Feasibility of Fetal Portal Venous System Ultrasound Assessment at the FT Anomaly Scan
Abstract
:1. Introduction
Aim
2. Materials and Methods
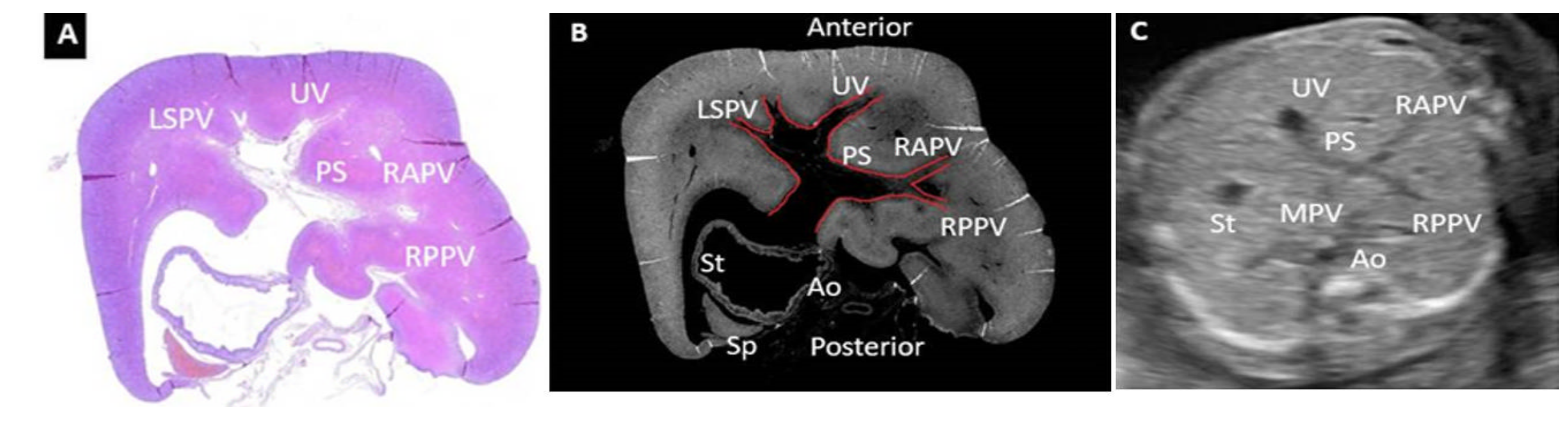
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yagel, S.; Kivilevitch, Z.; Cohen, S.M.; Valsky, D.V.; Messing, B.; Shen, O.; Achiron, R. The fetal venous system, part I: Normal embryology, anatomy, hemodynamics, ultrasound evaluation and Doppler investigation. Ultrasound Obstet. Gynecol. 2010, 35, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Strizek, B.; Zamprakou, A.; Gottschalk, I.; Roethlisberger, M.; Hellmund, A.; Müller, A.; Gembruch, U.; Geipel, A.; Berg, C. Prenatal diagnosis of agenesis of ductus venosus: A retrospective study of anatomic variants, associated anomalies and impact on postnatal outcome. Ultraschall Med. 2019, 40, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, D.G.; Cara, M.L.; Tudorache, S.; Antsaklis, P.; Novac, L.V.; Antsaklis, A.; Cernea, N. Agenesis of ductus venosus in sequential first and second trimester screening. Prenat. Diagn. 2014, 34, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, M.; Ohhama, Y.; Nishi, T.; Yamamoto, H.; Fujita, S.; Take, H.; Adachi, M.; Tachibana, K.; Aida, N.; Kato, K.; et al. Congenital absence of the portal vein and role of liver transplantation in children. J. Pediatr. Surg. 2001, 36, 1026–1031. [Google Scholar] [CrossRef]
- Guérin, F.; Blanc, T.; Gauthier, F.; Abella, S.F.; Branchereau, S. Congenital portosystemic vascular malformations. Semin. Pediatr. Surg. 2012, 21, 233–244. [Google Scholar] [CrossRef]
- Kerlan, R.K., Jr.; Sollenberger, R.D.; Palubinskas, A.J.; Raskin, N.H.; Callen, P.W.; Ehrenfeld, W.K. Portal-systemic encephalopathy due to a congenital portocaval shunt. AJR Am. J. Roentgenol. 1982, 139, 1013–1015. [Google Scholar] [CrossRef]
- Ohno, T.; Muneuchi, J.; Ihara, K.; Yuge, T.; Kanaya, Y.; Yamaki, S.; Hara, T. Pulmonary hypertension in patients with congenital portosystemic venous shunt: A previously unrecognized association. Pediatrics 2008, 121, e892–e899. [Google Scholar] [CrossRef]
- Jaeggi, E.T.; Fouron, J.C.; Hornberger, L.K.; Proulx, F.; Oberhänsli, I.; Yoo, S.J.; Fermont, L. Agenesis of the ductus venosus that is associated with extrahepatic umbilical vein drainage: Prenatal features and clinical outcome. Am. J. Obstet. Gynecol. 2002, 187, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Shen, O.; Valsky, D.V.; Messing, B.; Cohen, S.M.; Lipschuetz, M.; Yagel, S. Shunt diameter in agenesis of the ductus venosus with extrahepatic portosystemic shunt impacts on prognosis. Ultrasound Obstet. Gynecol. 2011, 37, 184–190. [Google Scholar] [CrossRef]
- Achiron, R.; Gindes, L.; Kivilevitch, Z.; Kuint, J.; Kidron, D.; Boyanover, Y.; Yakobson, J.; Heggesh, J. Prenatal diagnosis of congenital agenesis of the fetal portal venous system. Ultrasound Obstet. Gynecol. 2009, 34, 643–652. [Google Scholar] [CrossRef]
- Achiron, R.; Kivilevitch, Z. Fetal umbilical-portal-systemic venous shunt: In-utero classification and clinical significance. Ultrasound Obstet. Gynecol. 2016, 47, 739–747. [Google Scholar] [CrossRef] [Green Version]
- Iliescu, D.; Tudorache, S.; Comanescu, A.; Antsaklis, P.; Cotarcea, S.; Novac, L.; Cernea, N.; Antsaklis, A. Improved detection rate of structural abnormalities in the first trimester using an extended examination protocol. Ultrasound Obstet. Gynecol. 2013, 42, 300–309. [Google Scholar] [CrossRef]
- Yagel, S.; Kivilevitch, Z.; Achiron, R. The fetal venous system: Normal embryology anatomy, and physiology, and the development and appearance of anomalies. In Fetal Cardiology; Yagel, S., Silverman, N.H., Gembruch, U., Eds.; Martin Dunitz: London, UK, 2003; Chapter 27; pp. 321–332. [Google Scholar]
- Morgan, G.; Superina, R. Congenital absence of the portal vein: Two cases and a proposed classification system for portosystemic vascular anomalies. J. Pediatr. Surg. 1994, 29, 1239–1241. [Google Scholar] [CrossRef]
- Bellotti, M.; Pennati, G.; De Gasperi, C.; Bozzo, M.; Battaglia, F.C.; Ferrazzi, E. Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth-restricted human fetuses. Am. J. Obstet. Gynecol. 2004, 190, 1347–1358. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity and Overweight; Fact Sheet n° 311; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Hendler, I.; Blackwell, S.C.; Bujold, E.; Treadwell, M.C.; Mittal, P.; Sokol, R.J.; Sorokin, Y. Suboptimal second-trimester ultrasonographic visualization of the fetal heart in obese women should we repeat the examination? J. Ultrasound Med. 2005, 24, 1205–1209. [Google Scholar] [CrossRef] [Green Version]
- Salomon, L.J.; Alfirevic, Z.; Bilardo, C.M.; Chalouhi, G.E.; Ghi, T.; Kagan, K.O.; Lau, T.K.; Papageorghiou, A.T.; Raine-Fenning, N.J.; Stirnemann, J.; et al. ISUOG practice guidelines: Performance of first-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2013, 41, 102–113. [Google Scholar] [CrossRef]
- Zorn, A.M. Liver Development; StemBook: Athens, GA, USA, 2008. [Google Scholar] [CrossRef]
- Johnson, G.R.; Moore, M.A. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature 1975, 258, 726–728. [Google Scholar] [CrossRef]
- Rouiller, C.H. THE LIVER (Morphology, Biochemistry, Physiology 1), 1st ed.; Academic Press: New York, NY, USA, 1963. [Google Scholar]
- MacParland, S.A.; Liu, J.C.; Ma, X.Z.; Innes, B.T.; Bartczak, A.M.; Gage, B.K.; Manuel, J.; Khuu, N.; Echeverri, J.; Linares, I.; et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 2018, 9, 4383. [Google Scholar] [CrossRef] [Green Version]
- Moore, K. Clinically Oriented Anatomy, 8th ed.; LWW: Philadelphia, PA, USA, 2018; p. 501. [Google Scholar]
- Pernick, N. Stains % CD Markers. Cytokeratin AE1/AE3. Pathology Outlines. 2013. Available online: https://www.pathologyoutlines.com/topic/stainsae1ae3.html (accessed on 28 January 2021).
- Kmieć, Z. Cooperation of liver cells in health and disease. Adv. Anat. Embryol. Cell Biol. 2001, 161, 1–151. [Google Scholar] [CrossRef]
- Hajira, B.; Tan, M.L.; Webster, D.R. Histology, Kupffer Cell; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK493226/ (accessed on 25 August 2020).
- Nguyen-Lefebvre, A.T.; Horuzsko, A. Kupffer Cell Metabolism and Function. J. Enzymol. Metab. 2015, 1, 101. [Google Scholar]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer Cells in the Liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar]
- Allen, J.D. Human Physiology—The Basis of Medicine. Ulst. Med. J. 2008, 77, 216. [Google Scholar]
- Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001, 21, 311–335. [Google Scholar] [CrossRef]
- Winau, F.; Hegasy, G.; Weiskirchen, R.; Weber, S.; Cassan, C.; Sieling, P.A.; Modlin, R.L.; Liblau, R.S.; Gressner, A.M.; Kaufmann, S.H. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 2007, 26, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, F.J.; Friedman, S.L.; Fibrogenesis, I. New insights into hepatic stellate cell activation: The simple becomes complex. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G7–G11. [Google Scholar] [CrossRef]
- Achiron, R.; Hegesh, J.; Yagel, S.; Lipitz, S.; Cohen, S.B.; Rotstein, Z. Abnormalities of the fetal central veins and umbilico-portal system: Prenatal ultrasonographic diagnosis and proposed classification. Ultrasound Obstet. Gynecol. 2000, 16, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Mavrides, E.; Moscoso, G.; Carvalho, J.S.; Campbell, S.; Thilaganathan, B. The anatomy of the umbilical, portal and hepatic venous systems in the human fetus at 14-19 weeks of gestation. Ultrasound Obstet. Gynecol. 2001, 18, 598–604. [Google Scholar] [CrossRef] [PubMed]
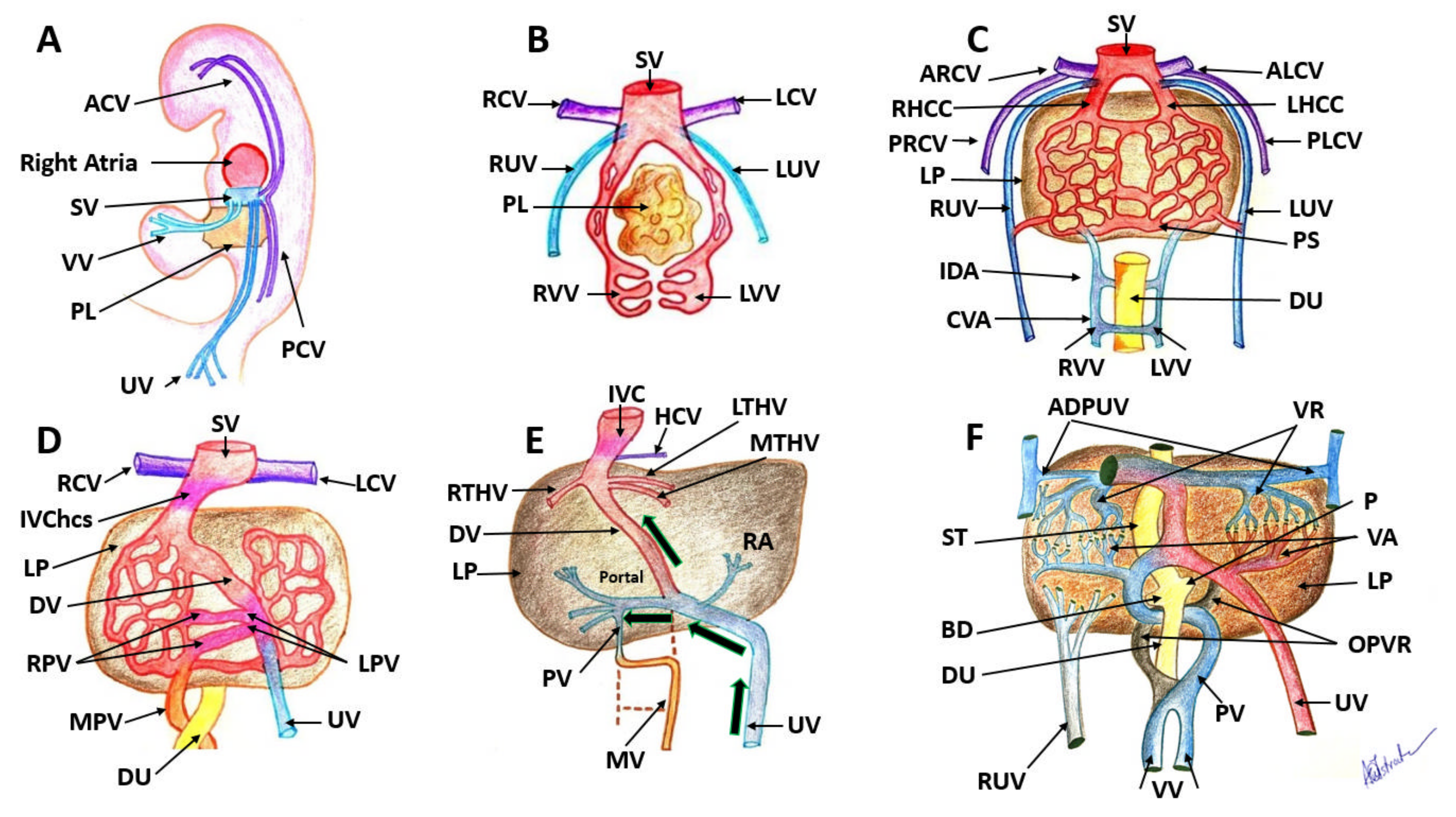
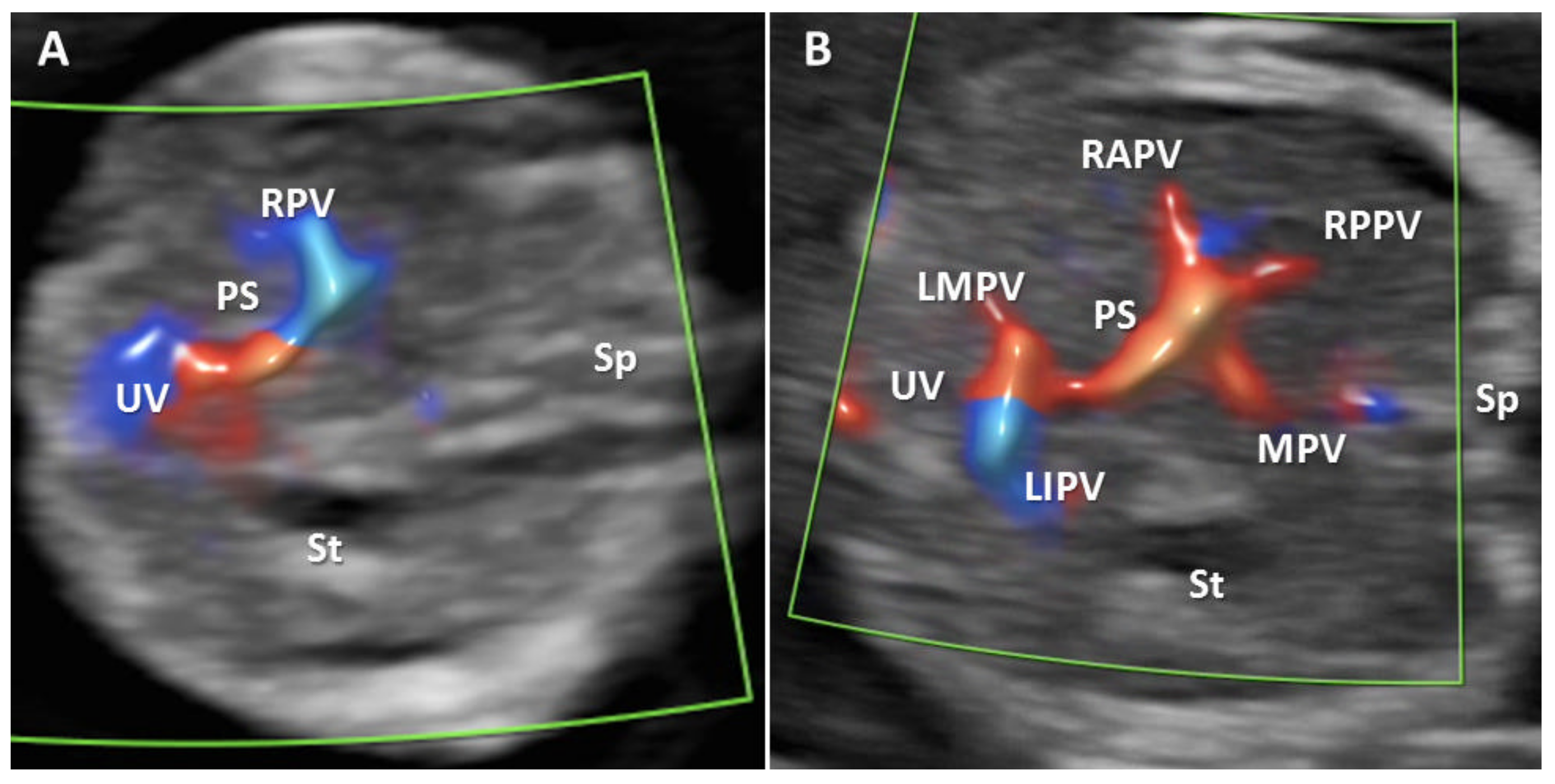

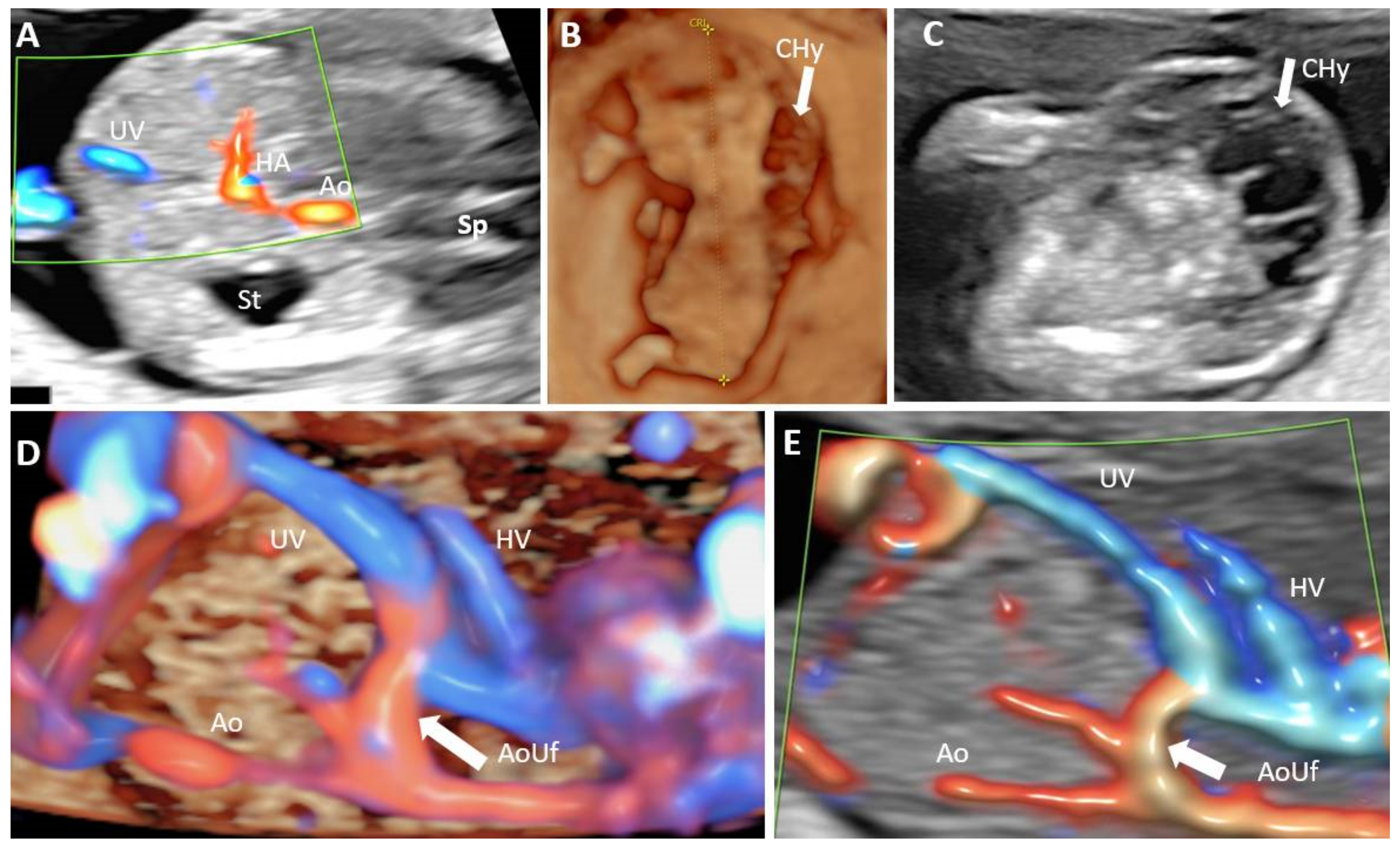

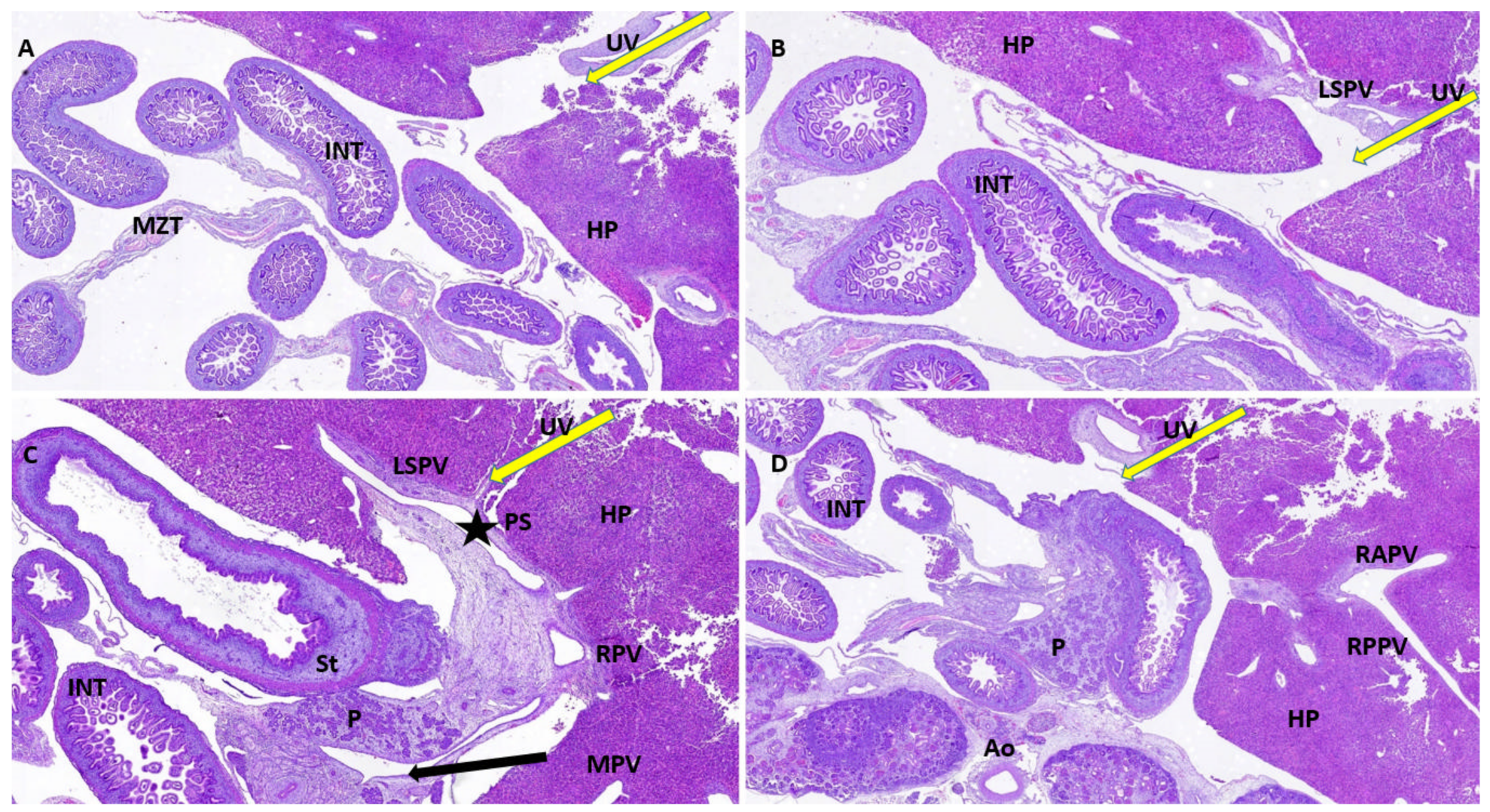



| Antibody | Manufacturer | Clone | Antigenic Exposure | Secondary Antibody | Dilution | Labeling |
|---|---|---|---|---|---|---|
| Anti-CK AE1-AE3 | Dako | AE1/AE3 | Citrate, pH 6 | Monoclonal Mouse Anti-Human Cytokeratin | 1:50 | Epithelial tissues |
| Anti-CD68 | Dako | KP1 | Citrate, pH 6 | Monoclonal Mouse Anti-Human CD68 | 1:100 | Macrophages |
| Anti-αSMA | Dako | 1A4 | Citrate, pH 6 | Monoclonal Mouse Anti-Human Smooth Muscle Actin | 1:100 | α actin smooth muscle |
| Identification of All PVS Features by TAUS | Associated Unfavorable Conditions | Visualization of the L-Shaped UV Confluence, TA US | Satisfactory Visualization of PVS Features after Reschedule/TV Evaluation | Associate Unfavorable Conditions at Reschedule/TV Evaluation | Visualization of the L-Shaped UV Confluence at Reschedule/TV Evaluation | Final Visualization Rate of PVS Features | Final Visualization Rate of L-Shaped UV Confluence | |
|---|---|---|---|---|---|---|---|---|
| Group I | 27 (of 100 cases), 27% | Fibroids 3 cases, 4.11% | 91 (of 100 cases) 91% (one atypical, one abnormal-TPVSA) | 61 (of 73 cases), 83.56% | persistent unfavorable fetal position- vertical lie, 5 cases 41.66%) | 7 of 9 cases (77.77%) | 88% | 98% |
| BMI > 24 19 cases, 26.02% | ||||||||
| unfavorable fetal position 9 cases, 12.32% | ||||||||
| retroverted uterus 9 cases, 12.32% | persistent unfavorable fetal position- far from the probe 6 cases (50%) | |||||||
| Abdominal scar 8 cases, 10.96% | Istmic fibromyoma 1 case (8.33%) | |||||||
| Fibroids and abdominal scar, 4 cases, 5.48% | ||||||||
| Unfavorable fetal position and increased BMI, 9 cases, 12.32% | ||||||||
| Increased BMI and abdominal scar, 12 cases, 16.43% | ||||||||
| Group II | 14 (of 100 cases), 14% | Fibroids 6 cases, 6.97% | 79 (of 100 cases), 79% | 58 (of 86 cases), 67.44% | low-lying fibroids 2 cases, 7.14% | 16 of 21 cases (76.19%) | 72% | 95% |
| BMI > 24 22 cases, 25.58% | ||||||||
| unfavorable fetal position 17 cases, 19.76% | persistent unfavorable fetal position -vertical lie, 12 cases, 42.85% | |||||||
| retroverted uterus 16 cases, 18.60% | persistent unfavorable fetal position- fetus far from the probe 14 cases, 50% | |||||||
| Abdominal scar 4 cases, 4.65% | ||||||||
| Fibroids and abdominal scar, 3 cases, 3.49% | ||||||||
| Unfavorable fetal position and increased BMI, 18 cases, 20.93% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, R.D.; Ruican, D.; Zorilă, G.-L.; Istrate-Ofiţeru, A.-M.; Badiu, A.M.; Iliescu, D.G. Feasibility of Fetal Portal Venous System Ultrasound Assessment at the FT Anomaly Scan. Diagnostics 2022, 12, 361. https://doi.org/10.3390/diagnostics12020361
Nagy RD, Ruican D, Zorilă G-L, Istrate-Ofiţeru A-M, Badiu AM, Iliescu DG. Feasibility of Fetal Portal Venous System Ultrasound Assessment at the FT Anomaly Scan. Diagnostics. 2022; 12(2):361. https://doi.org/10.3390/diagnostics12020361
Chicago/Turabian StyleNagy, Rodica Daniela, Dan Ruican, George-Lucian Zorilă, Anca-Maria Istrate-Ofiţeru, Anne Marie Badiu, and Dominic Gabriel Iliescu. 2022. "Feasibility of Fetal Portal Venous System Ultrasound Assessment at the FT Anomaly Scan" Diagnostics 12, no. 2: 361. https://doi.org/10.3390/diagnostics12020361
APA StyleNagy, R. D., Ruican, D., Zorilă, G.-L., Istrate-Ofiţeru, A.-M., Badiu, A. M., & Iliescu, D. G. (2022). Feasibility of Fetal Portal Venous System Ultrasound Assessment at the FT Anomaly Scan. Diagnostics, 12(2), 361. https://doi.org/10.3390/diagnostics12020361







