Urinary Activin A: A Novel Biomarker for Human Acute Kidney Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Patients
2.2. Sample and Data Collection
2.3. ELISA
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Patients
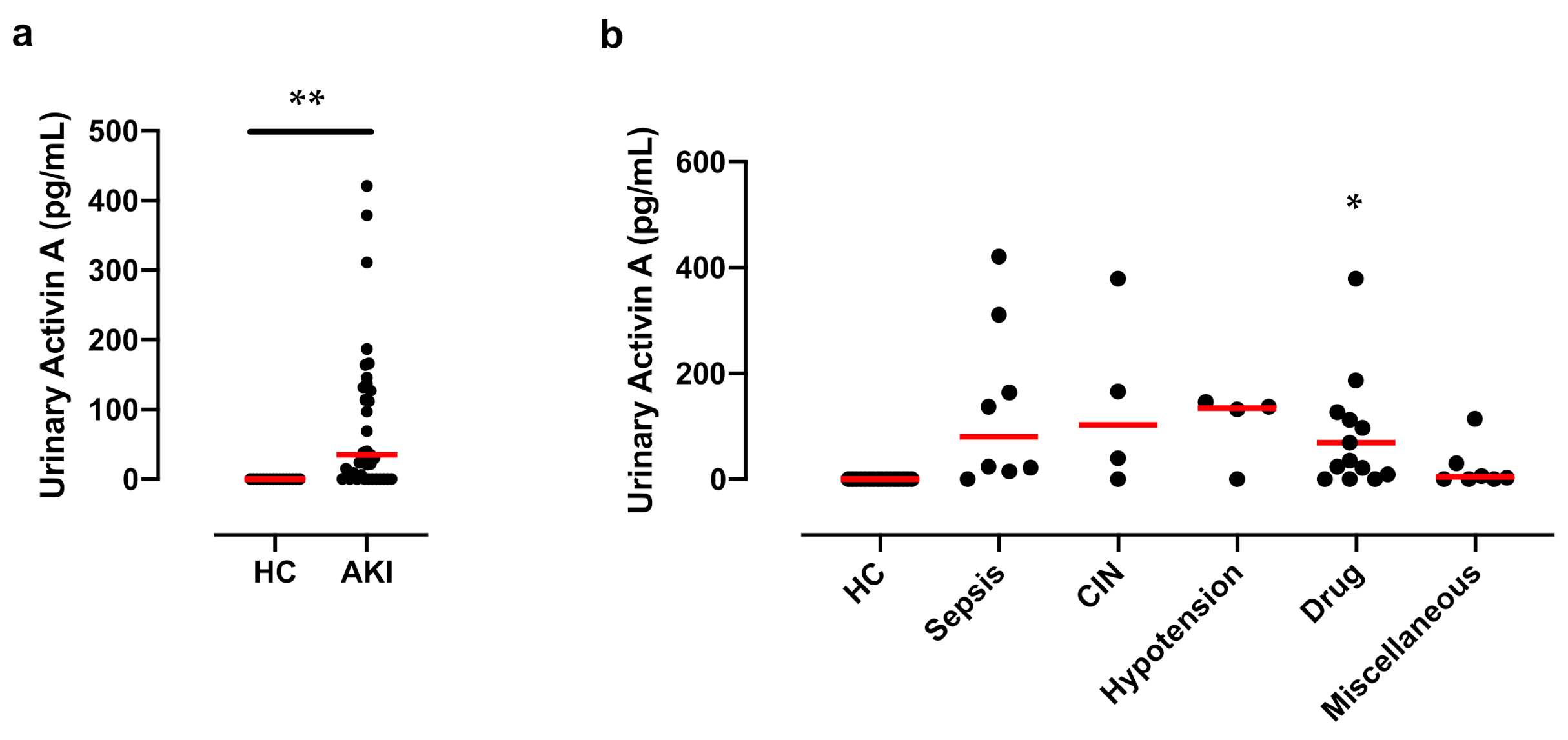
3.2. Significant Increase in Activin A in the Urine of Patients with AKI
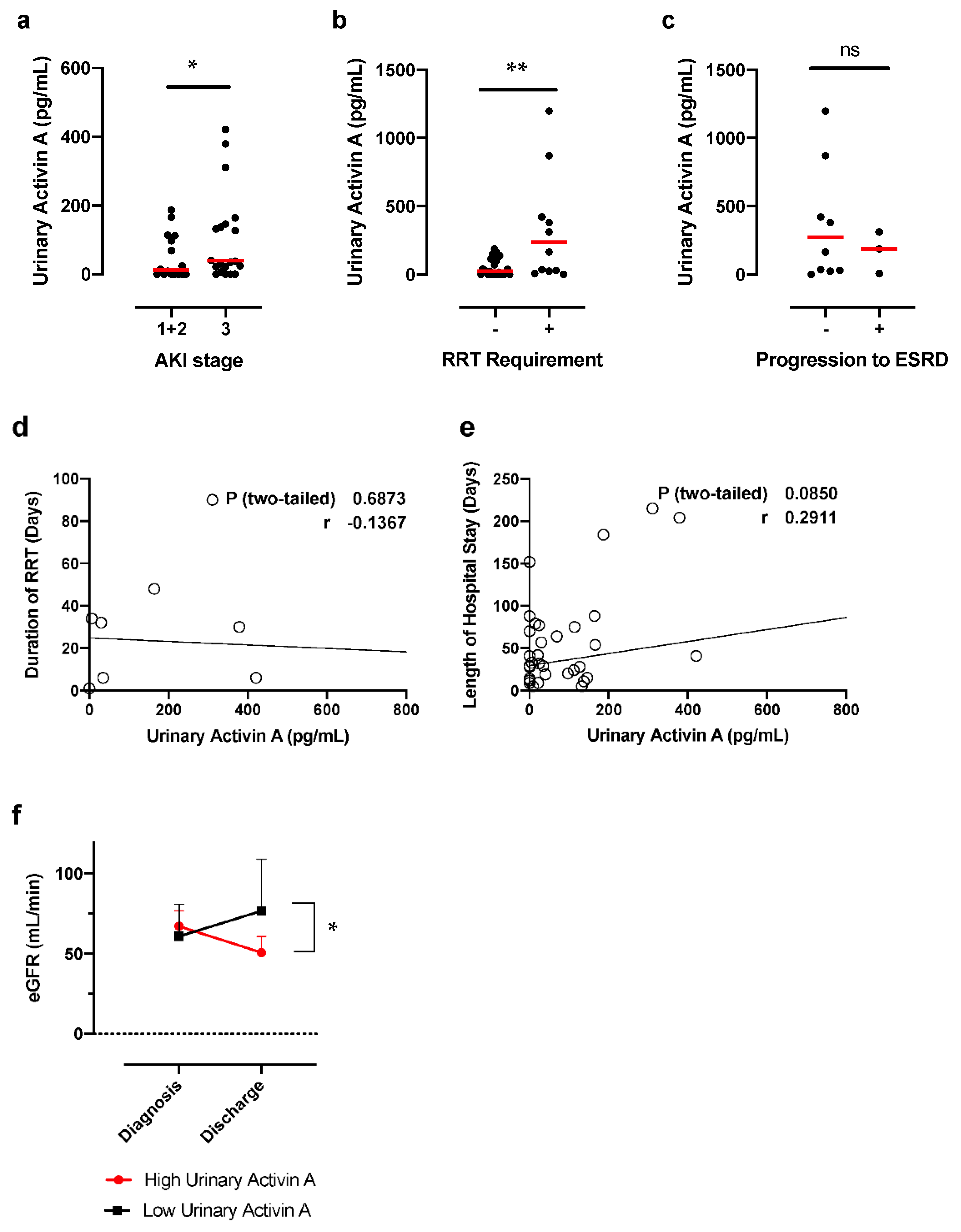
3.3. Urinary Activin A Is Associated with the Severity of AKI
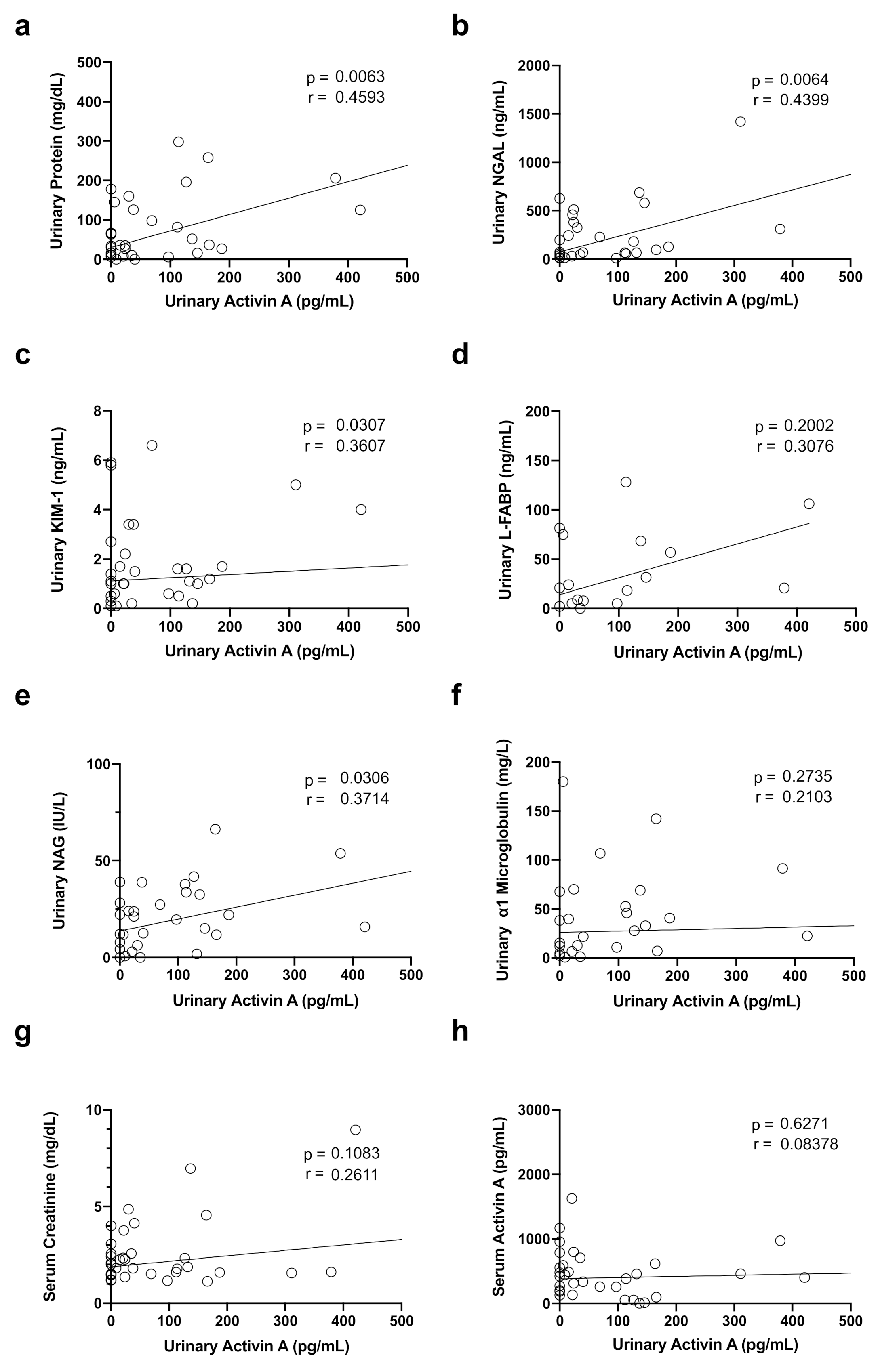
3.4. Correlation between Urinary Activin A Level and Renal Function and Markers of Tubular Injury
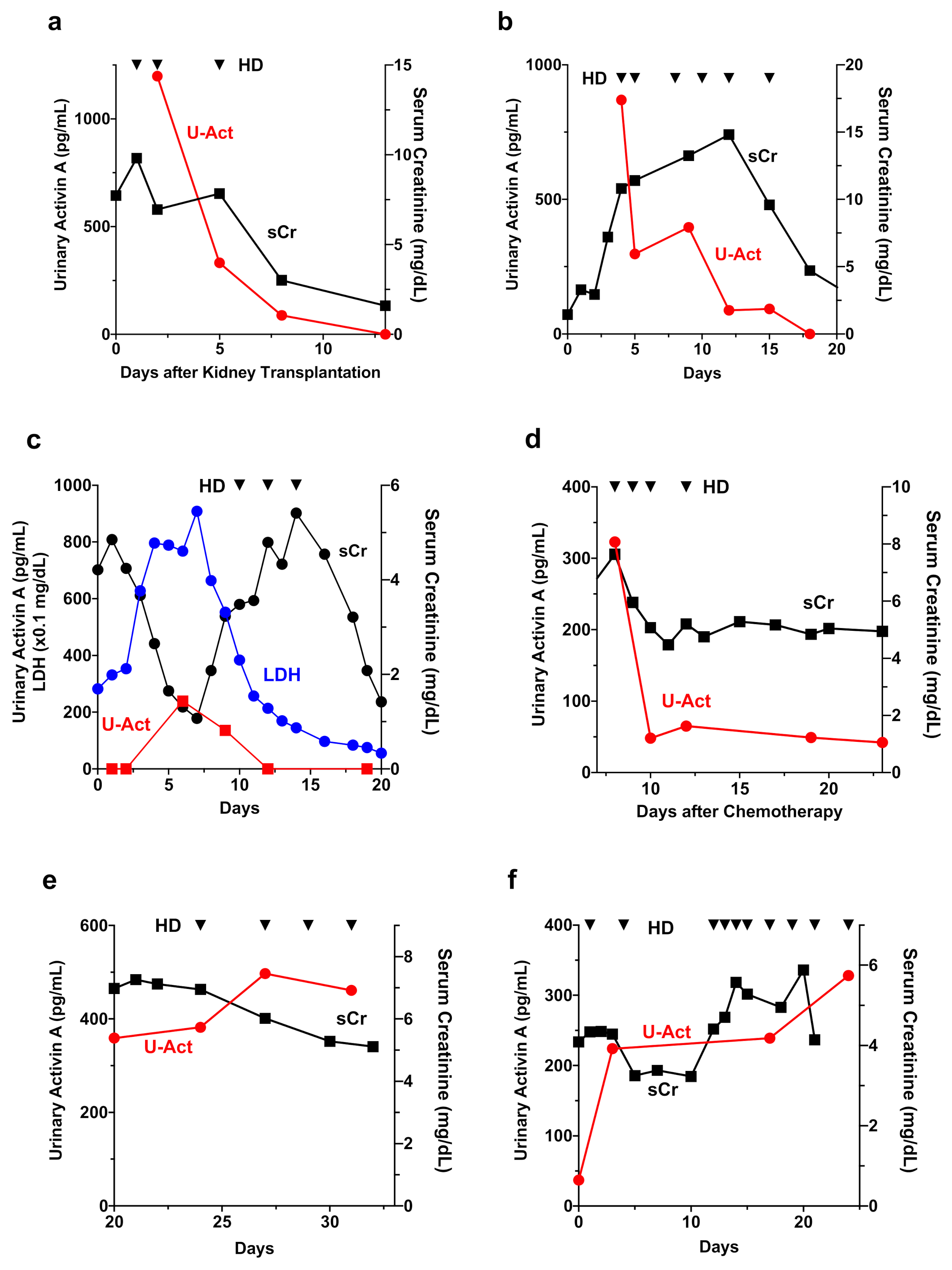
3.5. Time Course of Changes in Urinary Activin A and Other AKI Biomarkers in a Patient with AKI
3.6. Urinary Activin A in Patients with Different Causes of AKI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Agarwal, A.; Dong, Z.; Harris, R.; Murray, P.; Parikh, S.M.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Acute Dialysis Quality Initiative XIII Working Group. Cellular and Molecular Mechanisms of AKI. J. Am. Soc. Nephrol. 2016, 27, 1288–1299. [Google Scholar] [CrossRef]
- Zuk, A.; Bonventre, J.V. Acute kidney injury: Can remote ischaemic preconditioning prevent AKI? Nat. Rev. Nephrol. 2015, 11, 512–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A.; NGAL Meta-Analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, M.; Dent, C.L.; Ma, Q.; Dastrala, S.; Grenier, F.; Workman, R.; Syed, H.; Ali, S.; Barasch, J.; Devarajan, P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin. J. Am. Soc. Nephrol. 2008, 3, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Nakao, K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007, 71, 967–970. [Google Scholar] [CrossRef] [Green Version]
- Nickolas, T.L.; O’Rourke, M.J.; Yang, J.; Sise, M.E.; Canetta, P.A.; Barasch, N.; Buchen, C.; Khan, F.; Mori, K.; Giglio, J.; et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann. Intern. Med. 2008, 148, 810–819. [Google Scholar] [CrossRef]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]
- Parikh, C.R.; Abraham, E.; Ancukiewicz, M.; Edelstein, C.L. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J. Am. Soc. Nephrol. 2005, 16, 3046–3052. [Google Scholar] [CrossRef] [Green Version]
- Parikh, C.R.; Mishra, J.; Thiessen-Philbrook, H.; Dursun, B.; Ma, Q.; Kelly, C.; Dent, C.; Devarajan, P.; Edelstein, C.L. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006, 70, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Parikh, C.R.; Jani, A.; Melnikov, V.Y.; Faubel, S.; Edelstein, C.L. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am. J. Kidney Dis. 2004, 43, 405–414. [Google Scholar] [CrossRef]
- Parr, S.K.; Clark, A.J.; Bian, A.; Shintani, A.K.; Wickersham, N.E.; Ware, L.B.; Ikizler, T.A.; Siew, E.D. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2015, 87, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Portilla, D.; Dent, C.; Sugaya, T.; Nagothu, K.K.; Kundi, I.; Moore, P.; Noiri, E.; Devarajan, P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008, 73, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Negishi, K.; Noiri, E.; Doi, K.; Maeda-Mamiya, R.; Sugaya, T.; Portilla, D.; Fujita, T. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am. J. Pathol. 2009, 174, 1154–1159. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Noiri, E.; Ono, Y.; Doi, K.; Negishi, K.; Kamijo, A.; Kimura, K.; Fujita, T.; Kinukawa, T.; Taniguchi, H.; et al. Renal L-type fatty acid–binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 2007, 18, 2894–2902. [Google Scholar] [CrossRef] [Green Version]
- Liangos, O.; Perianayagam, M.C.; Vaidya, V.S.; Han, W.K.; Wald, R.; Tighiouart, H.; MacKinnon, R.W.; Li, L.; Balakrishnan, V.S.; Pereira, B.J.; et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J. Am. Soc. Nephrol. 2007, 18, 904–912. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ozer, J.S.; Dieterle, F.; Collings, F.B.; Ramirez, V.; Troth, S.; Muniappa, N.; Thudium, D.; Gerhold, D.; Holder, D.J.; et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 2010, 28, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, V.S.; Ramirez, V.; Ichimura, T.; Bobadilla, N.A.; Bonventre, J.V. Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal Physiol. 2006, 290, F517–F529. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Maeshima, A.; Vaughn, D.A.; Choi, Y.; Nigam, S.K. Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct. Dev. Biol. 2006, 295, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Maeshima, A.; Sakurai, H.; Choi, Y.; Kitamura, S.; Vaughn, D.A.; Tee, J.B.; Nigam, S.K. Glial cell-derived neurotrophic factor independent ureteric bud outgrowth from the Wolffian duct. J. Am. Soc. Nephrol. 2007, 18, 3147–3155. [Google Scholar] [CrossRef] [Green Version]
- Maeshima, A.; Shiozaki, S.; Tajima, T.; Nakazato, Y.; Naruse, T.; Kojima, I. Number of glomeruli is increased in the kidney of transgenic mice expressing the truncated type II activin receptor. Biochem. Biophys. Res. Commun. 2000, 268, 445–449. [Google Scholar] [CrossRef]
- Maeshima, A.; Zhang, Y.Q.; Furukawa, M.; Naruse, T.; Kojima, I. Hepatocyte growth factor induces branching tubulogenesis in MDCK cells by modulating the activin-follistatin system. Kidney Int. 2000, 58, 1511–1522. [Google Scholar] [CrossRef] [Green Version]
- Maeshima, A.; Yamashita, S.; Maeshima, K.; Kojima, I.; Nojima, Y. Activin a produced by ureteric bud is a differentiation factor for metanephric mesenchyme. J. Am. Soc. Nephrol. 2003, 14, 1523–1534. [Google Scholar] [CrossRef] [Green Version]
- Maeshima, A.; Zhang, Y.Q.; Nojima, Y.; Naruse, T.; Kojima, I. Involvement of the activin-follistatin system in tubular regeneration after renal ischemia in rats. J. Am. Soc. Nephrol. 2001, 12, 1685–1695. [Google Scholar] [CrossRef]
- Maeshima, A.; Maeshima, K.; Nojima, Y.; Kojima, I. Involvement of Pax-2 in the action of activin A on tubular cell regeneration. J. Am. Soc. Nephrol. 2002, 13, 2850–2859. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Nakasatomi, M.; Takei, Y.; Ikeuchi, H.; Sakairi, T.; Kaneko, Y.; Hiromura, K.; Nojima, Y.; Maeshima, A. Identification of Urinary Activin A as a Novel Biomarker Reflecting the Severity of Acute Kidney Injury. Sci. Rep. 2018, 8, 5176. [Google Scholar] [CrossRef] [Green Version]
- Palevsky, P.M.; Liu, K.D.; Brophy, P.D.; Chawla, L.S.; Parikh, C.R.; Thakar, C.V.; Tolwani, A.J.; Waikar, S.S.; Weisbord, S.D. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013, 61, 649–672. [Google Scholar] [CrossRef]
- Bian, X.; Griffin, T.P.; Zhu, X.; Islam, M.N.; Conley, S.M.; Eirin, A.; Tang, H.; O’Shea, P.M.; Palmer, A.K.; McCoy, R.G.; et al. Senescence marker activin A is increased in human diabetic kidney disease: Association with kidney function and potential implications for therapy. BMJ Open Diab. Res. Care 2019, 7, e000720. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Maeshima, A.; Kojima, I.; Nojima, Y. Activin A is a potent activator of renal interstitial fibroblasts. J. Am. Soc. Nephrol. 2004, 15, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeshima, A.; Mishima, K.; Yamashita, S.; Nakasatomi, M.; Miya, M.; Sakurai, N.; Sakairi, T.; Ikeuchi, H.; Hiromura, K.; Hasegawa, Y.; et al. Follistatin, an activin antagonist, ameliorates renal interstitial fibrosis in a rat model of unilateral ureteral obstruction. Biomed Res. Int. 2014, 2014, 376191. [Google Scholar] [CrossRef] [PubMed]
- Agapova, O.A.; Fang, Y.; Sugatani, T.; Seifert, M.E.; Hruska, K.A. Ligand trap for the activin type IIA receptor protects against vascular disease and renal fibrosis in mice with chronic kidney disease. Kidney Int. 2016, 89, 1231–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| AKI n = 39 | Healthy Control n = 16 | p Value | |
|---|---|---|---|
| Age, years, mean ± S.E. | 60.2 ± 1.9 | 54.9 ± 2.9 | 0.097 |
| BMI, kg/m2, mean ± S.E. | 24.2 ± 0.7 | 23.3 ± 0.7 | 0.711 |
| Male gender, n (%) | 26 (66.6) | 2 (12.5) | 0.058 |
| Weight, kg, mean ± S.E. | 63.9 ± 2.2 | 60.1 ± 3.2 | 0.383 |
| Complications, n (%) | |||
| Diabetes | 0 (0) | 1 (6.2) | 0.089 |
| Hypertension | 25 (64.1) | 7 (43.7) | 0.059 |
| Dyslipidemia | 9 (23) | 1 (6.2) | 0.089 |
| Old myocardial infarction | 0 (0) | 0 (0) | 0.438 |
| Old cerebral infarction | 3 (7.6) | 0 (0) | 0.264 |
| Angina pectoris | 2 (5.1) | 0 (0) | 0.433 |
| Chronic obstructive pulmonary disease | 0 (0) | 0 (0) | 0.587 |
| Liver disease | 2 (5.1) | 0 (0) | 0.438 |
| Hematological data, mean ± S.E. | |||
| Na, mEq/L | 138.3 ± 0.8 | 142 ± 0.4 | 0.007 |
| K, mEq/L | 4.5 ± 0.1 | 4.1 ± 0.1 | 0.069 |
| Cl, mEq/L | 104.2 ± 0.8 | 105.9 ± 0.6 | 0.138 |
| BUN, mg/dL | 47.9 ± 5.0 | 14 ± 0.8 | <0.0001 |
| Creatinine, mg/dL | 3.64 ± 0.58 | 0.68 ± 0.04 | 0.001 |
| eGFR, mL/min | 23.5 ± 2.6 | 79.9 ± 3.6 | <0.0001 |
| Hemoglobin, g/dL | 10.8 ± 0.4 | 13.2 ± 0.2 | 0.001 |
| Platelets, ×104/μL | 16.6 ± 1.7 | 26.0 ± 1.6 | 0.004 |
| WBC, ×103/μL | 10.2 ± 1.1 | 5.7 ± 0.4 | 0.021 |
| CRP, mg/dL | 8.0 ± 1.5 | 0.2 ± 0.1 | 0.001 |
| Urinalysis, mean ± S.E. | |||
| Urinary protein, mg/dL | 166.6 ± 57.0 | - | - |
| NAG, IU/L | 28.9 ± 5.5 | - | - |
| α1MG, mg/L | 57.8 ± 12.8 | - | - |
| β2MG, mg/mL | 13.7 ± 4.6 | - | - |
| NGAL, ng/mL | 783.4 ± 218.1 | - | - |
| KIM-1, ng/mL | 2.4 ± 0.4 | - | - |
| L-FABP, ng/mL | 213.5 ± 147.1 | - | - |
| FENa, % | 4.7 ± 1.4 | - | - |
| FEUrea, % | 35.5 ± 3.0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagayama, I.; Maeshima, A.; Nagata, D. Urinary Activin A: A Novel Biomarker for Human Acute Kidney Injury. Diagnostics 2022, 12, 661. https://doi.org/10.3390/diagnostics12030661
Nagayama I, Maeshima A, Nagata D. Urinary Activin A: A Novel Biomarker for Human Acute Kidney Injury. Diagnostics. 2022; 12(3):661. https://doi.org/10.3390/diagnostics12030661
Chicago/Turabian StyleNagayama, Izumi, Akito Maeshima, and Daisuke Nagata. 2022. "Urinary Activin A: A Novel Biomarker for Human Acute Kidney Injury" Diagnostics 12, no. 3: 661. https://doi.org/10.3390/diagnostics12030661
APA StyleNagayama, I., Maeshima, A., & Nagata, D. (2022). Urinary Activin A: A Novel Biomarker for Human Acute Kidney Injury. Diagnostics, 12(3), 661. https://doi.org/10.3390/diagnostics12030661





