Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients
Abstract
1. Introduction
2. Results
2.1. Preoperative Serum and Urine Levels of sCD163, sPD-L1, and sIL-10
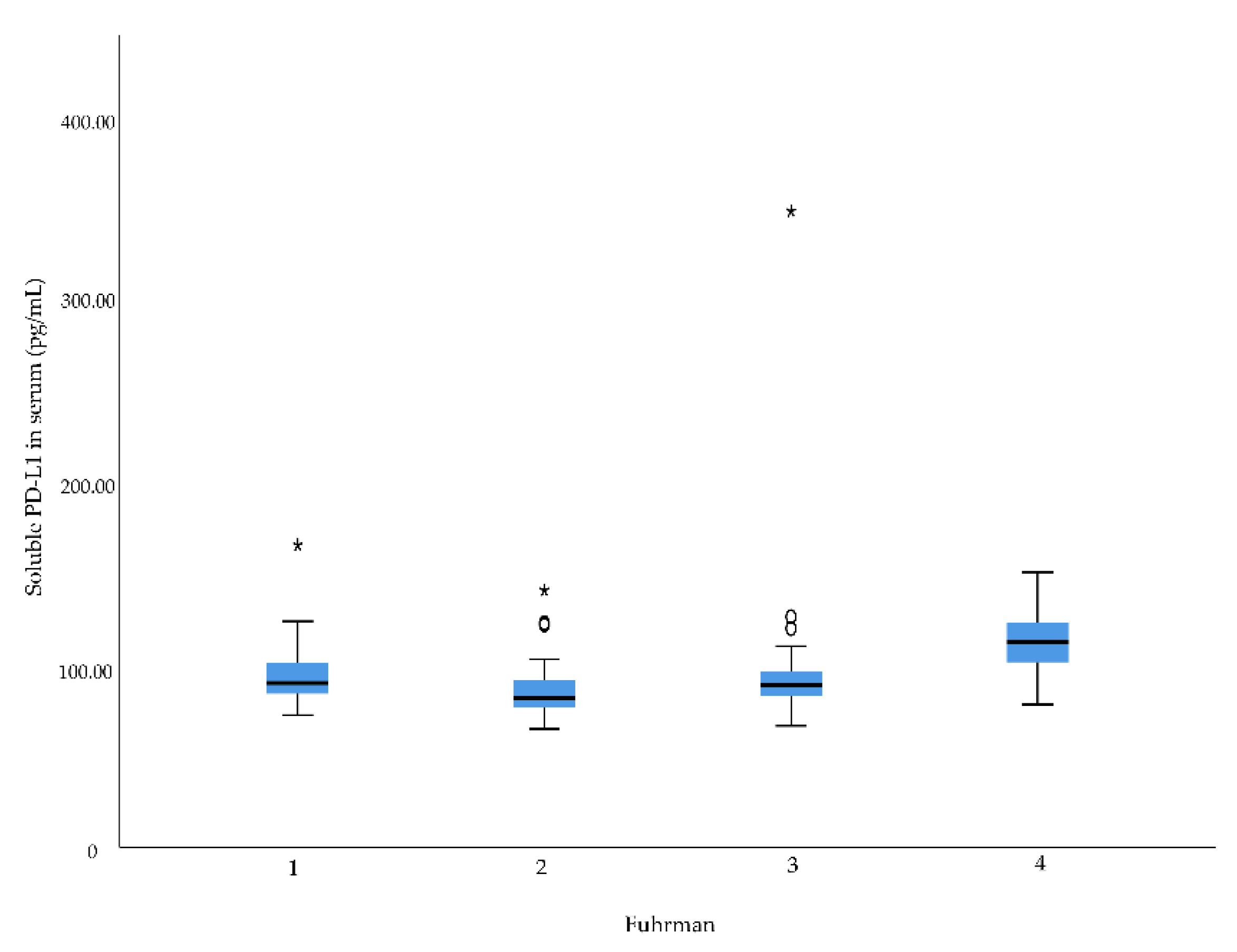
2.2. Associations between Preoperative sCD163 and sPD-L1 Levels and Clinico-Pathological Characteristics in RCC
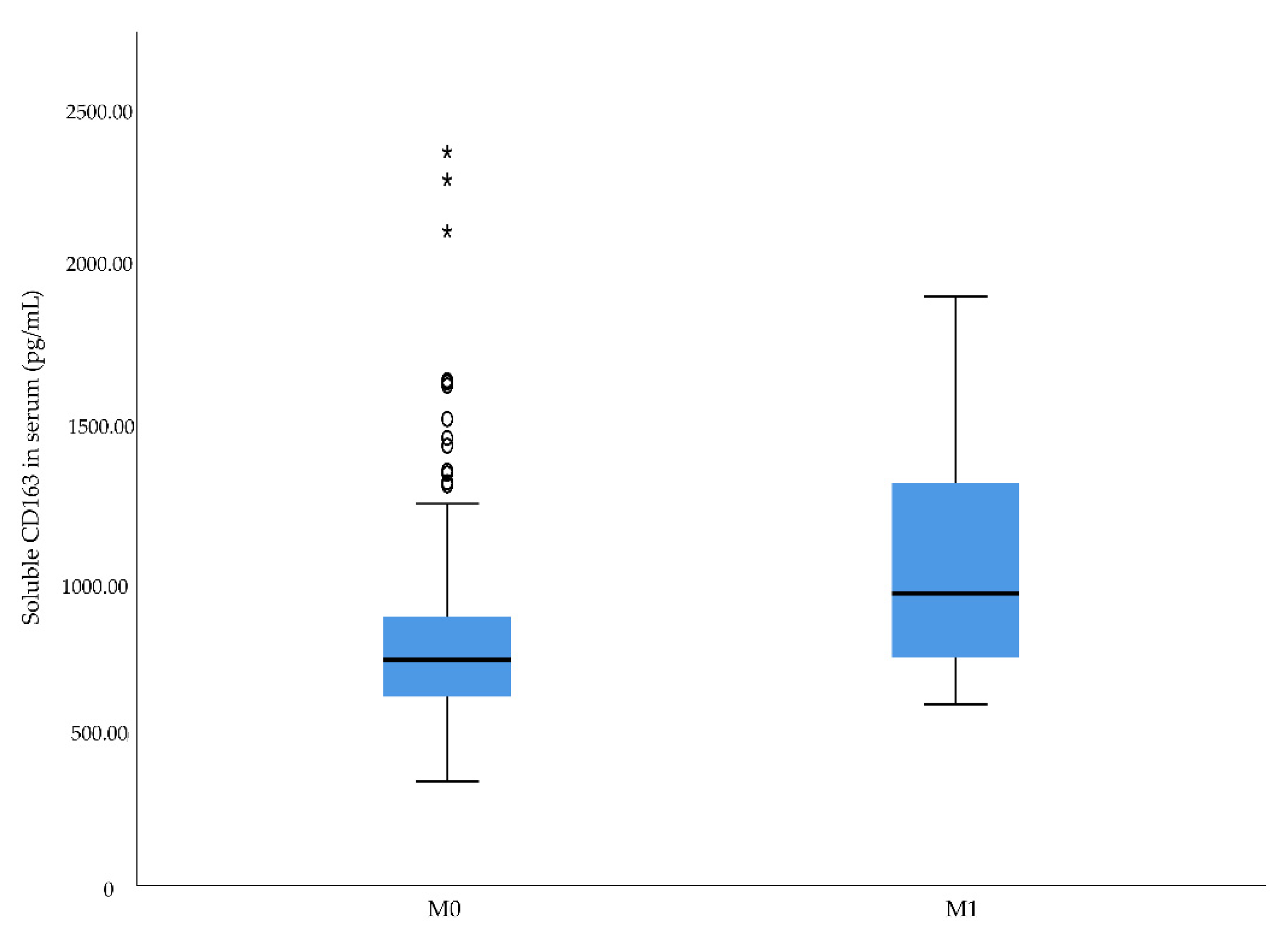
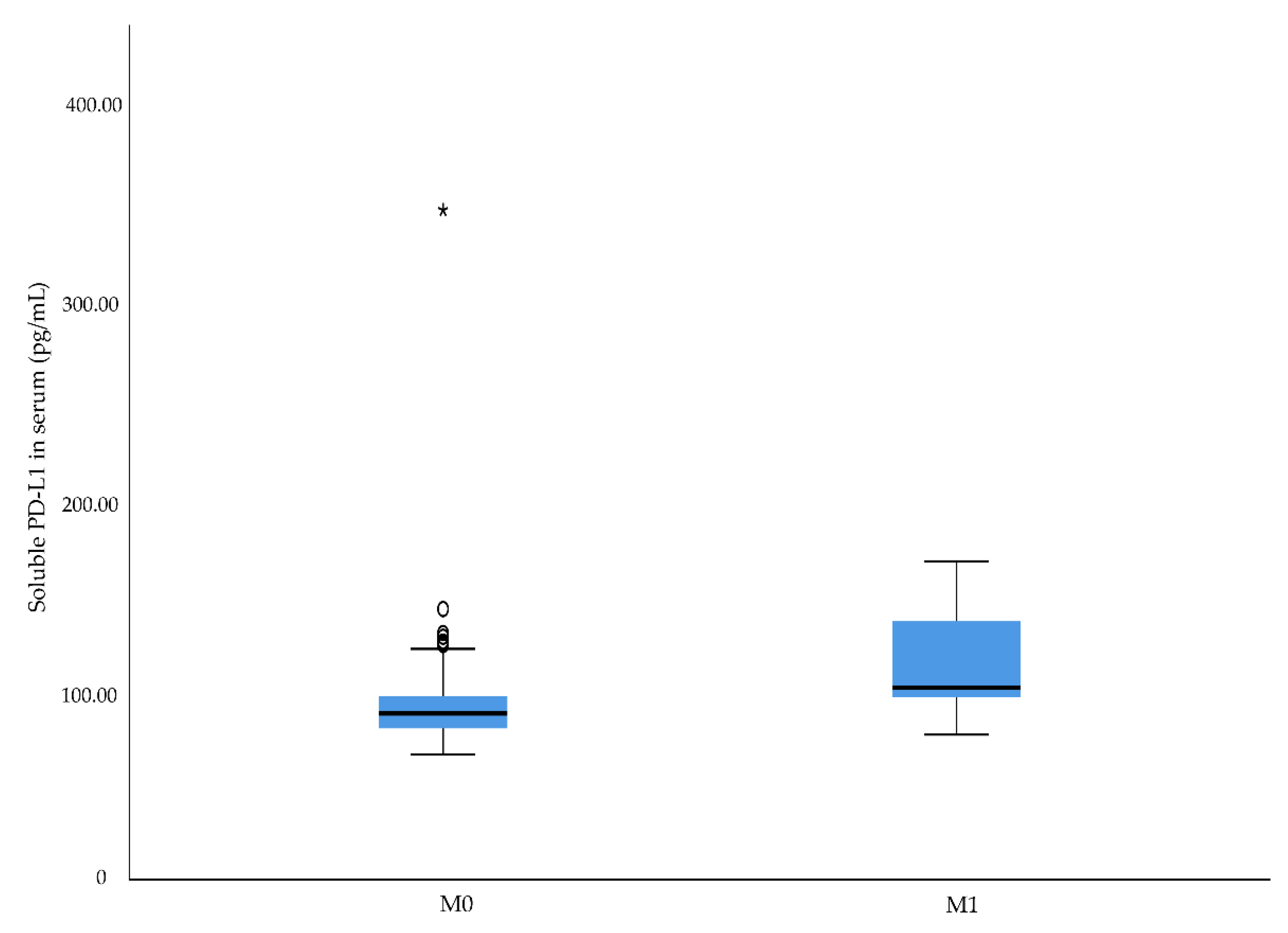
2.3. Associations between Preoperative sCD163 and sPD-L1 Levels and Cancer-Specific Death
3. Discussion
4. Materials and Methods
4.1. Measurement of sCD163, sPD-L1, and sIL-10 in Serum and Urine Samples
4.2. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Takeya, M.; Komohara, Y. Role of tumor-associated macrophages in human malignancies: Friend or foe? Pathol. Int. 2016, 66, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Moriyama, M.; Furukawa, S.; Rafiul, H.; Maruse, Y.; Jinno, T.; Tanaka, A.; Ohta, M.; Ishiguro, N.; Yamauchi, M.; et al. CD163+CD204+ tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1755. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.R.; Reis, S.T.; Junior, J.P.; Zerati, M.; Gomes Dde, O.; Camara-Lopes, L.H.; Srougi, M. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn. Pathol. 2015, 10, 189. [Google Scholar] [CrossRef]
- Gevensleben, H.; Dietrich, D.; Golletz, C.; Steiner, S.; Jung, M.; Thiesler, T.; Majores, M.; Stein, J.; Uhl, B.; Muller, S.; et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin. Cancer Res. 2016, 22, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Sundqvist, P.; Kosuta, V.; Falt, A.; Giunchi, F.; Fiorentino, M.; Davidsson, S. PD-L1 Expression is Associated With Poor Prognosis in Renal Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Carlsson, J.; Giunchi, F.; Harlow, A.; Kirrander, P.; Rider, J.; Fiorentino, M.; Andren, O. PD-L1 Expression in Men with Penile Cancer and its Association with Clinical Outcomes. Eur. Urol. Oncol. 2019, 2, 214–221. [Google Scholar] [CrossRef]
- Zhong, Q.; Shou, J.; Ying, J.; Ling, Y.; Yu, Y.; Shen, Z.; Zhang, Y.; Li, N.; Shi, Y.; Zhou, A. High PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor in urothelial carcinoma. Future Oncol. 2021, 17, 2893–2905. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, D.; Wu, P.; Wang, Z.; Huang, J. Serum IL-10 Predicts Worse Outcome in Cancer Patients: A Meta-Analysis. PLoS ONE 2015, 10, e0139598. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Marincola, F.; Rossi, C.R.; Nitti, D.; Lise, M. The multifaceted relationship between IL-10 and adaptive immunity: Putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004, 15, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Baba, Y.; Okadome, K.; Kiyozumi, Y.; Hiyoshi, Y.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; et al. Tumour-associated macrophages are associated with poor prognosis and programmed death ligand 1 expression in oesophageal cancer. Eur. J. Cancer 2019, 111, 38–49. [Google Scholar] [CrossRef] [PubMed]
- He, K.F.; Zhang, L.; Huang, C.F.; Ma, S.R.; Wang, Y.F.; Wang, W.M.; Zhao, Z.L.; Liu, B.; Zhao, Y.F.; Zhang, W.F.; et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed. Res. Int. 2014, 2014, 838632. [Google Scholar] [CrossRef]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Falt, A.; Andersson, S.O.; Andren, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Davidsson, S.; Fiorentino, M.; Giunchi, F.; Eriksson, M.; Erlandsson, A.; Sundqvist, P.; Carlsson, J. Infiltration of M2 Macrophages and Regulatory T Cells Plays a Role in Recurrence of Renal Cell Carcinoma. Eur. Urol. Open Sci. 2020, 20, 62–71. [Google Scholar] [CrossRef]
- Ueda, K.; Suekane, S.; Kurose, H.; Chikui, K.; Nakiri, M.; Nishihara, K.; Matsuo, M.; Kawahara, A.; Yano, H.; Igawa, T. Prognostic value of PD-1 and PD-L1 expression in patients with metastatic clear cell renal cell carcinoma. Urol. Oncol. 2018, 36, 499.e9–499.e416. [Google Scholar] [CrossRef]
- Chang, Y.L.; Yang, C.Y.; Huang, Y.L.; Wu, C.T.; Yang, P.C. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget 2017, 8, 18021–18030. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Li, R.; Cao, Y.; Gu, Y.; Lin, C.; Liu, X.; Lv, K.; He, X.; Fang, H.; Jin, K.; et al. Poor Clinical Outcomes and Immunoevasive Contexture in Intratumoral IL-10-Producing Macrophages Enriched Gastric Cancer Patients. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Dannenmann, S.R.; Thielicke, J.; Stockli, M.; Matter, C.; von Boehmer, L.; Cecconi, V.; Hermanns, T.; Hefermehl, L.; Schraml, P.; Moch, H.; et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology 2013, 2, e23562. [Google Scholar] [CrossRef]
- Umemoto, Y.; Okano, S.; Matsumoto, Y.; Nakagawara, H.; Matono, R.; Yoshiya, S.; Yamashita, Y.; Yoshizumi, T.; Ikegami, T.; Soejima, Y.; et al. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J. Gastroenterol. 2015, 50, 65–75. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Canli, O.; Tal, A.; Pleli, T.; Trojan, J.; Schmidt, M.; Kronenberger, B.; Zeuzem, S.; Piiper, A.; Greten, F.R.; et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur. J. Cancer 2016, 59, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Nakamura, T.; Hayashi, A.; Okamoto, T.; Iino, T.; Asanuma, Y.; Hagi, T.; Kita, K.; Nakamura, K.; Sudo, A. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci. Rep. 2020, 10, 9077. [Google Scholar] [CrossRef] [PubMed]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Gershtein, E.S.; Morozov, A.A.; Goryacheva, I.O.; Filipenko, M.L.; Alferov, A.A.; Bezhanova, S.D.; Bazaev, V.V.; Kazantseva, I.A. Soluble Ligand of the Immune Checkpoint Receptor (sPD-L1) in Blood Serum of Patients with Renal Cell Carcinoma. Bull. Exp. Biol. Med. 2019, 166, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kamai, T.; Masuda, A.; Nukui, A.; Abe, H.; Arai, K.; Yoshida, K. Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016, 5, 1810–1820. [Google Scholar] [CrossRef]
- Larrinaga, G.; Solano-Iturri, J.D.; Errarte, P.; Unda, M.; Loizaga-Iriarte, A.; Perez-Fernandez, A.; Echevarria, E.; Asumendi, A.; Manini, C.; Angulo, J.C.; et al. Soluble PD-L1 Is an Independent Prognostic Factor in Clear Cell Renal Cell Carcinoma. Cancers 2021, 13, 667. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Tu, H.; Liang, D.; Chang, D.W.; Ye, Y.; Wu, X. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J. Immunother. Cancer 2019, 7, 334. [Google Scholar] [CrossRef]
- Tosev, G.; Wahafu, W.; Reimold, P.; Damgov, I.; Schwab, C.; Aksoy, C.; Kaczorowski, A.; Stenzinger, A.; Nyarangi-Dix, J.; Hohenfellner, M.; et al. Detection of PD-L1 in the urine of patients with urothelial carcinoma of the bladder. Sci. Rep. 2021, 11, 14244. [Google Scholar] [CrossRef]
- Nederby, L.; Roug, A.S.; Knudsen, S.S.; Skovbo, A.; Kjeldsen, E.; Moller, H.J.; Hokland, M. Soluble CD163 as a prognostic biomarker in B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 3219–3221. [Google Scholar] [CrossRef]
- Andersen, M.N.; Abildgaard, N.; Maniecki, M.B.; Moller, H.J.; Andersen, N.F. Monocyte/macrophage-derived soluble CD163: A novel biomarker in multiple myeloma. Eur. J. Haematol. 2014, 93, 41–47. [Google Scholar] [CrossRef] [PubMed]
- No, J.H.; Moon, J.M.; Kim, K.; Kim, Y.B. Prognostic significance of serum soluble CD163 level in patients with epithelial ovarian cancer. Gynecol. Obstet. Invest. 2013, 75, 263–267. [Google Scholar] [CrossRef]
- Ding, D.; Song, Y.; Yao, Y.; Zhang, S. Preoperative serum macrophage activated biomarkers soluble mannose receptor (sMR) and soluble haemoglobin scavenger receptor (sCD163), as novel markers for the diagnosis and prognosis of gastric cancer. Oncol. Lett. 2017, 14, 2982–2990. [Google Scholar] [CrossRef] [PubMed]
- Wittke, F.; Hoffmann, R.; Buer, J.; Dallmann, I.; Oevermann, K.; Sel, S.; Wandert, T.; Ganser, A.; Atzpodien, J. Interleukin 10 (IL-10): An immunosuppressive factor and independent predictor in patients with metastatic renal cell carcinoma. Br. J. Cancer 1999, 79, 1182–1184. [Google Scholar] [CrossRef]
- Guida, M.; Casamassima, A.; Monticelli, G.; Quaranta, M.; Colucci, G. Basal cytokines profile in metastatic renal cell carcinoma patients treated with subcutaneous IL-2-based therapy compared with that of healthy donors. J. Transl. Med. 2007, 5, 51. [Google Scholar] [CrossRef] [PubMed]


| RCC Cases, n = 144 | |
|---|---|
| Subtypes of RCC, n (%) | |
| Clear cell RCC | 110 (76.4) |
| Chromophobe RCC | 11 (7.6) |
| Papillary RCC type I | 18 (12.5) |
| Papillary RCC type II | 5 (3.5) |
| Tumor diameter, mm (range) | 50.5 (13–230) |
| pT stage, n (%) | |
| 1 | 115 (79.9) |
| 2 | 18 (12.5) |
| 3 | 10 (6.9) |
| 4 | 1 (0.7) |
| pN stage, n (%) | |
| 0 | 142 (98.6) |
| 1 | 2 (1.4) |
| M stage, n (%) | |
| 0 | 136 (94.4) |
| 1 | 8 (5.6) |
| Fuhrman, n (%) | |
| 1 | 24 (17.1) |
| 2 | 66 (47.2) |
| 3 | 42 (30.0) |
| 4 | 8 (5.7) |
| Missing | 4 |
| Variable | sPD-L1 Serum | p-Value | sCD163 Serum | p-Value | sPD-L1 Urine | p-Value |
|---|---|---|---|---|---|---|
| Fuhrman grade | 0.001 | 0.065 | 0.559 | |||
| 1 | 90.0 | 731.4 | 68.1 | |||
| 2 | 81.8 | 740.3 | 66.3 | |||
| 3 | 89.0 | 698.0 | 68.9 | |||
| 4 | 112.2 | 1378.4 | 92.4 | |||
| pT stage | 0.001 | 0.066 | 0.136 | |||
| 1 | 83.8 | 731.5 | 65.6 | |||
| 2 | 93.3 | 941.1 | 69.1 | |||
| 3 | 102.1 | 757.4 | 99.4 | |||
| 4 | 117.8 | 1916.9 | ||||
| pN stage | 0.993 | 0.215 | 0.763 | |||
| 0 | 87.2 | 739.2 | 68.2 | |||
| 1 | 108.6 | 990.0 | ||||
| M stage | 0.011 | 0.041 | 0.023 | |||
| 0 | 86.3 | 734.3 | 67.2 | |||
| 1 | 99.8 | 950.7 | 72.4 | |||
| Cancer-specific death | 0.001 | 0.219 | 0.115 | |||
| No | 85.0 | 736.8 | 67.2 | |||
| Yes | 100.1 | 799.0 | 91.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidsson, S.; Huotilainen, S.; Carlsson, J.; Sundqvist, P. Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients. Diagnostics 2022, 12, 336. https://doi.org/10.3390/diagnostics12020336
Davidsson S, Huotilainen S, Carlsson J, Sundqvist P. Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients. Diagnostics. 2022; 12(2):336. https://doi.org/10.3390/diagnostics12020336
Chicago/Turabian StyleDavidsson, Sabina, Sofia Huotilainen, Jessica Carlsson, and Pernilla Sundqvist. 2022. "Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients" Diagnostics 12, no. 2: 336. https://doi.org/10.3390/diagnostics12020336
APA StyleDavidsson, S., Huotilainen, S., Carlsson, J., & Sundqvist, P. (2022). Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients. Diagnostics, 12(2), 336. https://doi.org/10.3390/diagnostics12020336






