Effect of Subclinical Hypothyroidism on the Association between Hemoglobin A1c and Reduced Renal Function: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Laboratory Measurments
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Study Population by Subclinical Hypothyroidism (SCH) Status
3.2. Correlations between Annual Change in Estimated Glomerular Filtration Rate (ΔeGFR) and Hemoglobin A1c (HbA1c) in Relation to Diabetes
3.3. Correlations between Annual ΔeGFR and HbA1c by SCH Status among Participants without Diabetes
3.4. Correlations between Annual ΔeGFR and HbA1c by SCH Status
3.5. Correlations between Annual ΔeGFR and HbA1c among Non-SCH Status by Using Age-Matched Model
3.6. Correlations between ΔeGFR and HbA1c by SCH Status among Participants Who Were Not Taking Glucose-Lowerung Medication
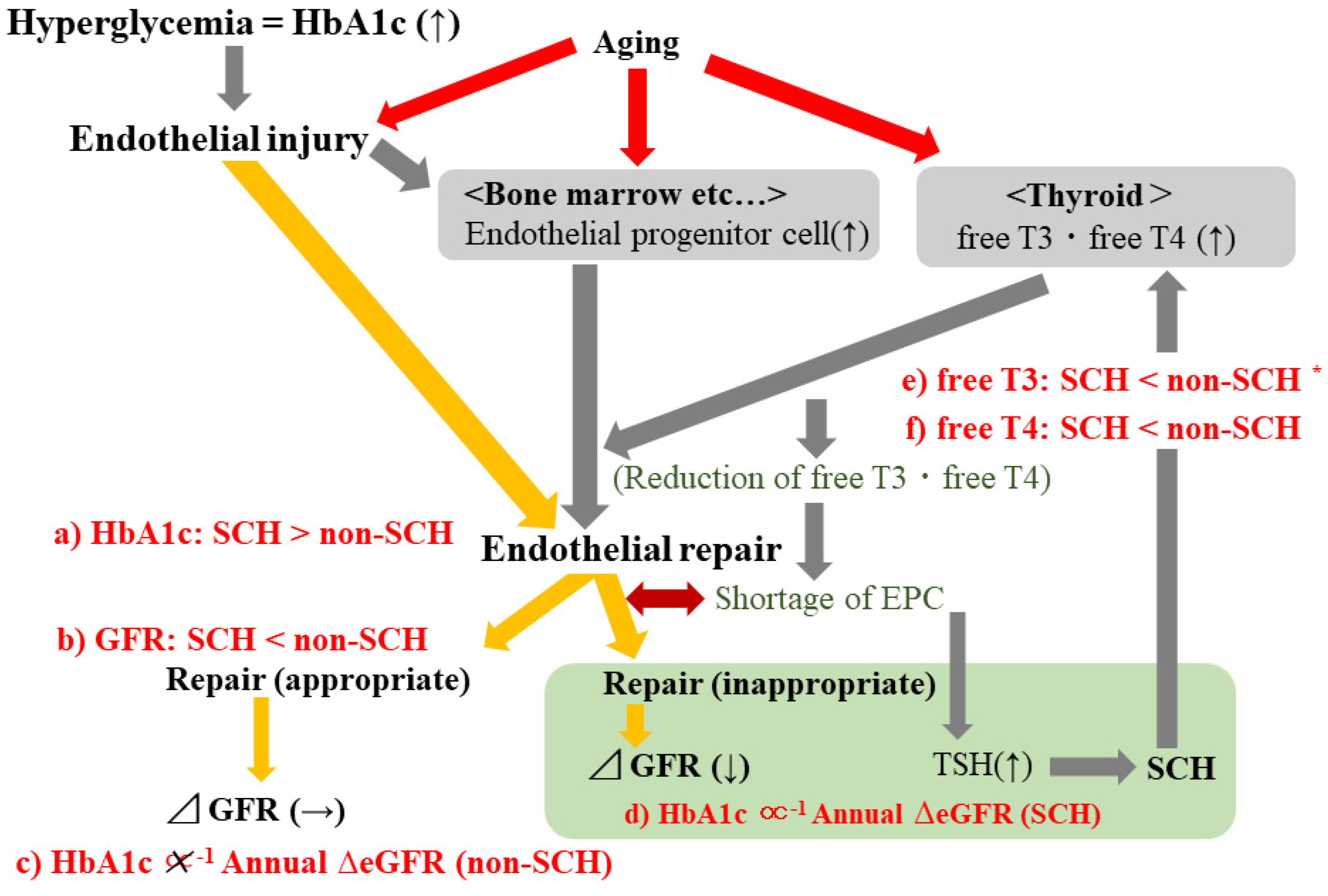
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, M.; Yang, C.-B.; Gao, L.; Zhao, J.-J. Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction (Review). Exp. Ther. Med. 2015, 9, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacek, A.; Rotkovská, D.; BartoniČková, A.; Pospísiil, M. Effect of hyperthyroidism on haemopoietic stem cell kinetics in mice. Cell Prolif. 1978, 11, 487–496. [Google Scholar] [CrossRef]
- Daub, K.; Langer, H.; Seizer, P.; Stellos, K.; May, A.E.; Goyal, P.; Bigalke, B.; Schönberger, T.; Geisler, T.; Siegel-Axel, D.; et al. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J. 2006, 20, 2559–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stellos, K.; Langer, H.; Daub, K.; Schoenberger, T.; Gauss, A.; Geisler, T.; Bigalke, B.; Mueller, I.; Schumm, M.; Schaefer, I.; et al. Platelet-derived stromal cell–derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 2008, 117, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Villacorte, M.; Delmarcelle, A.-S.; Lernoux, M.; Bouquet, M.; Lemoine, P.; Bolsée, J.; Umans, L.; Lopes, S.C.D.S.; van der Smissen, P.; Sasaki, T.; et al. Thyroid follicle development requires Smad1/5- and endothelial cell-dependent basement membrane assembly. Development 2016, 143, 1958–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degosserie, J.; Heymans, C.; Spourquet, C.; Halbout, M.; D’Auria, L.; van der Smissen, P.; Vertommen, D.; Courtoy, P.J.; Tyteca, D.; Pierreux, C.E. Extracellular vesicles from endothelial progenitor cells promote thyroid follicle formation. J. Extracell. Vesicles 2018, 7, 1487250. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Yamanashi, H.; Noguchi, Y.; Koyamatsu, J.; Nagayoshi, M.; Kiyoura, K.; Fukui, S.; Tamai, M.; Kawashiri, S.-Y.; Kondo, H.; et al. Association between chronic kidney disease and carotid intima-media thickness in relation to circulating CD34-positive cell count among community-dwelling elderly Japanese men. Atherosclerosis 2019, 283, 85–91. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.-H.; Hickner, R.C. Endothelial dysfunction: Is there a hyperglycemia-induced imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef] [Green Version]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Kang, S.H.; Jung, D.J.; Choi, E.W.; Cho, K.H.; Park, J.W.; Do, J.Y. HbA1c levels are associated with chronic kidney disease in a non-diabetic adult population: A nationwide survey (KNHANES 2011–2013). PLoS ONE 2015, 10, e0145827. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nabeshima-Kimura, Y.; Kawashiri, S.-Y.; Noguchi, Y.; Nagata, Y.; Maeda, T.; Hayashida, N. Anti-thyroid peroxidase antibody and thyroid cysts among the general Japanese population: A cross-sectional study. Environ. Health Prev. Med. 2020, 25, 7. [Google Scholar] [CrossRef] [Green Version]
- LSI Medience Corporation Information. Rinsyokensa Jugyo. 2017. 17-04 C-01. Available online: http://www.medience.co.jp/information/02.html (accessed on 12 January 2022).
- Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; Kasuga, M.; et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetol. Int. 2010, 1, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Imai, E.; Horio, M.; Watanabe, T.; Iseki, K.; Yamagata, K.; Hara, S.; Ura, N.; Kiyohara, Y.; Moriyama, T.; Ando, Y.; et al. Prevalence of chronic kidney disease in the Japanese general population. Clin. Exp. Nephrol. 2009, 13, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Jaseem, T.; Ambalavanan, J.; Hegde, A. Homeostatic model assessment-insulin resistance (HOMA-IR 2) in mild subclinical hypothyroid subjects. Indian J. Clin. Biochem. 2018, 33, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Huang, X. Association of subclinical thyroid dysfunction with chronic kidney disease: A systematic review and meta-analysis. Endocr. Res. 2019, 45, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-B.; Li, H.-B.; Zhu, X.-R.; Song, H.-L.; Zhao, Y.-Y.; Yang, J.-K. Subclinical hypothyroidism and the risk of chronic kidney disease in T2D subjects: A case control and dose-response analysis. Medicine 2017, 96, e6519. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; He, X.; Xia, X.; Li, Y.; Shi, X.; Shan, Z.; Teng, W. Subclinical hypothyroidism and type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0135233. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kawashiri, S.-Y.; Kiyoura, K.; Nobusue, K.; Yamanashi, H.; Nagata, Y.; Maeda, T. Gamma-glutamyl transpeptidase (γ-GTP) has an ambivalent association with hypertension and atherosclerosis among elderly Japanese men: A cross-sectional study. Environ. Health. Prev. Med. 2019, 24, 69. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Sato, S.; Koyamatsu, J.; Yamanashi, H.; Nagayoshi, M.; Kadota, K.; Kawashiri, S.-Y.; Inoue, K.; Nagata, Y.; Maeda, T. Platelets and circulating CD34-positive cells as an indicator of the activity of the vicious cycle between hypertension and endothelial dysfunction in elderly Japanese men. Atherosclerosis 2017, 259, 26–31. [Google Scholar] [CrossRef]
- Krenning, G.; Dankers, P.Y.; Drouven, J.W.; Waanders, F.; Franssen, C.F.; van Luyn, M.J.; Harmsen, M.C.; Popa, E.R. Endothelial progenitor cell dysfunction in patients with progressive chronic kidney disease. Am. J. Physiol. Renal. Physiol. 2009, 296, F1314–F1322. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Kawashiri, S.-Y.; Kiyoura, K.; Koyamatsu, J.; Fukui, S.; Tamai, M.; Nobusue, K.; Yamanashi, H.; Nagata, Y.; Maeda, T. Circulating CD34+ cells and active arterial wall thickening among elderly men: A prospective study. Sci. Rep. 2020, 10, 4656. [Google Scholar] [CrossRef] [PubMed]
- Garvin, K.; Feschuk, C.; Sharp, J.G.; Berger, A. Does the number or quality of pluripotent bone marrow stem cells decrease with age? Clin. Orthop. Relat. Res. 2007, 465, 202–207. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of vascular aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Gesing, A.; Lewiński, A.; Karbownik-Lewińska, M. The thyroid gland and the process of aging; what is new? Thyroid Res. 2012, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Kawashiri, S.-Y.; Noguchi, Y.; Nagata, Y.; Maeda, T.; Hayashida, N. Association between thyroid cysts and hypertension by atherosclerosis status: A cross-sectional study. Sci. Rep. 2021, 11, 13922. [Google Scholar] [CrossRef]
- Calsolaro, V.; Niccolai, F.; Pasqualetti, G.; Calabrese, A.M.; Polini, A.; Okoye, C.; Magno, S.; Caraccio, N.; Monzani, F. Overt and subclinical hypothyroidism in the elderly: When to treat? Front. Endocrinol. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed]
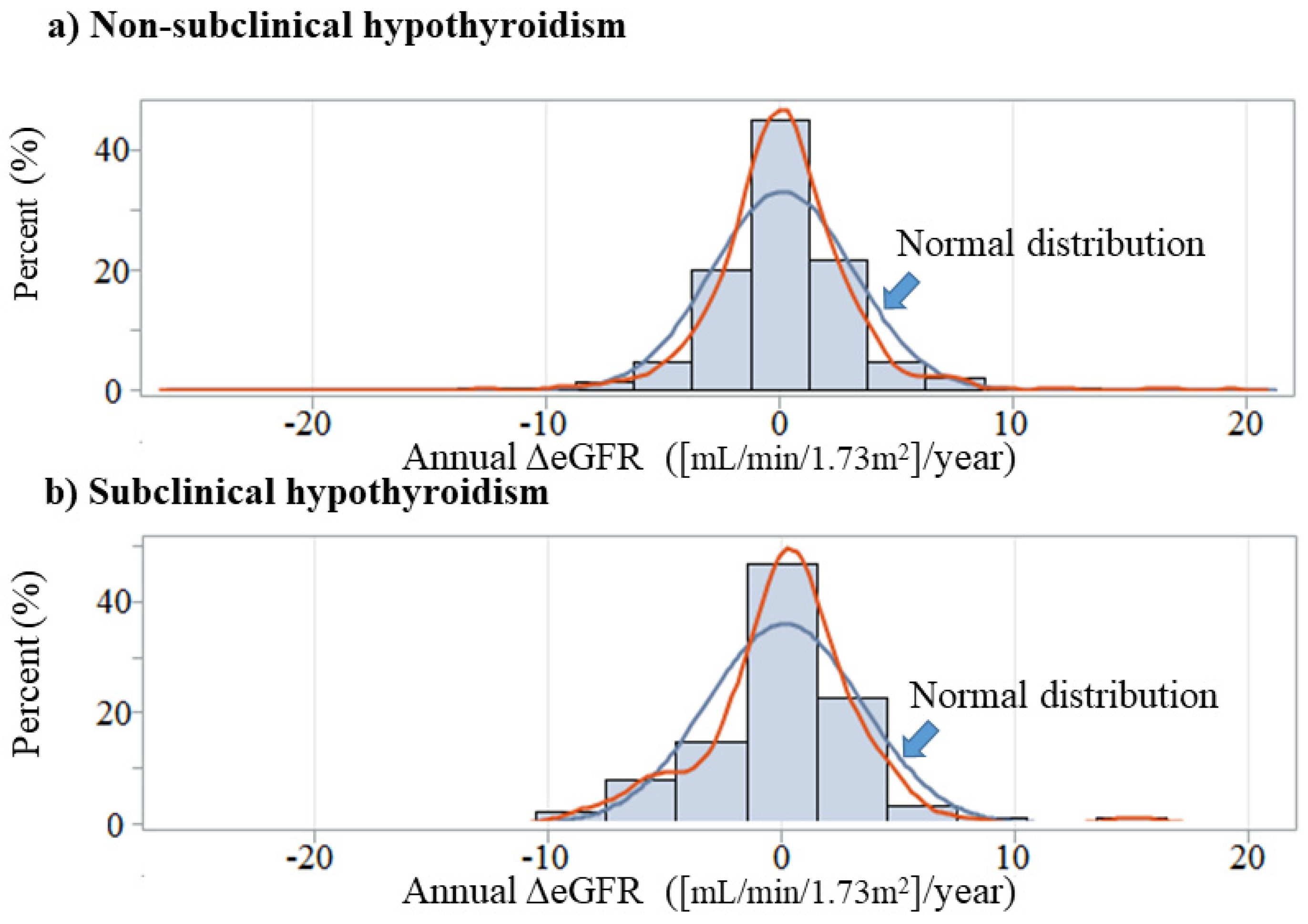

| Subclinical Hypothyroidism | p | ||
|---|---|---|---|
| (−) | (+) | ||
| No of participants | 1492 | 88 | |
| Men, % | 36.7 | 39.8 | 0.566 |
| Age, year | 60.8 ± 8.9 | 62.6 ± 8.9 | 0.074 |
| free T3, (2.1–4.1) pg/mL | 3.2 ± 0.3 | 3.1 ± 0.3 | 0.084 |
| free T4, (1.0–1.7) ng/dL | 1.3 ± 0.2 | 1.2 ± 0.2 | <0.001 |
| TSH, (0.39–4.01) μIU/mL | 1.7 ± 0.8 | 5.7 ± 1.7 | <0.001 |
| Diabetes, % | 8.2 | 20.5 | <0.001 |
| Glucose lowering medication use, % | 5.4 | 12.5 | <0.001 |
| HbA1c, % | 5.6 ± 0.6 | 6.0 ± 1.0 | <0.001 |
| Serum creatinine, mg/dL | 0.75 ± 0.33 | 0.82 ± 0.32 | 0.069 |
| eGFR, mL/min/1.76m2 | 71.4 ± 12.9 | 67.0 ± 15.0 | 0.002 |
| Simple Correlation Analysis | Multiple Linear Regression Analysis | |||
|---|---|---|---|---|
| r (p) | Β | β | p | |
| Total | ||||
| No of participants | 1580 | |||
| Sex (Men) | −0.07 (p = 0.008) | −0.51 | −0.08 | 0.020 |
| Age | −0.03 (p = 0.320) | −0.02 | −0.06 | 0.028 |
| free T3 | −0.01 (p = 0.646) | 0.30 | 0.03 | 0.240 |
| TSH | −0.002 (p = 0.943) | −0.05 | −0.02 | 0.396 |
| eGFR | 0.13 (p < 0.001) | −0.04 | −0.16 | <0.001 |
| HbA1c | −0.02 (p = 0.417) | −0.01 | −0.002 | 0.938 |
| Non-diabetes | ||||
| No of participants | 1440 | |||
| Sex (Men) | −0.07 (p = 0.013) | −0.45 | −0.07 | 0.080 |
| Age | −0.03 (p = 0.196) | −0.02 | −0.07 | 0.012 |
| free T3 | −0.01 (p = 0.695) | 0.33 | 0.04 | 0.202 |
| TSH | 0.02 (p = 0.528) | 0.002 | 0.001 | 0.979 |
| eGFR | −0.13 (p < 0.001) | −0.04 | −0.16 | <0.001 |
| HbA1c | 0.001 (p = 0.970) | −0.01 | −0.001 | 0.982 |
| Diabetes | ||||
| No of participants | 140 | |||
| Sex (Men) | −0.11 (p = 0.208) | −1.11 | −0.13 | 0.123 |
| Age | 0.09 (p = 0.284) | 0.02 | 0.03 | 0.710 |
| free T3 | −0.03 (p = 0.721) | 0.18 | 0.01 | 0.870 |
| TSH | −0.08 (p = 0.358) | −0.33 | −0.14 | 0.113 |
| eGFR | −0.17 (p = 0.048) | −0.06 | −0.21 | 0.021 |
| HbA1c | 0.01 (p = 0.884) | 0.26 | 0.06 | 0.530 |
| Simple Correlation Analysis | Multiple Linear Regression Analysis | |||
|---|---|---|---|---|
| r (p) | Β | β | p | |
| Non-Subclinical Hypothyroidism | ||||
| No of participants | 1370 | |||
| Sex (Men) | −0.08 (p = 0.004) | −0.51 | −0.09 | 0.003 |
| Age | −0.05 (p = 0.094) | −0.03 | −0.09 | 0.003 |
| free T3 | −0.01 (p = 0.718) | 0.38 | 0.04 | 0.152 |
| TSH | 0.02 (p = 0.574) | −0.02 | −0.01 | 0.857 |
| eGFR | −0.14 (p < 0.001) | −0.04 | −0.17 | <0.001 |
| HbA1c | 0.01 (p = 0.759) | 0.08 | 0.01 | 0.736 |
| Subclinical Hypothyroidism | ||||
| No of participants | 70 | |||
| Sex (Men) | 0.13 (p = 0.266) | 1.72 | 0.26 | 0.055 |
| Age | 0.14 (p = 0.239) | 0.10 | 0.27 | 0.038 |
| free T3 | 0.001 (p = 0.996) | −1.24 | −0.11 | 0.386 |
| TSH | −0.12 (p = 0.338) | −0.29 | −0.14 | 0.248 |
| eGFR | 0.07 (p = 0.557) | 0.03 | 0.11 | 0.416 |
| HbA1c | −0.17 (p = 0.167) | −2.58 | −0.25 | 0.049 |
| Simple Correlation Analysis | Multiple Linear Regression Analysis | |||
|---|---|---|---|---|
| r (p) | Β | β | p | |
| Non-Subclinical Hypothyroidism | ||||
| No of participants | 1492 | |||
| Sex (Men) | −0.08 (p = 0.002) | −0.57 | −0.09 | <0.001 |
| Age | −0.04 (p = 0.161) | −0.03 | −0.08 | 0.003 |
| free T3 | −0.01 (p = 0.592) | 0.34 | 0.04 | 0.194 |
| TSH | 0.01 (p = 0.670) | −0.05 | −0.01 | 0.641 |
| eGFR | −0.15 (p < 0.001) | −0.04 | −0.18 | <0.001 |
| HbA1c | 0.01 (p = 0.804) | 0.16 | 0.03 | 0.250 |
| Subclinical Hypothyroidism | ||||
| No of participants | 88 | |||
| Sex (Men) | 0.06 (p = 0.580) | 0.55 | 0.08 | 0.472 |
| Age | 0.15 (p = 0.163) | 0.07 | 0.19 | 0.095 |
| free T3 | 0.03 (p = 0.809) | −0.53 | −0.05 | 0.674 |
| TSH | −0.10 (p = 0.360) | −0.10 | −0.05 | 0.625 |
| eGFR | 0.09 (p = 0.429) | 0.04 | 0.16 | 0.166 |
| HbA1c | −0.26 (p = 0.014) | −0.86 | −0.26 | 0.014 |
| Simple Correlation Analysis | Multiple Linear Regression Analysis | |||
|---|---|---|---|---|
| r (p) | Β | β | p | |
| Non-Subclinical Hypothyroidism | ||||
| No of participants | 176 | |||
| Sex (Men) | −0.11 (p = 0.138) | −0.83 | −0.14 | 0.107 |
| Age | 0.03 (p = 0.690) | 0.002 | 0.01 | 0.937 |
| free T3 | −0.09 (p = 0.239) | −0.26 | −0.03 | 0.745 |
| TSH | 0.004 (p = 0.959) | −0.09 | −0.02 | 0.774 |
| eGFR | −0.14 (p = 0.080) | −0.03 | −0.13 | 0.112 |
| HbA1c | 0.11 (p = 0.173) | 0.55 | 0.13 | 0.105 |
| Simple Correlation Analysis | Multiple Linear Regression Analysis | |||
|---|---|---|---|---|
| r (p) | Β | β | p | |
| Non-Subclinical Hypothyroidism | ||||
| No of participants | 1411 | |||
| Sex (Men) | −0.08 (p = 0.002) | −0.54 | −0.09 | 0.002 |
| Age | −0.04 (p = 0.115) | −0.03 | −0.08 | 0.003 |
| free T3 | −0.02 (p = 0.566) | 0.30 | 0.03 | 0.267 |
| TSH | 0.01 (p = 0.746) | −0.04 | −0.11 | 0.690 |
| eGFR | −0.13 (p < 0.001) | −0.04 | −0.16 | <0.001 |
| HbA1c | 0.02 (p = 0.424) | 0.19 | 0.03 | 0.266 |
| Subclinical Hypothyroidism | ||||
| No of participants | 77 | |||
| Sex (Men) | 0.17 (p = 0.139) | 1.63 | 0.24 | 0.049 |
| Age | 0.19 (p = 0.101) | 0.09 | 0.24 | 0.050 |
| free T3 | 0.04 (p = 0.760) | −1.52 | −0.14 | 0.261 |
| TSH | −0.07 (p = 0.550) | −0.33 | −0.16 | 0.172 |
| eGFR | −0.001 (p = 0.993) | 0.03 | 0.12 | 0.331 |
| HbA1c | −0.28 (p = 0.015) | −1.14 | −0.32 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, Y.; Kawashiri, S.-Y.; Noguchi, Y.; Nakamichi, S.; Nagata, Y.; Maeda, T.; Hayashida, N. Effect of Subclinical Hypothyroidism on the Association between Hemoglobin A1c and Reduced Renal Function: A Prospective Study. Diagnostics 2022, 12, 462. https://doi.org/10.3390/diagnostics12020462
Shimizu Y, Kawashiri S-Y, Noguchi Y, Nakamichi S, Nagata Y, Maeda T, Hayashida N. Effect of Subclinical Hypothyroidism on the Association between Hemoglobin A1c and Reduced Renal Function: A Prospective Study. Diagnostics. 2022; 12(2):462. https://doi.org/10.3390/diagnostics12020462
Chicago/Turabian StyleShimizu, Yuji, Shin-Ya Kawashiri, Yuko Noguchi, Seiko Nakamichi, Yasuhiro Nagata, Takahiro Maeda, and Naomi Hayashida. 2022. "Effect of Subclinical Hypothyroidism on the Association between Hemoglobin A1c and Reduced Renal Function: A Prospective Study" Diagnostics 12, no. 2: 462. https://doi.org/10.3390/diagnostics12020462
APA StyleShimizu, Y., Kawashiri, S.-Y., Noguchi, Y., Nakamichi, S., Nagata, Y., Maeda, T., & Hayashida, N. (2022). Effect of Subclinical Hypothyroidism on the Association between Hemoglobin A1c and Reduced Renal Function: A Prospective Study. Diagnostics, 12(2), 462. https://doi.org/10.3390/diagnostics12020462





