MicroRNAs Related to TACE Treatment Response: A Review of the Literature from a Radiological Point of View
Abstract
:1. Introduction
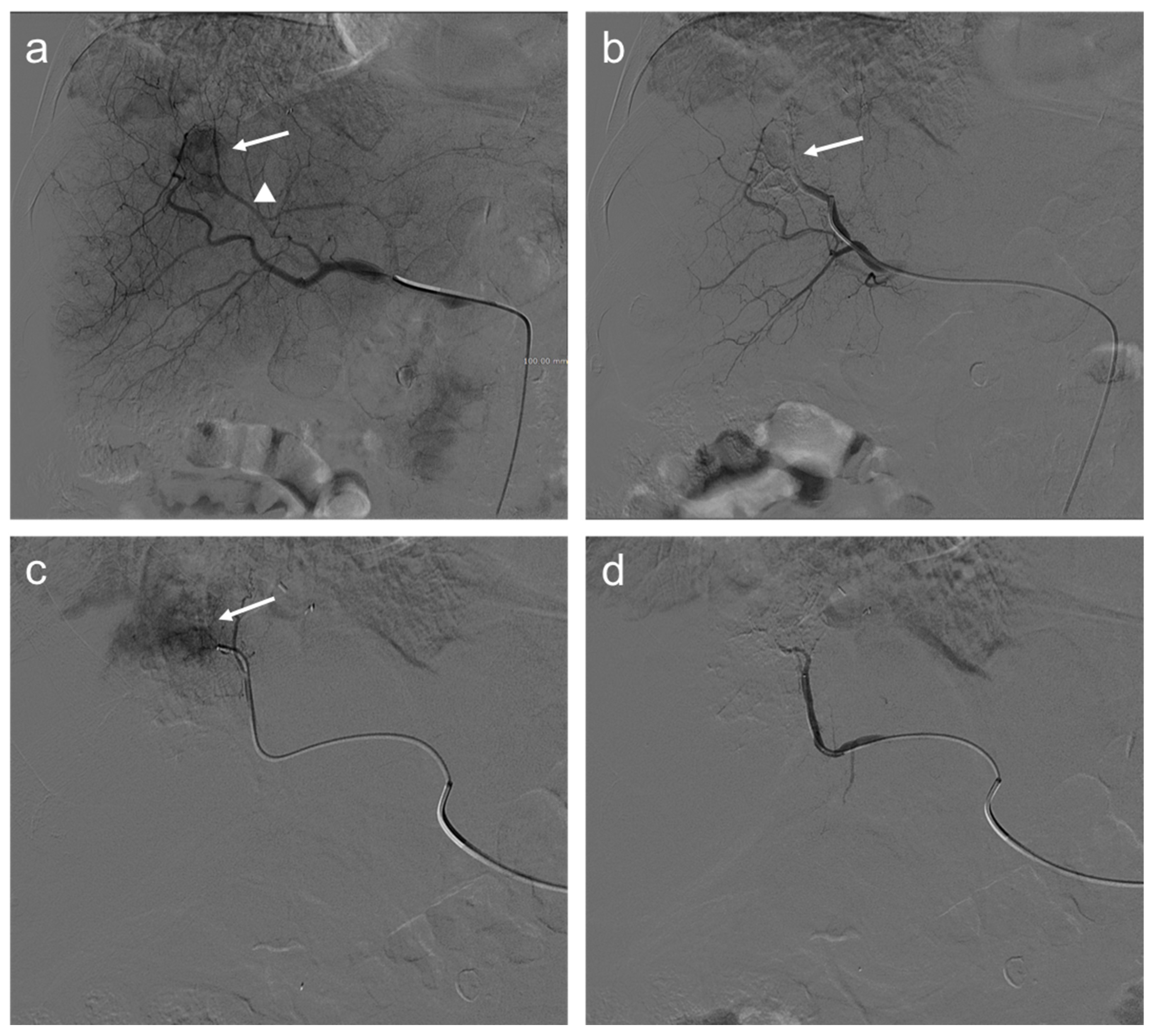
2. TACE Technique
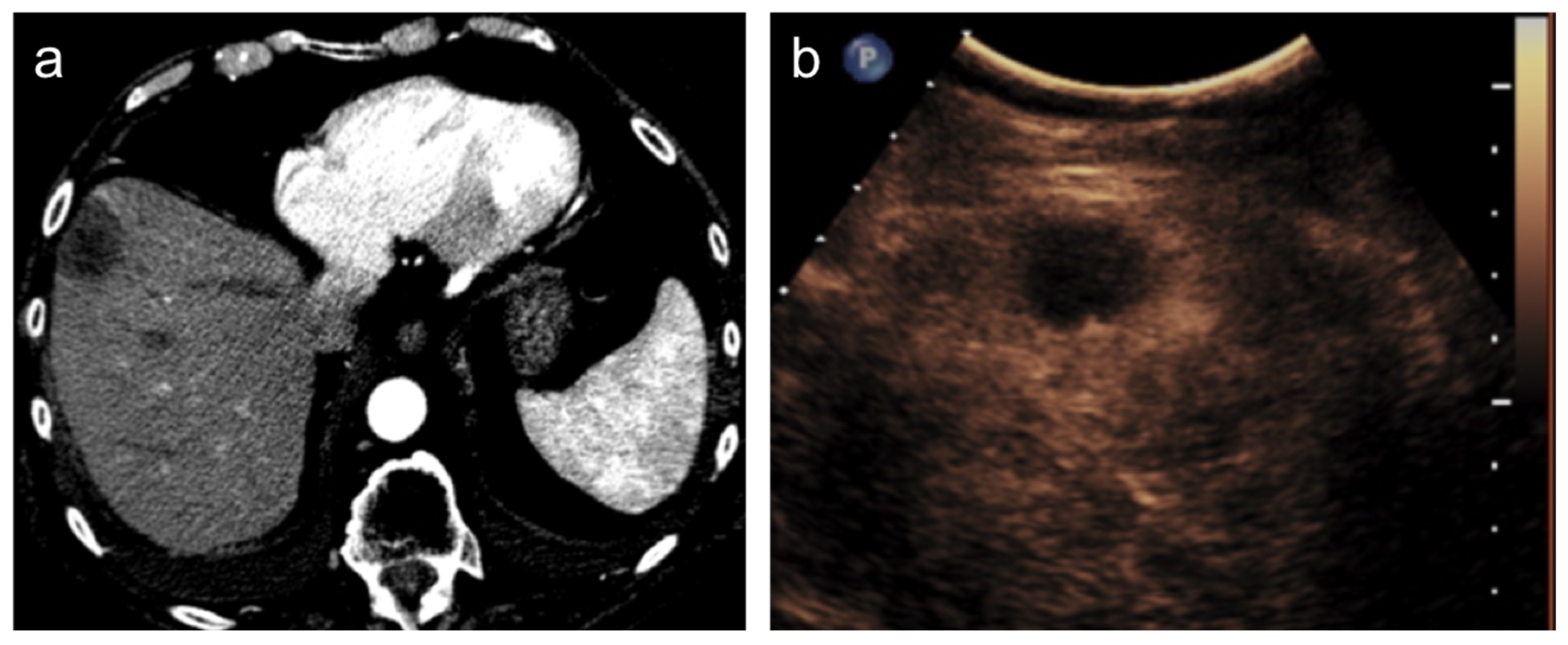
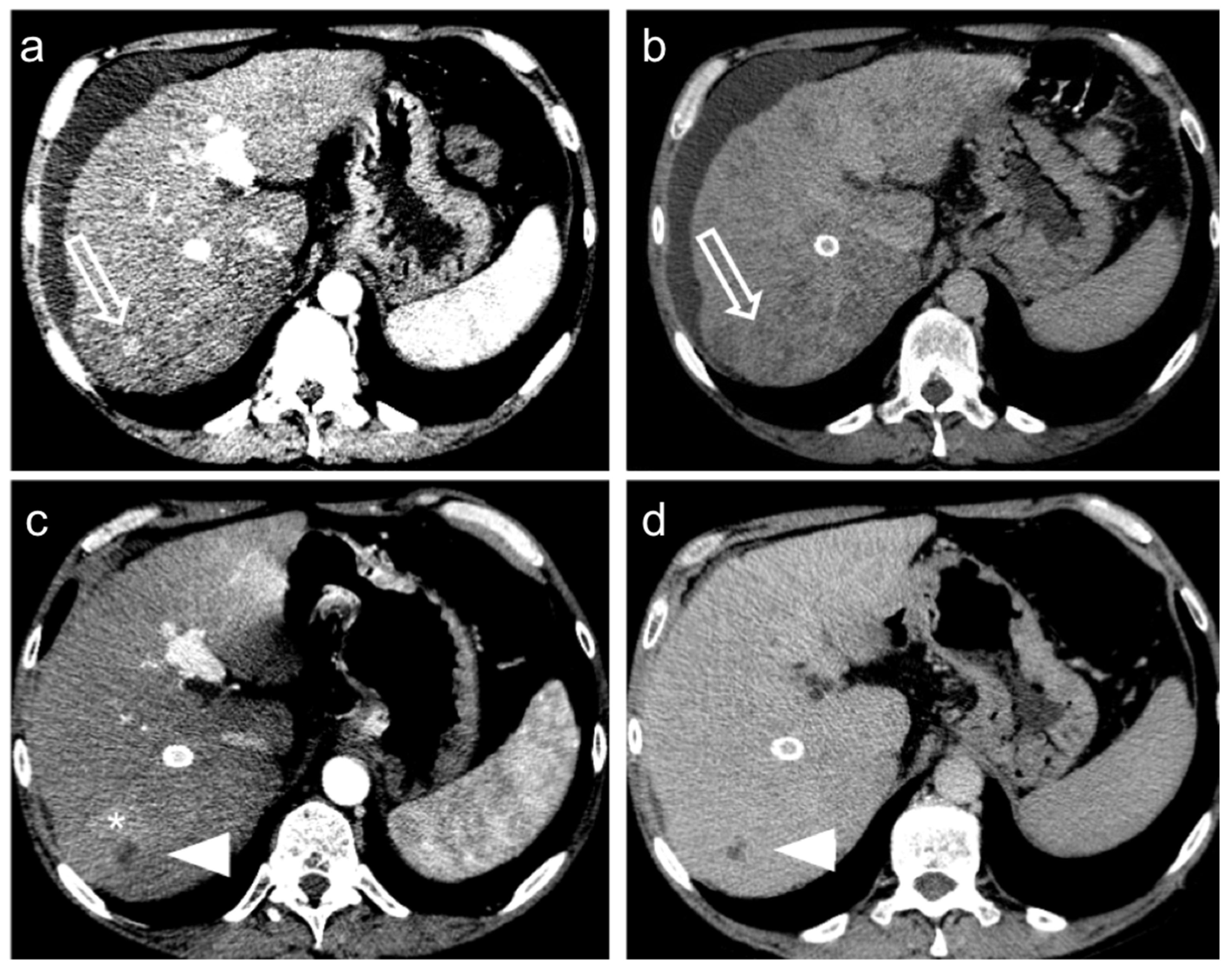
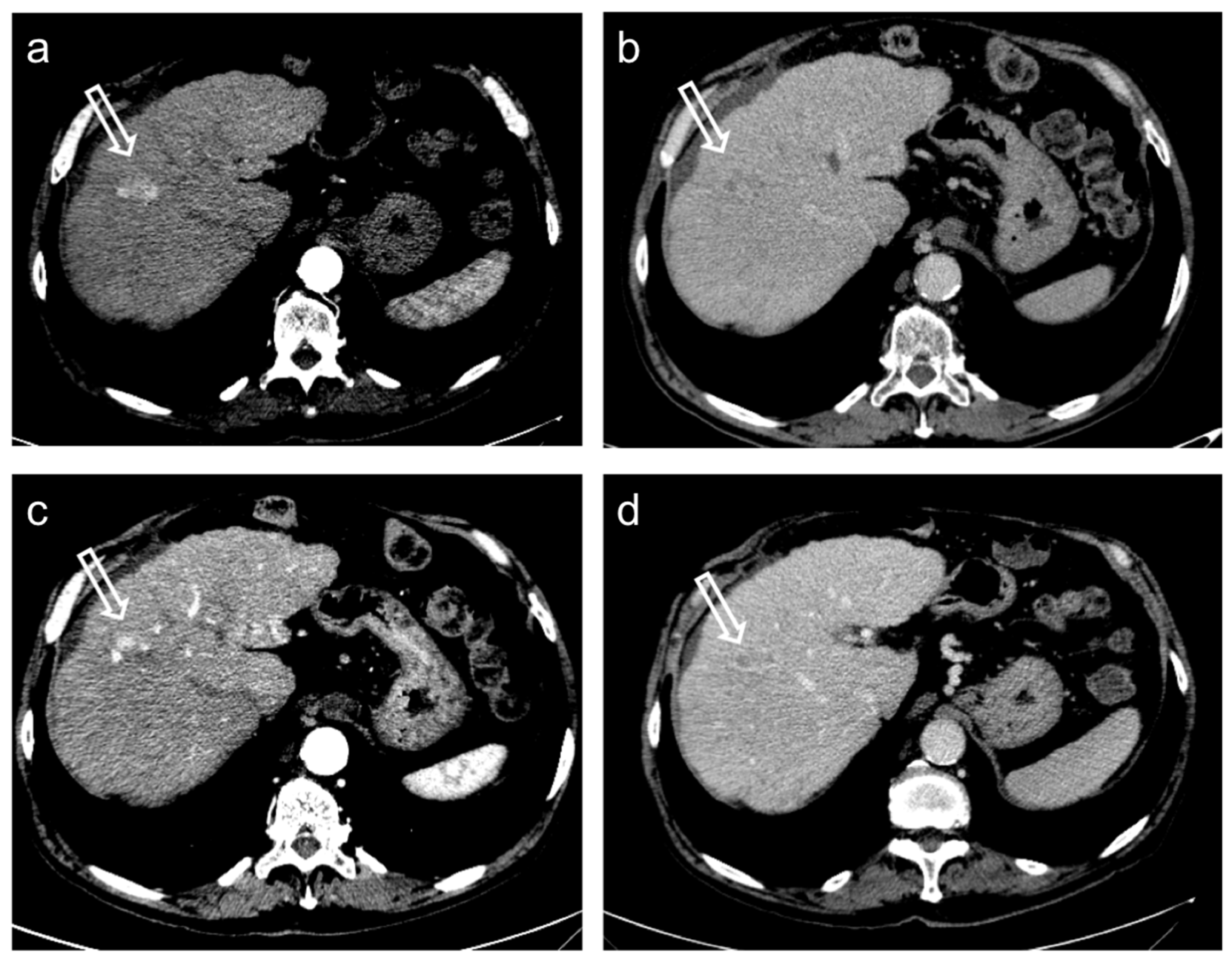
3. Treatment Response Criteria
4. MiRNA and TACE
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018, 22, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma: An Emphasis on Demographic and Regional Variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Campigotto, M.; Giuffrè, M.; Colombo, A.; Visintin, A.; Aversano, A.; Budel, M.; Masutti, F.; Abazia, C.; Crocé, L.S. Comparison between hepatocellular carcinoma prognostic scores: A 10-year single-center experience and brief review of the current literature. World J. Hepatol. 2020, 12, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.-Y.; Li, S.-M.; Fan, H.-Y.; Zhang, L.; Zhao, H.-J.; Li, S.-M. Transarterial chemoembolization extends long-term survival in patients with unresectable hepatocellular carcinoma. Medicine 2018, 97, e11872. [Google Scholar] [CrossRef]
- Lopez, P.M.; Villanueva, A.; Llovet, J.M. Systematic review: Evidence-based management of hepatocellular carcinoma—An updated analysis of randomized controlled trials. Aliment. Pharmacol. Ther. 2006, 23, 1535–1547. [Google Scholar] [CrossRef]
- Lencioni, R.; Chen, X.-P.; Dagher, L.; Venook, A.P. Treatment of Intermediate/Advanced Hepatocellular Carcinoma in the Clinic: How Can Outcomes Be Improved? Oncologist 2010, 15, 42–52. [Google Scholar] [CrossRef]
- Xu, X.; Tao, Y.; Shan, L.; Chen, R.; Jiang, H.; Qian, Z.; Cai, F.; Ma, L.; Yu, Y. The role of MicroRNAs in hepatocellular carcinoma. J. Cancer 2018, 9, 3557–3569. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Slack, F.J.; Basson, M.; Pasquinelli, A.E.; Bettinger, J.C.; Rougvie, A.E.; Horvitz, H.R.; Ruvkun, G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000, 403, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in cancer. Annu. Rev. Pathol. 2014, 9, 287–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Wang, J.; Li, L.; Liu, R.; Fang, J.; Tie, B. Value of miR-1271 and glypican-3 in evaluating the prognosis of patients with hepatocellular carcinoma after transcatheter arterial chemoembolization. World J. Clin. Cases 2020, 8, 3493–3502. [Google Scholar] [CrossRef]
- Pratama, M.Y.; Visintin, A.; Crocè, L.S.; Tiribelli, C.; Pascut, D. Circulatory miRNA as a biomarker for therapy response and disease-free survival in hepatocellular carcinoma. Cancers 2020, 12, 2810. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, X.M. Serum TSGF and miR-214 levels in patients with hepatocellular carcinoma and their predictive value for the curative effect of transcatheter arterial chemoembolization. Ann. Palliat. Med. 2020, 9, 2111–2117. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, L.; Ren, R.; Ji, F.; Xue, S.; Zhang, J.; Liu, Z.; Ma, Z.; Wang, X.W.; Wong, L. MiR-125b Loss Activated HIF1α/pAKT Loop, Leading to Transarterial Chemoembolization Resistance in Hepatocellular Carcinoma. Hepatology 2021, 73, 1381–1398. [Google Scholar] [CrossRef]
- Ali, H.E.A.; Emam, A.A.; Zeeneldin, A.A.; Srour, R.; Tabashy, R.; El-Desouky, E.D.; Abd Elmageed, Z.Y.; Abdel-Wahab, A.A. Circulating miR-26a, miR-106b, miR-107 and miR-133b stratify hepatocellular carcinoma patients according to their response to transarterial chemoembolization. Clin. Biochem. 2019, 65, 45–52. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Tu, J.; Zhao, Z.; Fan, X.; Mao, J.; Weng, Q.; Wu, X.; Huang, L.; Xu, M.; et al. miR-590-5p suppresses hepatocellular carcinoma chemoresistance by targeting YAP1 expression. BioMedicine 2018, 35, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Cho, H.J.; Nam, J.S.; Kim, H.J.; Kang, D.R.; Won, J.H.; Kim, J.; Kim, J.K.; Lee, J.H.; Kim, B.H.; et al. Plasma microRNA-21, 26a, and 29a-3p as predictive markers for treatment response following transarterial chemoembolization in patients with hepatocellular carcinoma. J. Korean Med. Sci. 2018, 33, e6. [Google Scholar] [CrossRef] [Green Version]
- Suehiro, T.; Miyaaki, H.; Kanda, Y.; Shibata, H.; Honda, T.; Ozawa, E.; Miuma, S.; Taura, N.; Nakao, K. Serum exosomal microRNA-122 and microRNA-21 as predictive biomarkers in transarterial chemoembolization-treated hepatocellular carcinoma patients. Oncol. Lett. 2018, 16, 3267–3273. [Google Scholar] [CrossRef]
- Kim, S.S.; Nam, J.S.; Cho, H.J.; Won, J.H.; Kim, J.W.; Ji, J.H.; Yang, M.J.; Park, J.H.; Noh, C.-K.; Shin, S.J.; et al. Plasma micoRNA-122 as a predictive marker for treatment response following transarterial chemoembolization in patients with hepatocellular carcinoma. J. Gastroenterol. Hepat. 2017, 32, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.L.; Yao, J.G.; Huang, X.Y.; Wang, C.; Wu, X.M.; Xia, Q.; Long, X.D. Prognostic significance of miR-1268a expression and its beneficial effects for post-operative adjuvant transarterial chemoembolization in hepatocellular carcinoma. Sci. Rep. 2016, 6, 36104. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Feng, C.; Hu, P.; Chen, Y.; He, X.F.; Li, Y.; Zhao, J. Serum microRNA-199a/b-3p as a predictive biomarker for treatment response in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Onco Targets Ther. 2016, 9, 2667–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, G.P.; Liu, J. MicroRNA gene polymorphisms in evaluating therapeutic efficacy after transcatheter arterial chemoembolization for primary hepatocellular carcinoma. Genet. Test. Mol. Biomarkers 2016, 20, 579–586. [Google Scholar] [CrossRef]
- Cui, L.; Hu, Y.; Bai, B.; Zhang, S. Serum miR-335 level is associated with the treatment response to trans-arterial chemoembolization and prognosis in patients with hepatocellular carcinoma. Cell Physiol. Biochem. 2015, 37, 276–283. [Google Scholar] [CrossRef]
- El-Halawany, M.S.; Ismail, H.M.; Zeeneldin, A.A.; Elfiky, A.; Tantawy, M.; Kobaisi, M.H.; Hamed, I.; Abdel Wahab, H.A. Investigating the pretreatment miRNA expression patterns of advanced hepatocellular carcinoma patients in association with response to TACE treatment. BioMed Res. Int. 2015, 2015, 649750. [Google Scholar] [CrossRef]
- Liu, J.; Yan, J.; Zhou, C.; Ma, Q.; Jin, Q.; Yang, Z. miR-1285-3p acts as a potential tumor suppressor miRNA via downregulating JUN expression in hepatocellular carcinoma. Tumor Biol. 2015, 36, 219–225. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Wang, L.; Wu, H.; Zhou, C.; Zhu, H.; Xu, N.; Xie, Y. Association of serum microRNA expression in hepatocellular carcinomas treated with transarterial chemoembolization and patient survival. PLoS ONE 2014, 9, e109347. [Google Scholar] [CrossRef]
- Zhan, M.; Li, Y.; Hu, B.; He, X.; Huang, J.; Zhao, Y.; Fu, S.; Lu, L. Serum microRNA-210 as a predictive biomarker for treatment response and prognosis in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J. Vasc. Interv. Radiol. 2014, 25, 1279–1287. [Google Scholar] [CrossRef]
- Forner, A.; Gilabert, M.; Bruix, J.; Raoul, J.L. Treatment of intermediate-stage hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2014, 11, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Desai, K.; Hickey, R.; Thornburg, B.; Lewandowski, R.; Salem, R. Transarterial approaches to primary and secondary hepatic malignancies. Nat. Rev. Clin. Oncol. 2015, 12, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Arimoto, A.; Wakasa, T.; Kita, R.; Kimura, T.; Osaki, Y. Effect of transcatheter arterial chemoembolization prior to surgical resection for hepatocellular carcinoma. Int. J. Oncol. 2013, 42, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhang, X.; Wu, L.; Ye, F.; Su, X.; Shi, L.; Li, B. Meta-analysis: Preoperative transcatheter arterial chemoembolization does not improve prognosis of patients with resectable hepatocellular carcinoma. BMC Gastroenterol. 2013, 13, 51. [Google Scholar] [CrossRef] [Green Version]
- Kan, Z.; Wright, K.; Wallace, S. Ethiodized oil emulsions in hepatic microcirculation: In vivo microscopy in animal models. Acad. Radiol. 1997, 4, 275–282. [Google Scholar] [CrossRef]
- Hecq, J.D.; Lewis, A.L.; Vanbeckbergen, D.; Athanosopoulos, A.; Galanti, L.; Jamart, J.; Czuczman, P.; Chung, T. Doxorubicin-loaded drug-eluting beads (DC Bead®) for use in transarterial chemoembolization: A stability assessment. J. Oncol. Pharm. Pract. 2013, 19, 65–74. [Google Scholar] [CrossRef]
- Malagari, K.; Pomoni, M.; Moschouris, H.; Bouma, E.; Koskinas, J.; Stefaniotou, A.; Marinis, A.; Kelekis, A.; Alexopoulou, E.; Chatziioannou, A.; et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: Five-year survival analysis. Cardiovasc. Interv. Radiol. 2012, 35, 1119–1128. [Google Scholar] [CrossRef]
- Spreafico, C.; Cascella, T.; Facciorusso, A.; Sposito, C.; Rodolfo, L.; Morosi, C.; Civelli, E.M.; Vaiani, M.; Bhoori, S.; Pellegrinelli, A.; et al. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: Clinical-radiological outcomes and safety profile. Cardiovasc. Interv. Radiol. 2015, 38, 129–134. [Google Scholar] [CrossRef]
- Monier, A.; Guiu, B.; Duran, R.; Aho, S.; Bize, P.; Deltenre, P.; Dunet, V.; Denys, A. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: Comparison between drug-eluting beads and lipiodol emulsion. Eur. Radiol. 2017, 27, 1431–1439. [Google Scholar] [CrossRef]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc. Int. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Sacco, R.; Bargellini, I.; Bertini, M.; Bozzi, E.; Romano, A.; Petruzzi, P.; Tumino, E.; Gianni, B.; Federici, G.; Cioni, R.; et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2011, 22, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Golifieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenbrey, J.R.; Gabriel, H.; Savsani, E.; Lyshchik, A. Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies. Abdom. Radiol. 2021, 46, 3579–3595. [Google Scholar] [CrossRef] [PubMed]
- Kielar, A.; Fowler, K.J.; Lewis, S.; Yaghmai, V.; Miller, F.H.; Yarmohammadi, H.; Kim, C.; Chernyak, V.; Yokoo, T.; Meyer, J.; et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom. Radiol. 2018, 43, 218–230. [Google Scholar] [CrossRef]
- Gregory, J.; Dioguardi Burgio, M.; Corrias, G.; Vilgrain, V.; Ronot, M. Evaluation of liver tumour response by imaging. JHEP Rep. 2020, 2, 100100. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- American College of Radiology. Liver Imaging Reporting and Data System (LI-RADS). American College of Radiology Web site. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-DataSystems/LI-RADS/CT-MRI-LI-RADS-v2017 (accessed on 5 May 2021).

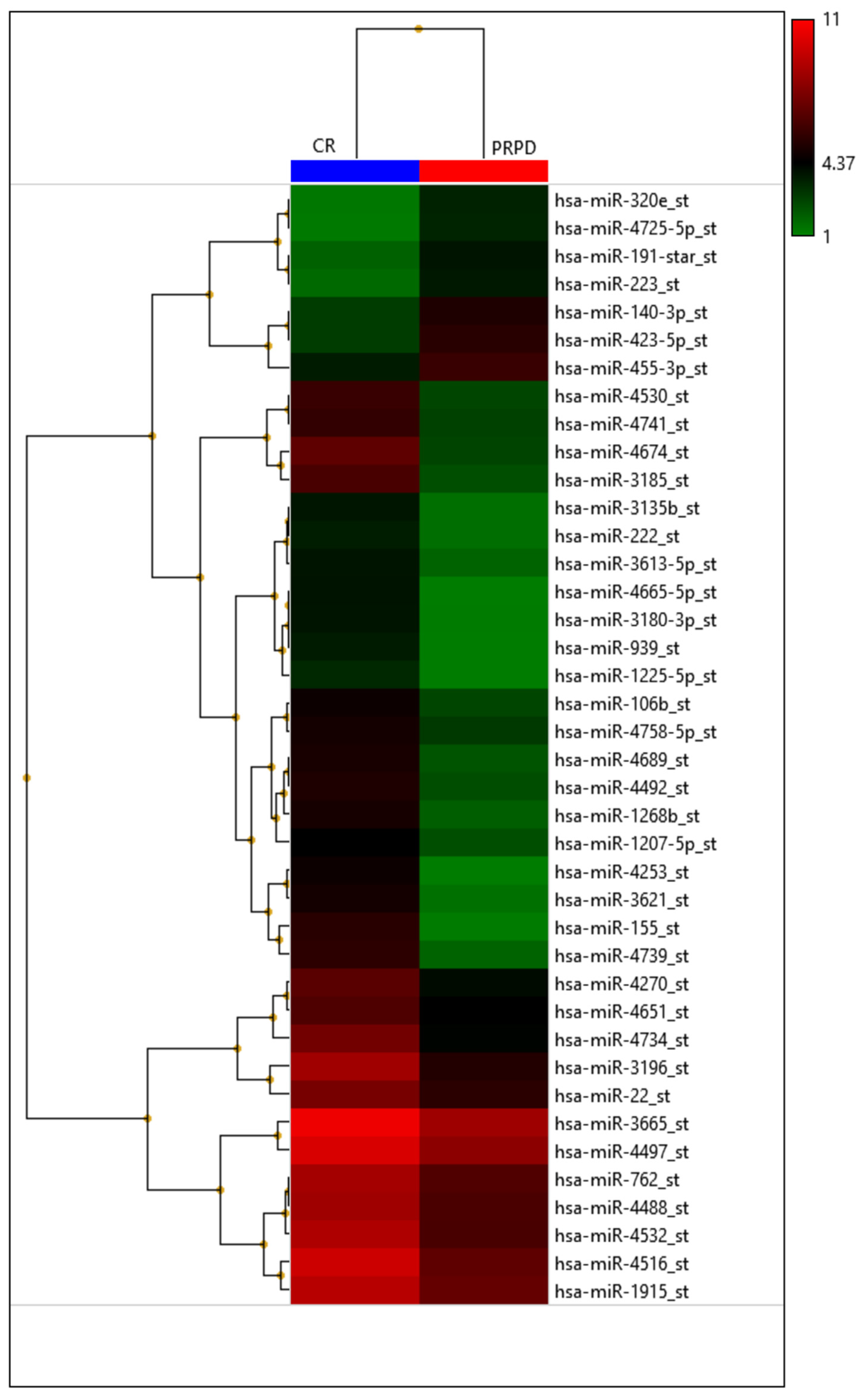

| Authors (Year) | Number of Patients | miRNA | Sample Source | Method | TACE Technique | Treatment Response Criteria | Main Endpoint |
|---|---|---|---|---|---|---|---|
| Guo Z. et al. (2020) [14] | 162 | miR-1271 | serum | qRT-PCR | ethiodized oil, fluorourea glycoside, oxaliplatin, and polyvinyl alcohol | mRECIST | The level of miR-1271 in remission patients was significantly increased after TACE |
| Pratama M.Y. et al. (2020) [15] | 46 | miR-4492 | serum | qRT-PCR | NM | mRECIST | Serum miR-4492 levels were associated with TACE response; indeed, miR-4492 levels at the time of diagnosis of HCC were 2.67-folds higher in patients with complete response to TACE compared to patients with partial response or progressive disease |
| Tang L. et al. (2020) [16] | 87 | miR-214 | serum | qRT-PCR | chemotherapy drugs and super-liquid iodized oil-mixed emulsion | RECIST | High serum miR-214 levels one day before TACE were associated with disease control (complete remission, partial remission, and stability) one month after TACE, whereas low serum miR-214 levels one day before the procedure were related to disease progression one month after TACE. Nevertheless, one month after the treatment, the serum miR-214 levels were increased in both groups (patients with disease control and patients with disease progression) compared with before treatment |
| Wei X. et al. (2020) [17] | 61 | miR-125b | tissue samples | qRT-PCR | chemotherapeutic agents (including doxorubicin, epidoxorubicin, cisplatin, and mitomycin C) and ethiodized oil (5–10 mL) | mRECIST | High miR-125b levels were associated with survival benefit from adjuvant TACE |
| Ali H.E. et al. (2019) [18] | 51 | miR-26a, 106b, 107, and 133b | serum | qRT-PCR | doxorubicin (100 mg) and ethiodized oil | RECIST 1.1 | High serum miRNA-106b, miRNA-107, and miRNA-133b levels were associated with TACE response, while high serum miRNA-26a levels were associated with no response to TACE |
| Chen M. et al. (2018) [19] | 20 | miR-590-5p | tissue samples | qRT-PCR | NM | NM | High miR-590-5p expression was associated with a favorable chemotherapy response |
| Kim S.S. et al. (2018) [20] | 198 | combination of miR-21, 26a, and 29a-3p | plasma | qRT-PCR | doxorubicin (50 mg) and ethiodized oil (10 mL), followed by embolization with gelatin sponge particles | mRECIST | Individual or combined plasma miR-21, miR-26a, and miR-29a-3p expression levels before the treatment were not significantly associated with liver transplantation-free survival or overall TACE refractoriness. Nevertheless, the combination of high miR21, high miR-26a, and low miR-29a-3p plasma levels were independent predictive factors for early TACE refractoriness, defined as TACE refractoriness within 1 year from the first TACE treatment |
| Suehiro T. et al. (2018) [21] | 75 | miR-122 | serum | qRT-PCR | epirubicin hydrochloride or miriplatin hydrate, mitomycin C, ethiodized oil, and contrast agent, followed by embolization with gelatin sponge particles | NM | Low miR-122 ratio (serum miR expression after TACE/serum miR expression before TACE) was associated with significantly lower disease-specific survival |
| Kim S.S. et al. (2017) [22] | 177 | miR-122 | plasma | qRT-PCR | doxorubicin (50 mg) and ethiodized oil (10 mL), followed by embolization with gelatin sponge particles | mRECIST | High plasma miR-122 expression before TACE was correlated with early TACE refractoriness, defined as TACE refractoriness within 1 year from the first TACE treatment |
| Lu Y.L. et al. (2016) [23] | 411 | miR-1268a | tissue samples | qRT-PCR | doxorubicin, cisplatin, and ethiodized oil, followed by embolization with gelatin foam or polyvinyl alcohol | NM | Low miRNA-1268a expression four weeks before the procedure predicted increased overall survival and relapse-free survival |
| Luo Z. et al. (2016) [24] | 132 | miR-199a/b-3p | serum | qRT-PCR | adriamycin (20–50 mg), ethiodized oil (5–20 mL), and contrast agent, followed by embolization with polyvinyl alcohol particles | mRECIST | High miR-199a/b-3p expression levels before TACE were related with CR and PR. Moreover, the decrease of miR-199a/b-3p levels 3–5 days and four weeks after the procedure were higher in patients with CR and PR than in the other ones |
| Qiu G.P. et al. (2016) [25] | 507 | CC genotype and C allele in miR-196a2 rs11614913, and GG genotype and G allele in miR-499a rs3746444 | whole-blood | qRT-PCR | Epirubicin (40–80 mg) and hydroxycamptothecin (20–30 mg). Ethiodized oil (5–20 mL), and gelatin sponge particles were also injected in patients with less than six points of the Child-Pugh score | RECIST 1.1 | The frequency of CC genotype and C allele in miR-196a2 rs11614913, as well as the GG genotype and G allele in miR-499a rs3746444 was higher in patients with stable disease and progression disease after TACE |
| Cui L. et al. (2015) [26] | 125 | miR-335 | serum | qRT-PCR | carboplatin (300 mg), epirubicin (50 mg), mitomycin C (8 mg), and ethiodized oil (5 mL), followed by embolization with gelatin sponge particles | RECIST | The serum miR-335 expression thirty days after TACE may help in distinguishing good responders (CR and PR) from poor responders (SD and PD) with AUC of 0,922, specificity of 87%, and sensitivity of 77%. Indeed, low serum miR-335 levels thirty days after the procedure were correlated with poor response as well as decreased overall survival and time to progression in these patients |
| El-Halawany M.S. et al. (2015) [27] | 15 | miR-10a, 23a, 24, 26a, 27a, 30c, 30e, 31, 106b, 133-b, 199a-3p, and miR-200 b | tissue samples | qRT-PCR | cisplatin (50 mg), doxorubicin (50 mg), and ethiodized oil | RECIST | Pretreatment expression of this panel of 12 miRNAs was higher in non-responders to TACE |
| Liu J. et al. (2015) [28] | 97 | miR-1285-3p and miR-4741 | plasma | qRT-PCR | epirubicin (30 mg), cyano-camptothecin (30 mg), and cisplatin (40 mg) | NM | Low miR-1285-3p and miR-4741 levels in HCC patients before the TACE procedure were correlated with poor response to the treatment |
| Liu M. et al. (2014) [29] | 136 | miR-200a | serum | qRT-PCR | adriamycin (20–50 mg), ethiodized oil (5–20 mL), and contrast medium, followed by embolization with gelatin sponge particles | NM | The potential role of miR-200a as an independent prognostic factor associated with disease outcome in HCC patients treated with TACE was described. Indeed, high serum miR-200a levels in patients with HCC before TACE treatment were associated with decreased survival |
| Zhan M. et al. (2014) [30] | 113 | miR-210 | serum | qRT-PCR | Oxaliplatin (135 mg) and epirubicin (30–40 mg). In case of incomplete embolization, gelatin sponge particles were also injected | mRECIST | High miR-210 serum levels before TACE were associated with SD and PD, and the increase of miR-210 serum levels four weeks after the treatment were higher in patients with SD and PD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzato, A.M.; Martingano, P.; Pozzi Mucelli, R.A.; Cavallaro, M.F.M.; Cesarotto, M.; Marcello, C.; Tiribelli, C.; Pascut, D.; Pizzolato, R.; Pozzi Mucelli, F.; et al. MicroRNAs Related to TACE Treatment Response: A Review of the Literature from a Radiological Point of View. Diagnostics 2022, 12, 374. https://doi.org/10.3390/diagnostics12020374
Bozzato AM, Martingano P, Pozzi Mucelli RA, Cavallaro MFM, Cesarotto M, Marcello C, Tiribelli C, Pascut D, Pizzolato R, Pozzi Mucelli F, et al. MicroRNAs Related to TACE Treatment Response: A Review of the Literature from a Radiological Point of View. Diagnostics. 2022; 12(2):374. https://doi.org/10.3390/diagnostics12020374
Chicago/Turabian StyleBozzato, Alessandro Marco, Paola Martingano, Roberta Antea Pozzi Mucelli, Marco Francesco Maria Cavallaro, Matteo Cesarotto, Cristina Marcello, Claudio Tiribelli, Devis Pascut, Riccardo Pizzolato, Fabio Pozzi Mucelli, and et al. 2022. "MicroRNAs Related to TACE Treatment Response: A Review of the Literature from a Radiological Point of View" Diagnostics 12, no. 2: 374. https://doi.org/10.3390/diagnostics12020374
APA StyleBozzato, A. M., Martingano, P., Pozzi Mucelli, R. A., Cavallaro, M. F. M., Cesarotto, M., Marcello, C., Tiribelli, C., Pascut, D., Pizzolato, R., Pozzi Mucelli, F., Giuffrè, M., Crocè, L. S., & Cova, M. A. (2022). MicroRNAs Related to TACE Treatment Response: A Review of the Literature from a Radiological Point of View. Diagnostics, 12(2), 374. https://doi.org/10.3390/diagnostics12020374







