Google Scholar] [CrossRef]
- Rolls, E.T. A Non-Reward Attractor Theory of Depression. Neurosci. Biobehav. Rev. 2016, 68, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Jauhar, S.; McCutcheon, R.; Borgan, F.; Veronese, M.; Nour, M.; Pepper, F.; Rogdaki, M.; Stone, J.; Egerton, A.; Turkheimer, F.; et al. The Relationship between Cortical Glutamate and Striatal Dopamine in First-Episode Psychosis: A Cross-Sectional Multimodal PET and Magnetic Resonance Spectroscopy Imaging Study. Lancet Psychiatry 2018, 5, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Kringelbach, M.L. The Human Orbitofrontal Cortex: Linking Reward to Hedonic Experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Fettes, P.; Schulze, L.; Downar, J. Cortico-Striatal-Thalamic Loop Circuits of the Orbitofrontal Cortex: Promising Therapeutic Targets in Psychiatric Illness. Front. Syst. Neurosci. 2017, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, A.; Bodnar, M.; Malla, A.K.; Joober, R.; Lepage, M. The Structural Neural Substrates of Persistent Negative Symptoms in First-Episode of Non-Affective Psychosis: A Voxel-Based Morphometry Study. Front. Psychiatry 2012, 3, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spalletta, G.; Tomaiuolo, F.; Marino, V.; Bonaviri, G.; Trequattrini, A.; Caltagirone, C. Chronic Schizophrenia as a Brain Misconnection Syndrome: A White Matter Voxel-Based Morphometry Study. Schizophr. Res. 2003, 64, 15–23. [Google Scholar] [CrossRef]
- Walther, S.; Lefebvre, S.; Conring, F.; Gangl, N.; Nadesalingam, N.; Alexaki, D.; Wüthrich, F.; Rüter, M.; Viher, P.V.; Federspiel, A.; et al. Limbic Links to Paranoia: Increased Resting-State Functional Connectivity between Amygdala, Hippocampus and Orbitofrontal Cortex in Schizophrenia Patients with Paranoia. Eur. Arch. Psychiatry Clin. Neurosci. 2021. [Google Scholar] [CrossRef]
- Sindermann, L.; Redlich, R.; Opel, N.; Böhnlein, J.; Dannlowski, U.; Leehr, E.J. Systematic Transdiagnostic Review of Magnetic-Resonance Imaging Results: Depression, Anxiety Disorders and Their Co-Occurrence. J. Psychiatr. Res. 2021, 142, 226–239. [Google Scholar] [CrossRef]
- Smith, M.L.; Milner, B. Right Hippocampal Impairment in the Recall of Spatial Location: Encoding Deficit or Rapid Forgetting? Neuropsychologia 1989, 27, 71–81. [Google Scholar] [CrossRef]
- Squire, L.R. Memory and the Hippocampus: A Synthesis from Findings with Rats, Monkeys, and Humans. Psychol. Rev. 1992, 99, 195–231. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.M.; Schultz, A.P.; Huijbers, W.; Van Dijk, K.R.A.; Hedden, T.; Sperling, R.A. The Parahippocampal Gyrus Links the Default-Mode Cortical Network with the Medial Temporal Lobe Memory System. Hum. Brain Mapp. 2014, 35, 1061–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razi, K.; Greene, K.P.; Sakuma, M.; Ge, S.; Kushner, M.; DeLisi, L.E. Reduction of the Parahippocampal Gyrus and the Hippocampus in Patients with Chronic Schizophrenia. Br. J. Psychiatry 1999, 174, 512–519. [Google Scholar] [CrossRef]
- Diederen, K.M.J.; Neggers, S.F.W.; Daalman, K.; Blom, J.D.; Goekoop, R.; Kahn, R.S.; Sommer, I.E.C. Deactivation of the Parahippocampal Gyrus Preceding Auditory Hallucinations in Schizophrenia. Am. J. Psychiatry 2010, 167, 427–435. [Google Scholar] [CrossRef]
- Vigo, D.; Thornicroft, G.; Atun, R. Estimating the True Global Burden of Mental Illness. Lancet Psychiatry 2016, 3, 171–178. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Management of Physical Health Conditions in Adults with Severe Mental Disorders: WHO Guidelines; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Rolls, E.T. The Affective and Cognitive Processing of Touch, Oral Texture, and Temperature in the Brain. Neurosci. Biobehav. Rev. 2010, 34, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.M.; Matheson, S.L.; Laurens, K.R.; Carr, V.J.; Green, M.J. Systematic Meta-Analysis of Insula Volume in Schizophrenia. Biol. Psychiatry 2012, 72, 775–784. [Google Scholar] [CrossRef]
- Ebisch, S.J.H.; Salone, A.; Ferri, F.; De Berardis, D.; Romani, G.L.; Ferro, F.M.; Gallese, V. Out of Touch with Reality? Social Perception in First-Episode Schizophrenia. Soc. Cogn. Affect. Neurosci. 2013, 8, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Ebisch, S.J.H.; Mantini, D.; Northoff, G.; Salone, A.; De Berardis, D.; Ferri, F.; Ferro, F.M.; Di Giannantonio, M.; Romani, G.L.; Gallese, V. Altered Brain Long-Range Functional Interactions Underlying the Link between Aberrant Self-Experience and Self-Other Relationship in First-Episode Schizophrenia. Schizophr. Bull. 2014, 40, 1072–1082. [Google Scholar] [CrossRef] [Green Version]
- Petrova, I.; Mateev, H.; Alexandrov, A.; Vladimirov, G.; Bankova, A.; Vassilev, D.; Paskaleva, I.; Gotcheva, N.; Gelev, V. TCT-385 The Role of Neutrophil Gelatinase-Associated Lipocalin (NGAL) for Evaluation of Kidney Function in Patients Undergoing Coronary Angiography. J. Am. Coll. Cardiol. 2016, 68, B157. [Google Scholar] [CrossRef]
- Petrova, I.; Bogov, I.; Alexandrov, A.; Vladimirov, G.; Avramov, D.; Mateev, H.; Paskaleva, I.; Georgiev, B.; Gotcheva, N. Neutrophil Gelatinase-Associated Lipocalin (NGAL) Predict Higher Risk of Serious Renal Dysfunction in Patients with CI-AKI. Eur. Heart J. 2020, 41 (Suppl. 2), ehaa946-2556. [Google Scholar] [CrossRef]
- Stojanov, D.; Korf, J.; De Jonge, P.; Popov, G. The Possibility of Evidence-Based Psychiatry: Depression as a Case. Clin. Epigenet. 2011, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, D. The Reification of Diagnosis in Psychiatry. Neurotox. Res. 2020, 37, 772–774. [Google Scholar] [CrossRef]
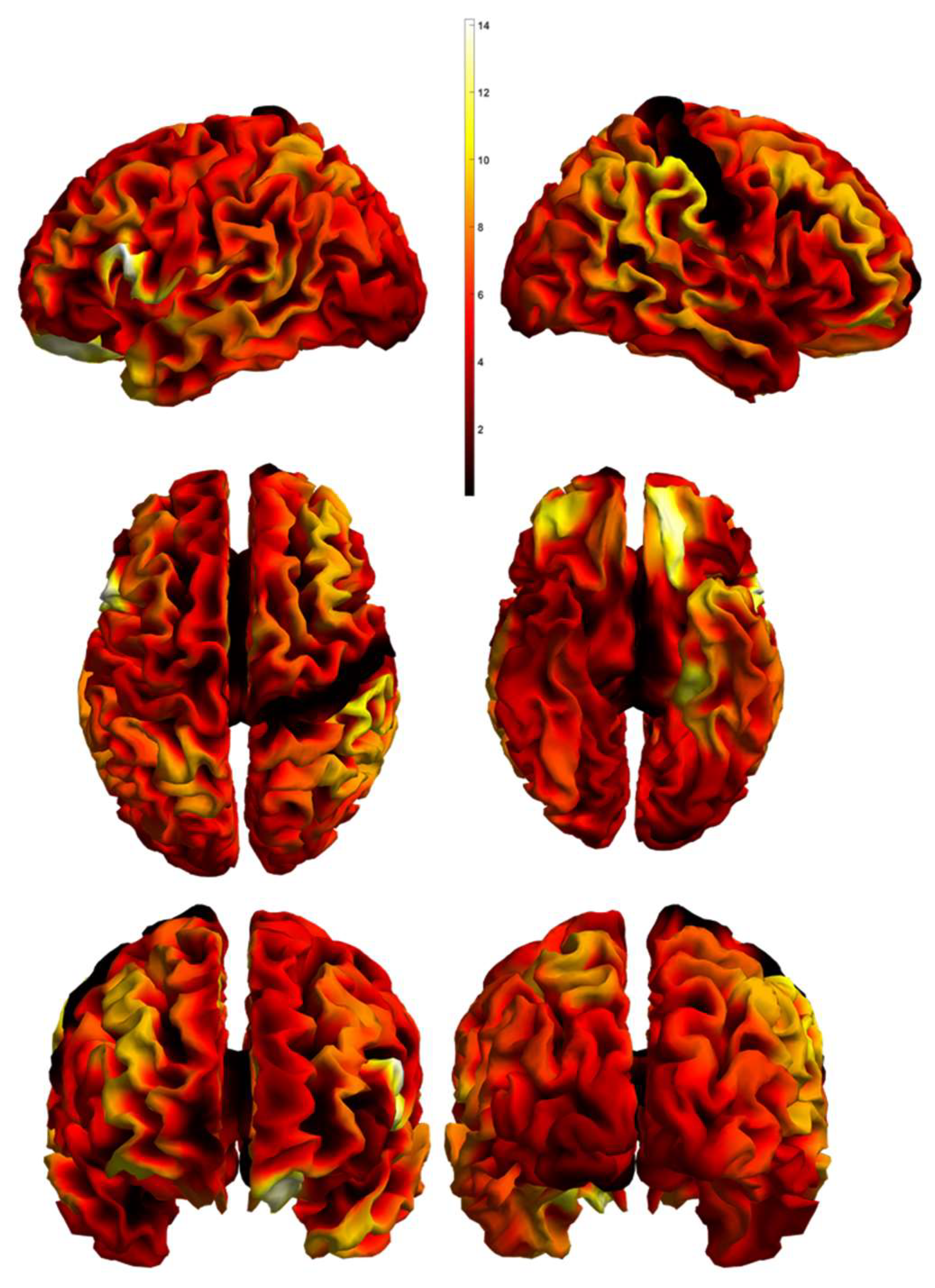

| Schizophrenia Patients (n = 19) | Depressed Patients (n = 25) | Statistical Significance | |
|---|---|---|---|
| Age (mean ± SD) | 39.3 ± 14.8 | 44.2 ± 12.1 | 0.231 a |
| Sex (M/F) | 9/10 | 9/16 | 0.542 b |
| Education (years) | 13.5 ± 2.8 | 14.1 ± 3.5 | 0.548 a |
| Age at onset (years) | 27.1 ± 9.1 | 33.8 ± 12.4 | 0.139 a |
| Illness duration (months) | 142.8 ± 121.6 | 121.8 ± 84.5 | 0.505 a |
| Episode duration (weeks) | 15.4 ± 14.1 | 11.9 ± 10.4 | 0.403 a |
| MADRS score | - | 30.5 ± 6.0 | - |
| PANSS score | 58.5 ± 13.6 | - | - |
| CGI-S score | 4.14 ± 0.66 | 4.18 ± 0.75 | 0.891 a |
| Regions | p-Value | Chi Statistics | Canonical Vector | |||
|---|---|---|---|---|---|---|
| CV1 | CV2 | CV3 | ||||
| 1 | Left planum polare | 0.0008 | 16.7299 | 0.6319 | 0.7613 | −0.1453 |
| 2 | Left opercular part of the inferior frontal gyrus | 0.0022 | 14.5809 | −0.4026 | −0.8402 | 0.3633 |
| 3 | Left medial orbital gyrus | 0.0039 | 13.3569 | −0.2124 | −0.9564 | 0.2005 |
| 4 | Left posterior insula | 0.0077 | 11.9015 | −0.3233 | −0.9339 | 0.1530 |
| 5 | Left parahippocampal gyrus | 0.0092 | 11.5353 | −0.3229 | −0.9040 | 0.2800 |
| 6 | Right lateral orbital gyrus | 0.0121 | 10.9252 | 0.2551 | 0.8937 | −0.3690 |
| 7 | Right supramarginal gyrus | 0.0134 | 10.7141 | 0.0964 | −0.6804 | 0.7265 |
| 8 | Right anterior orbital gyrus | 0.0169 | 10.2093 | 0.1428 | −0.7306 | 0.6677 |
| 9 | Right supplementary motor cortex | 0.0197 | 9.8746 | −0.0688 | −0.7482 | 0.6599 |
| 10 | Left supplementary motor cortex | 0.0203 | 9.8031 | −0.0859 | 0.7082 | −0.7007 |
| 11 | Left superior temporal gyrus | 0.0208 | 9.7535 | −0.2775 | −0.8334 | 0.4779 |
| 12 | Left temporal pole | 0.0211 | 9.7250 | 0.3911 | 0.8486 | −0.3563 |
| 13 | Left anterior orbital gyrus | 0.0238 | 9.4530 | −0.1262 | 0.7232 | −0.6790 |
| 14 | Right middle frontal gyrus | 0.0243 | 9.4139 | 0.3836 | −0.4898 | 0.7829 |
| 15 | Left Amygdala | 0.0263 | 9.2404 | −0.2334 | −0.9612 | 0.1474 |
| 16 | Left frontal operculum | 0.0267 | 9.2025 | 0.2479 | 0.9685 | 0.0226 |
| 17 | Right angular gyrus | 0.0274 | 9.1444 | 0.0716 | −0.7811 | 0.6202 |
| 18 | Right middle temporal gyrus | 0.0277 | 9.1216 | 0.2095 | −0.9312 | 0.2982 |
| 19 | Left superior frontal gyrus medial segment | 0.0285 | 9.0578 | 0.0298 | −0.7775 | 0.6282 |
| 20 | Left superior parietal lobule | 0.0305 | 8.9095 | 0.4082 | −0.4644 | 0.7859 |
| 21 | Left Hippocampus | 0.0307 | 8.9001 | −0.2239 | −0.9630 | 0.1502 |
| 22 | Right superior temporal gyrus | 0.0332 | 8.7205 | 0.1746 | −0.8503 | 0.4964 |
| 23 | Right posterior insula | 0.0341 | 8.6677 | −0.0524 | −0.9878 | 0.1469 |
| 24 | Left central operculum | 0.0348 | 8.6178 | −0.2178 | −0.9620 | 0.1649 |
| 25 | Left fusiform gyrus | 0.0350 | 8.6045 | −0.4066 | −0.8550 | 0.3219 |
| 26 | Left middle cingulate gyrus | 0.0351 | 8.5999 | −0.1485 | −0.9698 | 0.1935 |
| 27 | Left medial frontal cortex | 0.0366 | 8.5070 | 0.0482 | −0.9819 | 0.1833 |
| 28 | Right parietal operculum | 0.0378 | 8.4347 | −0.0461 | −0.9966 | 0.0686 |
| 29 | Right middle cingulate gyrus | 0.0388 | 8.3761 | −0.2403 | −0.9456 | 0.2196 |
| 30 | Left middle frontal gyrus | 0.0395 | 8.3418 | 0.2894 | −0.5013 | 0.8154 |
| 31 | Left gyrus rectus | 0.0403 | 8.2928 | −0.3306 | −0.8902 | 0.3133 |
| 32 | Left entorhinal area | 0.0406 | 8.2803 | −0.7626 | −0.5786 | 0.2894 |
| 33 | Left posterior cingulate gyrus | 0.0441 | 8.0939 | −0.2762 | −0.9312 | 0.2378 |
| 34 | Left middle temporal gyrus | 0.0451 | 8.0429 | −0.0043 | −0.9256 | 0.3785 |
| 35 | Right superior frontal gyrus | 0.0453 | 8.0345 | 0.6047 | −0.3385 | 0.7209 |
| 36 | Left anterior cingulate gyrus | 0.0471 | 7.9465 | −0.1187 | −0.8082 | 0.5768 |
| 37 | Right anterior cingulate gyrus | 0.0493 | 7.8452 | −0.0797 | −0.6843 | 0.7249 |
| 38 | Right medial orbital gyrus | 0.0501 | 7.8105 | −0.0785 | −0.9286 | 0.3626 |
| 39 | Left Basal Forebrain | 0.0508 | 7.7787 | −0.6022 | −0.7983 | 0.0046 |
| 40 | Right gyrus rectus | 0.0513 | 7.7589 | −0.4299 | −0.8586 | 0.2792 |
| 41 | Right superior frontal gyrus medial segment | 0.0521 | 7.7212 | 0.1002 | −0.7965 | 0.5963 |
| 42 | Right CO central operculum | 0.0526 | 7.7016 | −0.0305 | −0.9853 | 0.1684 |
| 43 | Right medial frontal cortex | 0.0544 | 7.6275 | 0.0393 | 0.9953 | 0.0889 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paunova, R.; Kandilarova, S.; Todeva-Radneva, A.; Latypova, A.; Kherif, F.; Stoyanov, D. Application of Mass Multivariate Analysis on Neuroimaging Data Sets for Precision Diagnostics of Depression. Diagnostics 2022, 12, 469. https://doi.org/10.3390/diagnostics12020469
Paunova R, Kandilarova S, Todeva-Radneva A, Latypova A, Kherif F, Stoyanov D. Application of Mass Multivariate Analysis on Neuroimaging Data Sets for Precision Diagnostics of Depression. Diagnostics. 2022; 12(2):469. https://doi.org/10.3390/diagnostics12020469
Chicago/Turabian StylePaunova, Rositsa, Sevdalina Kandilarova, Anna Todeva-Radneva, Adeliya Latypova, Ferath Kherif, and Drozdstoy Stoyanov. 2022. "Application of Mass Multivariate Analysis on Neuroimaging Data Sets for Precision Diagnostics of Depression" Diagnostics 12, no. 2: 469. https://doi.org/10.3390/diagnostics12020469
APA StylePaunova, R., Kandilarova, S., Todeva-Radneva, A., Latypova, A., Kherif, F., & Stoyanov, D. (2022). Application of Mass Multivariate Analysis on Neuroimaging Data Sets for Precision Diagnostics of Depression. Diagnostics, 12(2), 469. https://doi.org/10.3390/diagnostics12020469







