Shear-Wave-Elastography in Neurofibromatosis Type I
Abstract
:1. Introduction
2. Materials and Methods
3. Results
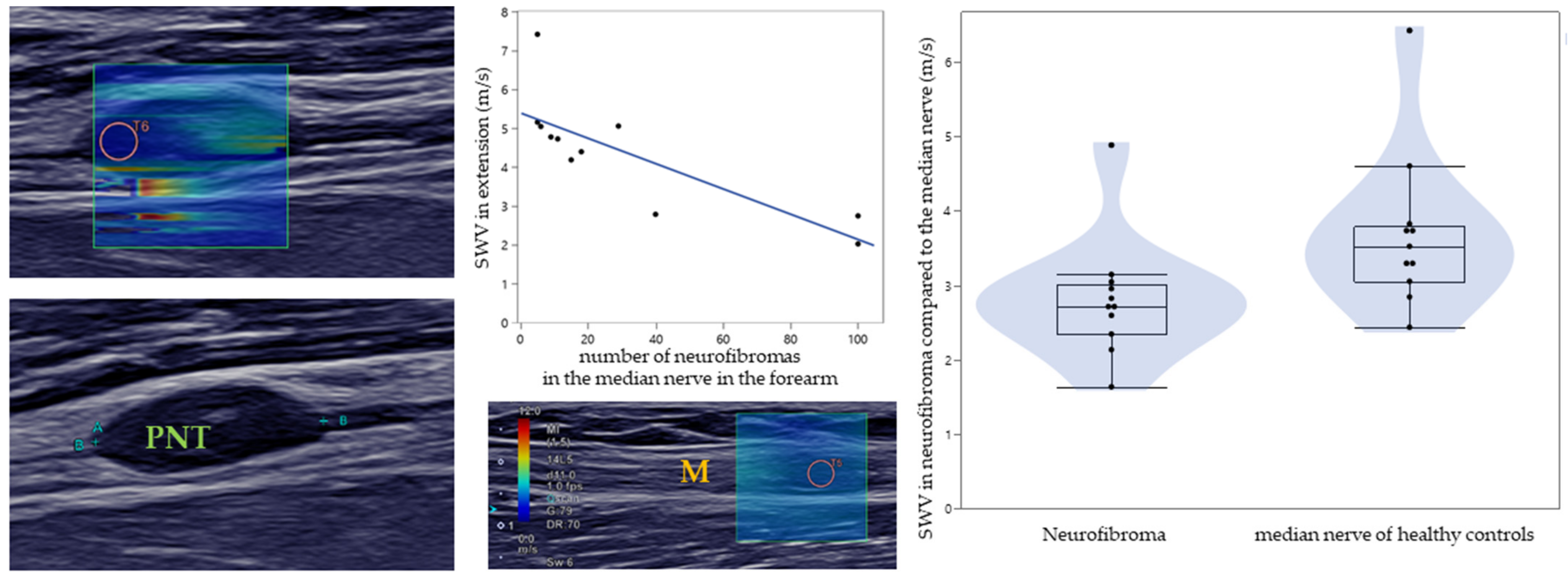
3.1. SWE of PNTs in NF1
3.2. Comparison between Patients and Healthy Controls
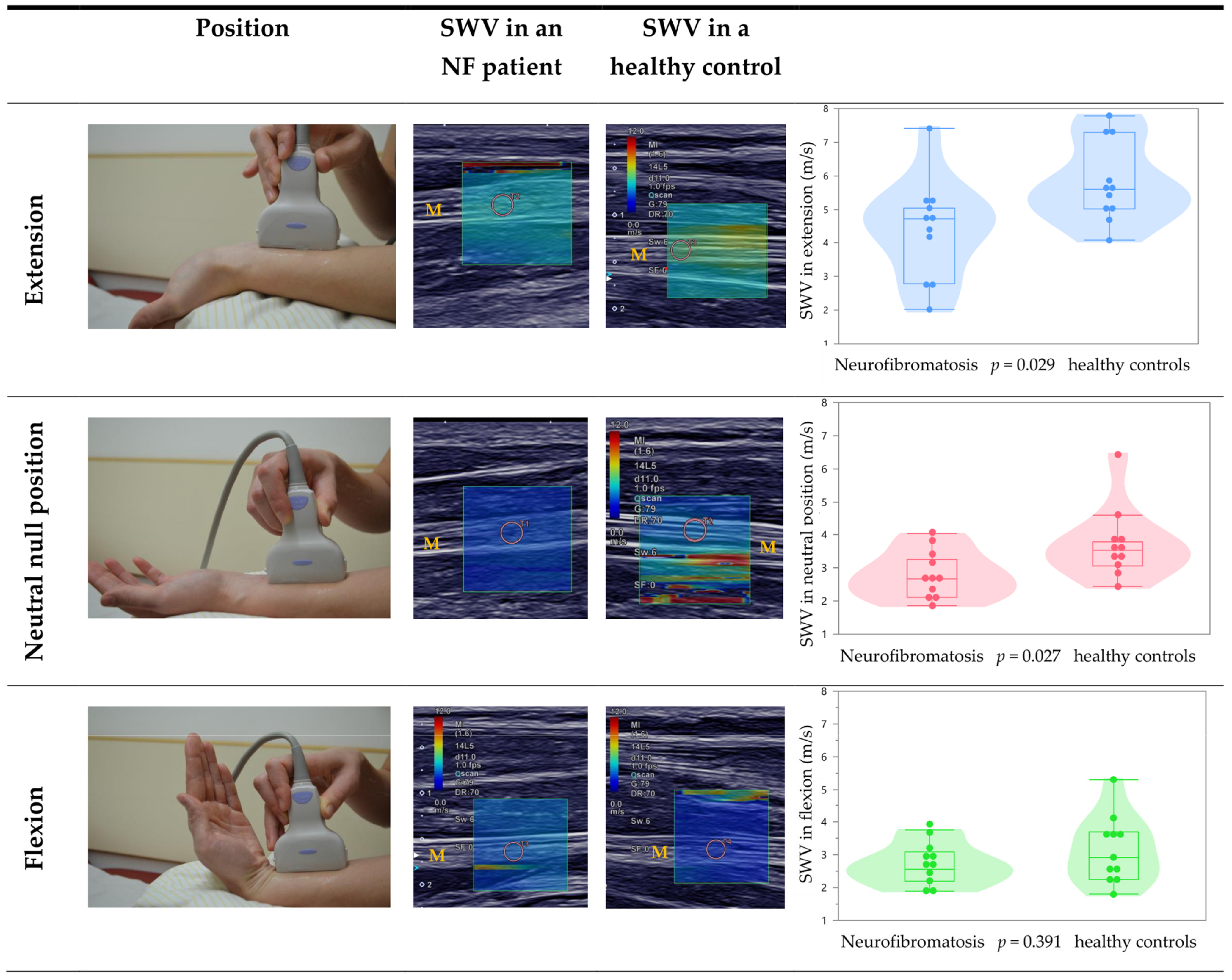
3.3. Effect of Different Wrist Positions on the Median Nerve
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gürün, E.; Akdulum, I.; Akyüz, M.; Oktar, S. Özhan Shear wave elastography evaluation of brachial plexus in multiple sclerosis. Acta Radiol. 2021, 2841851211002828. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G. Shear wave liver elastography. Abdom. Radiol. 2018, 43, 800–807. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, J.Y.; Lee, N.K.; Lee, J.W.; Song, Y.S.; Park, S.Y.; Shin, J.K. Three-dimensional versus two-dimensional shear-wave elastography: Associations of mean elasticity values with prognostic factors and tumor subtypes of breast cancer. Clin. Imaging 2018, 48, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Seo, B.K.; Park, E.K.; Kwon, M.; Jeong, H.; Cho, K.R.; Woo, O.H.; Song, S.E.; Cha, J. Tumor stiffness measured by shear wave elastography correlates with tumor hypoxia as well as histologic biomarkers in breast cancer. Cancer Imaging 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.S.S.; Tong, C.S.L.; Cho, C.C.M.; Yuen, E.H.Y.; Lee, Y.Y.P.; Ahuja, A.T. Shear wave elastography of thyroid nodules in routine clinical practice: Preliminary observations and utility for detecting malignancy. Eur. Radiol. 2012, 22, 2397–2406. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. RadioGraphics 2017, 37, 855–870. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.; Peterson, S.M.; Wessner, C.E.; Machado, P.; Forsberg, F. Diagnosis of Carpal Tunnel Syndrome using Shear Wave Elastography and High-frequency Ultrasound Imaging. Acad. Radiol. 2021, 28, e278–e287. [Google Scholar] [CrossRef]
- Paluch, Ł.; Noszczyk, B.; Nitek, Ż.; Walecki, J.; Osiak, K.; Pietruski, P. Shear-wave elastography: A new potential method to diagnose ulnar neuropathy at the elbow. Eur. Radiol. 2018, 28, 4932–4939. [Google Scholar] [CrossRef] [Green Version]
- Winter, N.; Rattay, T.W.; Axer, H.; Schäffer, E.; Décard, B.F.; Gugel, I.; Schuhmann, M.; Grimm, A. Ultrasound assessment of peripheral nerve pathology in neurofibromatosis type 1 and 2. Clin. Neurophysiol. 2017, 128, 702–706. [Google Scholar] [CrossRef]
- Stuart, R.M.; Koh, E.S.C.; Breidahl, W.H. Sonography of Peripheral Nerve Pathology. Am. J. Roentgenol. 2004, 182, 123–129. [Google Scholar] [CrossRef]
- Fornage, B.D. Peripheral nerves of the extremities: Imaging with US. Radiology 1988, 167, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.L.; Jacobson, J.; Inampudi, P.; Jamadar, D.A.; Ebrahim, F.S.; Hayes, C.W. Sonographic Characteristics of Peripheral Nerve Sheath Tumors. Am. J. Roentgenol. 2004, 182, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Farschtschi, S.; Mautner, V.-F.; McLean, A.C.L.; Schulz, A.; Friedrich, R.E.; Rosahl, S.K. The Neurofibromatoses. Dtsch. Aerzteblatt Online 2020, 117, 354–360; [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Dietemann, J. Imagerie de la neurofibromatose de type 1. J. Neuroradiol. 2005, 32, 180–197. [Google Scholar] [CrossRef]
- Azizi, G.; Piper, K.; Keller, J.M.; Mayo, M.L.; Puett, D.; Earp, K.M.; Malchoff, C.D. Shear wave elastography and parathyroid adenoma: A new tool for diagnosing parathyroid adenomas. Eur. J. Radiol. 2016, 85, 1586–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.-C.; Wu, M.-H.; Chen, K.-Y.; Hsieh, M.-S.; Chen, A.; Chen, C.-N. Ultrasonographic features for differentiating follicular thyroid carcinoma and follicular adenoma. Asian J. Surg. 2020, 43, 339–346. [Google Scholar] [CrossRef]
- Liu, C.; Li, T.T.; Hu, Z.; Li, Y.; Cheng, X.; Zhu, Y.; Lu, M. Transvaginal Real-time Shear Wave Elastography in the Diagnosis of Cervical Disease. J. Ultrasound Med. 2019, 38, 3173–3181. [Google Scholar] [CrossRef]
- Ozturk, M.; Selcuk, M.B.; Polat, A.V.; Ozbalci, A.B.; Baris, Y.S. The diagnostic value of ultrasound and shear wave elastography in the differentiation of benign and malignant soft tissue tumors. Skelet. Radiol. 2020, 49, 1795–1805. [Google Scholar] [CrossRef]
- Zhong, L.-C.; Yang, T.; Gu, L.-P.; Ma, F. The diagnostic performance of shear wave velocity ratio for the differential diagnosis of benign and malignant breast lesions: Compared with VTQ, and mammography. Clin. Hemorheol. Microcirc. 2021, 77, 123–131. [Google Scholar] [CrossRef]
- Kantarci, F.; Ustabasioglu, F.E.; Delil, S.; Olgun, D.C.; Korkmazer, B.; Dikici, A.S.; Tutar, O.; Nalbantoglu, M.; Uzun, N.; Mihmanli, I. Median nerve stiffness measurement by shear wave elastography: A potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur. Radiol. 2014, 24, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yan, F.; He, Y.; Wang, L.; Xiang, X.; Tang, Y.; Yang, Y.; Qiu, L. Evaluation of the healthy median nerve elasticity. Medicine 2018, 97, e12956. [Google Scholar] [CrossRef] [PubMed]
- Greening, J.; Dilley, A. Posture-induced changes in peripheral nerve stiffness measured by ultrasound shear-wave elastography. Muscle Nerve 2017, 55, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugel, C.L.; Franz, C.K.; Lee, S.S.M. Influence of limb position on assessment of nerve mechanical properties by using shear wave ultrasound elastography. Muscle Nerve 2020, 61, 616–622. [Google Scholar] [CrossRef]
| Parameter | Neurofibromatosis Type I | Healthy Controls |
|---|---|---|
| Age (years) | 30.0 ± 10.6, 21/27/33 (21–51) | 30.6 ± 11.0, 21/27/37 (20–53) |
| Body height (cm) | 168.7 ± 11.3, 160/168/178 (153–187) | 175.9 ± 8.0, 169/176/183 (163–189) |
| Body weight (kg) | 69.6 ± 17.7, 57/64/78 (48–110) | 74.6 ± 14.8, 62/75/85 (54–104) |
| Number of PNTs in the median nerve (forearm) * | 30.7 ± 35.9, 6/15/40 (5–100) Median: 15 |
| Parameter | Healthy Controls (HC) | Neurofibromatosis Type I (NF I) | Comparision NF vs. HC |
|---|---|---|---|
| SWV of PNT/median nerve in lower arm (m/s) | 2.8 ± 0.8, 2.4/2.7/3.0 (1.6–4.9) | U = 21.0, p = 0.008 | |
| SWV of median nerve in extension (m/s) + | 5.8 ± 1.2, 5.0/5.6/7.3 (4.1–7.8) | 4.4 ± 1.5, 2.8/4.7/5.1 (2.0–7.4) | U = 27.5, p = 0.029 |
| SWV of median nerve in neutral position (m/s) + | 3.8 ± 1.1, 3.1/3.5/3.8 (2.4–6.4) | 2.8 ± 0.7, 2.1/2.7/3.3 (1.9–4.0) | U = 27.0, p = 0.027 |
| SWV of median nerve in flexion (m/s) + | 3.2 ± 1.0, 2.3/2.9/3.7 (1.8–5.3) | 2.7 ± 0.6, 2.2/2.6/3.1 (1.9–3.8) | U = 47.0, p = 0.391 |
| Selected PNT length in lower arm (mm) | 11.9 ± 8.0, 7.0/9.8/14.3 (1.4–27.8) | ||
| Selected PNT height in lower arm (mm) | 3.0 ± 0.4, 2.7/3.0/3.4 (2.5–3.7) | 3.1 ± 2.1, 1.4/1.9/14.3 (1.0–7.4) | U = 54.5, p = 0.711 |
| Selected width PNT in lower arm (mm) | 4.4 ± 0.5, 4.1/4.3/4.7 (3.7–5.3) | 4.5 ± 3.0, 2.4/3.0/6.0 (1.4–12.2) | U = 52.5, p = 0.617 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staber, D.; Oppold, J.; Grimm, A.; Schuhmann, M.U.; Romano, A.; Marquetand, J.; Kleiser, B. Shear-Wave-Elastography in Neurofibromatosis Type I. Diagnostics 2022, 12, 360. https://doi.org/10.3390/diagnostics12020360
Staber D, Oppold J, Grimm A, Schuhmann MU, Romano A, Marquetand J, Kleiser B. Shear-Wave-Elastography in Neurofibromatosis Type I. Diagnostics. 2022; 12(2):360. https://doi.org/10.3390/diagnostics12020360
Chicago/Turabian StyleStaber, Deborah, Julia Oppold, Alexander Grimm, Martin U. Schuhmann, Alyssa Romano, Justus Marquetand, and Benedict Kleiser. 2022. "Shear-Wave-Elastography in Neurofibromatosis Type I" Diagnostics 12, no. 2: 360. https://doi.org/10.3390/diagnostics12020360
APA StyleStaber, D., Oppold, J., Grimm, A., Schuhmann, M. U., Romano, A., Marquetand, J., & Kleiser, B. (2022). Shear-Wave-Elastography in Neurofibromatosis Type I. Diagnostics, 12(2), 360. https://doi.org/10.3390/diagnostics12020360






