Iron in Porphyrias: Friend or Foe?
Abstract
:1. Introduction
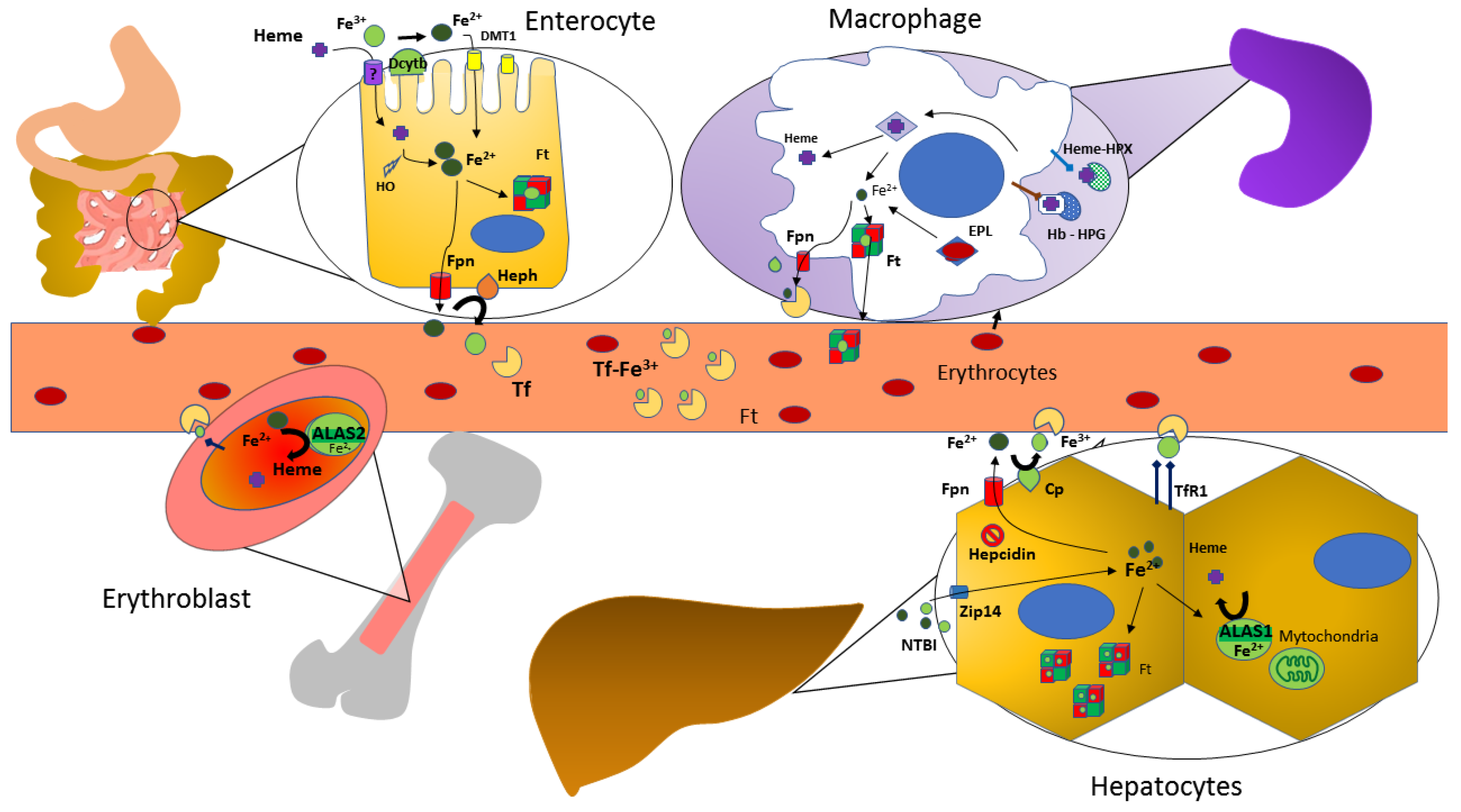
2. Overview on Iron Metabolism
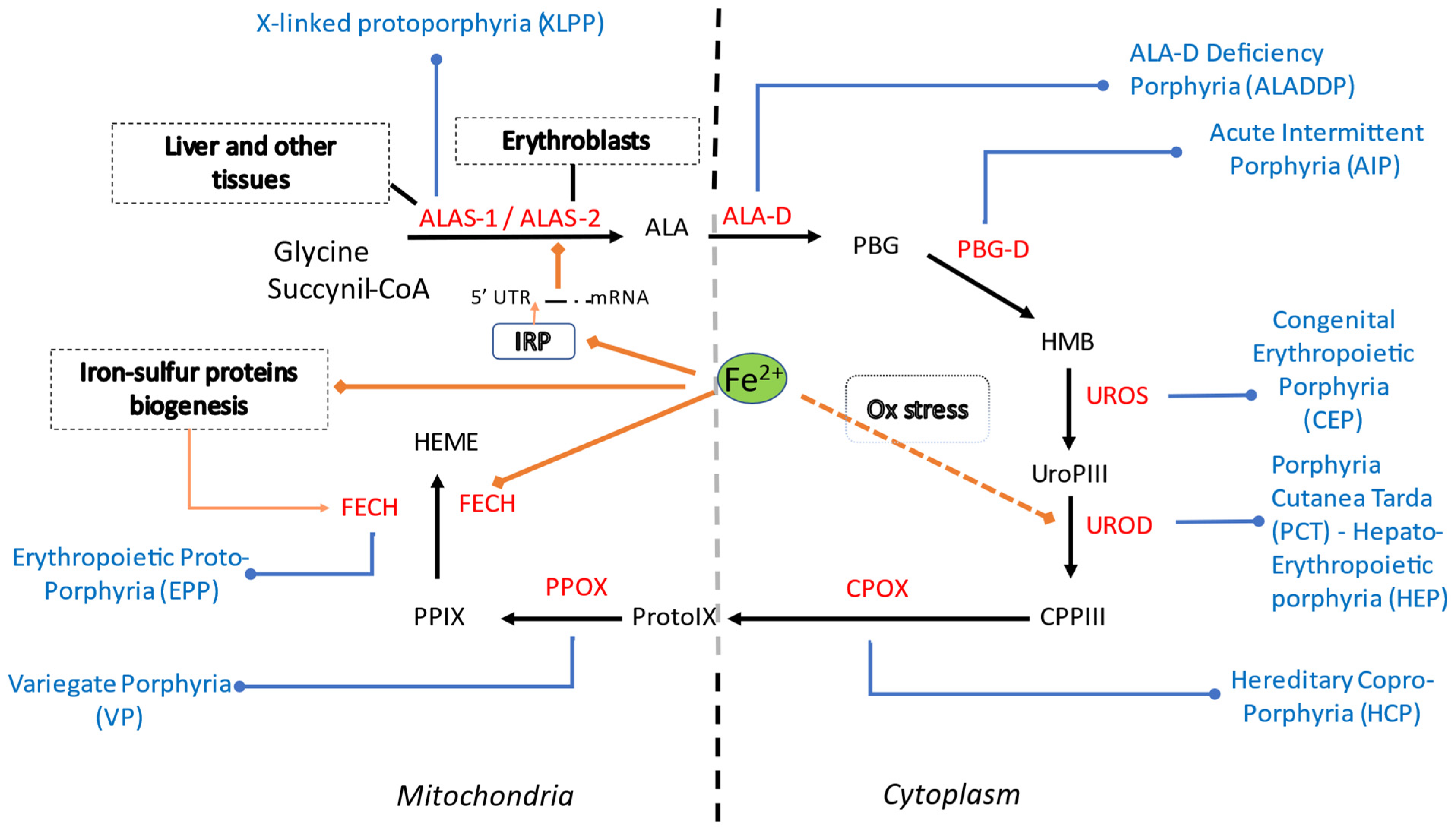
3. Heme Metabolism, Iron and Reciprocal Influences
4. Iron in Erythropoietic Porphyria
5. Iron in Congenital Erythropoietic Porphyria
6. Iron in Porphyria Cutanea Tarda
7. Iron in Acute Hepatic Porphyrias
8. Other Evidence from Experimental Models
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Pierro, E.; De Canio, M.; Mercadante, R.; Savino, M.; Granata, F.; Tavazzi, D.; Nicolli, A.M.; Trevisan, A.; Marchini, S.; Fustinoni, S. Laboratory Diagnosis of Porphyria. Diagnostics 2021, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Galano, J.M.; Durand, T.; Le Guennec, J.Y.; Lee, J.C. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrangelo, A. Mechanisms of iron hepatotoxicity. J. Hepatol. 2016, 65, 226–227. [Google Scholar] [CrossRef] [PubMed]
- West, A.R.; Oates, P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Buzzetti, E.; Pietrangelo, A. Genetic iron overload disorders. Mol. Aspects Med. 2020, 75, 100896. [Google Scholar] [CrossRef]
- Fleming, M.D.; Romano, M.A.; Su, M.A.; Garrick, L.M.; Garrick, M.D.; Andrews, N.C. Nramp2 is mutated in the anemic Belgrade (b) rat: Evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl. Acad. Sci. USA 1998, 95, 1148–1153. [Google Scholar] [CrossRef] [Green Version]
- Theil, E.C.; Chen, H.; Miranda, C.; Janser, H.; Elsenhans, B.; Nunez, M.T.; Pizarro, F.; Schumann, K. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J. Nutr. 2012, 142, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Knutson, M.D. Non-transferrin-bound iron transporters. Free Radic. Biol. Med. 2019, 133, 101–111. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Zumbrennen, K.B.; Paw, B.H.; Ward, D.M.; Kaplan, J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell. Biol. 2009, 29, 1007–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.T.; Manz, D.H.; Torti, F.M.; Torti, S.V. Mitochondria and Iron: Current questions. Expert Rev. Hematol. 2017, 10, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanatori, I.; Richardson, D.R.; Toyokuni, S.; Kishi, F. The new role of poly (rC)-binding proteins as iron transport chaperones: Proteins that could couple with inter-organelle interactions to safely traffic iron. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129685. [Google Scholar] [CrossRef]
- Chen, W.; Paradkar, P.N.; Li, L.; Pierce, E.L.; Langer, N.B.; Takahashi-Makise, N.; Hyde, B.B.; Shirihai, O.S.; Ward, D.M.; Kaplan, J.; et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc. Natl. Acad. Sci. USA 2009, 106, 16263–16268. [Google Scholar] [CrossRef] [Green Version]
- Berdoukas, V.; Coates, T.D.; Cabantchik, Z.I. Iron and oxidative stress in cardiomyopathy in thalassemia. Free Radic. Biol. Med. 2015, 88, 3–9. [Google Scholar] [CrossRef]
- Mariani, R.; Pelucchi, S.; Paolini, V.; Belingheri, M.; di Gennaro, F.; Faverio, P.; Riva, M.; Pesci, A.; Piperno, A. Prolonged exposure to welding fumes as a novel cause of systemic iron overload. Liver Int. 2021, 41, 1600–1607. [Google Scholar] [CrossRef]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Ruwe, T.A.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K. Inherited Disorders of Iron Overload. Front. Nutr. 2018, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.L.; Babitt, J.L. Coordination of iron homeostasis by bone morphogenetic proteins: Current understanding and unanswered questions. Dev. Dyn. 2022, 251, 26–46. [Google Scholar] [CrossRef]
- Lim, P.J.; Duarte, T.L.; Arezes, J.; Garcia-Santos, D.; Hamdi, A.; Pasricha, S.R.; Armitage, A.E.; Mehta, H.; Wideman, S.; Santos, A.G.; et al. Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat. Metab. 2019, 1, 519–531. [Google Scholar] [CrossRef]
- Silvestri, L.; Pagani, A.; Nai, A.; De Domenico, I.; Kaplan, J.; Camaschella, C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008, 8, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Meynard, D.; Vaja, V.; Sun, C.C.; Corradini, E.; Chen, S.; Lopez-Otin, C.; Grgurevic, L.; Hong, C.C.; Stirnberg, M.; Gutschow, M.; et al. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood 2011, 118, 747–756. [Google Scholar] [CrossRef]
- Ganz, T. The role of hepcidin in iron sequestration during infections and in the pathogenesis of anemia of chronic disease. Isr. Med. Assoc. J. 2002, 4, 1043–1045. [Google Scholar]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Srole, D.N.; Ganz, T. Erythroferrone structure, function, and physiology: Iron homeostasis and beyond. J. Cell. Physiol. 2021, 236, 4888–4901. [Google Scholar] [CrossRef]
- Pantopoulos, K. Iron metabolism and the IRE/IRP regulatory system: An update. Ann. N. Y. Acad. Sci. 2004, 1012, 1–13. [Google Scholar] [CrossRef]
- Zhang, D.L.; Hughes, R.M.; Ollivierre-Wilson, H.; Ghosh, M.C.; Rouault, T.A. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009, 9, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Lakhal-Littleton, S.; Wolna, M.; Carr, C.A.; Miller, J.J.; Christian, H.C.; Ball, V.; Santos, A.; Diaz, R.; Biggs, D.; Stillion, R.; et al. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc. Natl. Acad. Sci. USA 2015, 112, 3164–3169. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.J.; Jiang, B.H.; Chin, B.Y.; Iyer, N.V.; Alam, J.; Semenza, G.L.; Choi, A.M. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renassia, C.; Peyssonnaux, C. New insights into the links between hypoxia and iron homeostasis. Curr. Opin. Hematol. 2019, 26, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Santana-Codina, N.; Mancias, J.D. The Role of NCOA4-Mediated Ferritinophagy in Health and Disease. Pharmaceuticals 2018, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef]
- Coffey, R.; Ganz, T. Iron homeostasis: An anthropocentric perspective. J. Biol. Chem. 2017, 292, 12727–12734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.D. Physiology of iron metabolism. Transfus. Med. Hemother. 2014, 41, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.C. When is a heme transporter not a heme transporter? When it’s a folate transporter. Cell Metab. 2007, 5, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Le Blanc, S.; Garrick, M.D.; Arredondo, M. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am. J. Physiol. Cell. Physiol. 2012, 302, C1780–C1785. [Google Scholar] [CrossRef]
- Crooks, D.R.; Ghosh, M.C.; Haller, R.G.; Tong, W.H.; Rouault, T.A. Posttranslational stability of the heme biosynthetic enzyme ferrochelatase is dependent on iron availability and intact iron-sulfur cluster assembly machinery. Blood 2010, 115, 860–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Dailey, H.A.; Paw, B.H. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood 2010, 116, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Delaby, C.; Pilard, N.; Puy, H.; Canonne-Hergaux, F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: Early mRNA induction by haem, followed by iron-dependent protein expression. Biochem. J. 2008, 411, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balwani, M. Erythropoietic Protoporphyria and X-Linked Protoporphyria: Pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019, 128, 298–303. [Google Scholar] [CrossRef]
- Delaby, C.; Lyoumi, S.; Ducamp, S.; Martin-Schmitt, C.; Gouya, L.; Deybach, J.C.; Beaumont, C.; Puy, H. Excessive erythrocyte PPIX influences the hematologic status and iron metabolism in patients with dominant erythropoietic protoporphyria. Cell. Mol. Biol. 2009, 55, 45–52. [Google Scholar]
- Wahlin, S.; Floderus, Y.; Stal, P.; Harper, P. Erythropoietic protoporphyria in Sweden: Demographic, clinical, biochemical and genetic characteristics. J. Intern. Med. 2011, 269, 278–288. [Google Scholar] [CrossRef]
- Holme, S.A.; Thomas, C.L.; Whatley, S.D.; Bentley, D.P.; Anstey, A.V.; Badminton, M.N. Symptomatic response of erythropoietic protoporphyria to iron supplementation. J. Am. Acad. Dermatol. 2007, 56, 1070–1072. [Google Scholar] [CrossRef]
- Bentley, D.P.; Meek, E.M. Clinical and biochemical improvement following low-dose intravenous iron therapy in a patient with erythropoietic protoporphyria. Br. J. Haematol. 2013, 163, 289–291. [Google Scholar] [CrossRef]
- Minder, E.I.; Haldemann, A.R.; Schneider-Yin, X. Exacerbation of erythropoietic protoporphyria by hyperthyroidism. J. Inherit. Metab. Dis. 2010, 33 (Suppl. 3), S465–S469. [Google Scholar] [CrossRef] [Green Version]
- Milligan, A.; Graham-Brown, R.A.; Sarkany, I.; Baker, H. Erythropoietic protoporphyria exacerbated by oral iron therapy. Br. J. Dermatol. 1988, 119, 63–66. [Google Scholar] [CrossRef]
- Barman-Aksozen, J.; Minder, E.I.; Schubiger, C.; Biolcati, G.; Schneider-Yin, X. In ferrochelatase-deficient protoporphyria patients, ALAS2 expression is enhanced and erythrocytic protoporphyrin concentration correlates with iron availability. Blood Cells Mol. Dis. 2015, 54, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bossi, K.; Lee, J.; Schmeltzer, P.; Holburton, E.; Groseclose, G.; Besur, S.; Hwang, S.; Bonkovsky, H.L. Homeostasis of iron and hepcidin in erythropoietic protoporphyria. Eur. J. Clin. Investig. 2015, 45, 1032–1041. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. To induce or not to induce: The fight over hepcidin regulation. Haematologica 2019, 104, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Lyoumi, S.; Abitbol, M.; Andrieu, V.; Henin, D.; Robert, E.; Schmitt, C.; Gouya, L.; de Verneuil, H.; Deybach, J.C.; Montagutelli, X.; et al. Increased plasma transferrin, altered body iron distribution, and microcytic hypochromic anemia in ferrochelatase-deficient mice. Blood 2007, 109, 811–818. [Google Scholar] [CrossRef]
- Holme, S.A.; Worwood, M.; Anstey, A.V.; Elder, G.H.; Badminton, M.N. Erythropoiesis and iron metabolism in dominant erythropoietic protoporphyria. Blood 2007, 110, 4108–4110. [Google Scholar] [CrossRef] [Green Version]
- Barman-Aksoezen, J.; Girelli, D.; Aurizi, C.; Schneider-Yin, X.; Campostrini, N.; Barbieri, L.; Minder, E.I.; Biolcati, G. Disturbed iron metabolism in erythropoietic protoporphyria and association of GDF15 and gender with disease severity. J. Inherit. Metab. Dis. 2017, 40, 433–441. [Google Scholar] [CrossRef]
- Yasuda, M.; Chen, B.; Desnick, R.J. Recent advances on porphyria genetics: Inheritance, penetrance & molecular heterogeneity, including new modifying/causative genes. Mol. Genet. Metab. 2019, 128, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, I.; Xu, W.; Gurgey, A.; Tuncer, M.; Cetin, M.; Oner, C.; Yetgin, S.; Ersoy, F.; Aizencang, G.; Astrin, K.H.; et al. Congenital erythropoietic porphyria successfully treated by allogeneic bone marrow transplantation. Blood 1998, 92, 4053–4058. [Google Scholar] [CrossRef]
- Shaw, P.H.; Mancini, A.J.; McConnell, J.P.; Brown, D.; Kletzel, M. Treatment of congenital erythropoietic porphyria in children by allogeneic stem cell transplantation: A case report and review of the literature. Bone Marrow Transpl. 2001, 27, 101–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blouin, J.M.; Ged, C.; Lalanne, M.; Lamrissi-Garcia, I.; Morice-Picard, F.; Costet, P.; Daher, R.; Moreau-Gaudry, F.; Bedel, A.; Puy, H.; et al. Iron chelation rescues hemolytic anemia and skin photosensitivity in congenital erythropoietic porphyria. Blood 2020, 136, 2457–2468. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br. J. Haematol. 2016, 172, 512–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elder, G.H. Porphyria cutanea tarda. Semin. Liver Dis. 1998, 18, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ryan Caballes, F.; Sendi, H.; Bonkovsky, H.L. Hepatitis C, porphyria cutanea tarda and liver iron: An update. Liver Int. 2012, 32, 880–893. [Google Scholar] [CrossRef] [Green Version]
- Singal, A.K. Porphyria cutanea tarda: Recent update. Mol. Genet. Metab. 2019, 128, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.M.; Oliva, H.; Paradinas, F.J.; Hernandez-Guio, C. The pathology of the liver in porphyria cutanea tarda. Histopathology 1980, 4, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Lundvall, O.; Weinfeld, A.; Lundin, P. Iron storage in porphyria cutanea tarda. Acta Med. Scand. 1970, 1–2, 37–53. [Google Scholar] [CrossRef]
- Phillips, J.D.; Bergonia, H.A.; Reilly, C.A.; Franklin, M.R.; Kushner, J.P. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc. Natl. Acad. Sci. USA 2007, 104, 5079–5084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiella, A.; Zapata, E.; Otazua, P.; Zubiaurre, L. Porphyria cutanea tarda in the Basque Country: Significance of HFE gene mutations and of external factors. Liver Int. 2012, 32, 1597. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.G.; Whatley, S.D.; Morgan, R.R.; Worwood, M.; Elder, G.H. Increased frequency of the haemochromatosis Cys282Tyr mutation in sporadic porphyria cutanea tarda. Lancet 1997, 349, 321–323. [Google Scholar] [CrossRef]
- Phillips, J.D.; Kushner, J.P.; Bergonia, H.A.; Franklin, M.R. Uroporphyria in the Cyp1a2-/- mouse. Blood Cells Mol. Dis. 2011, 47, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, M.; Desnick, R.J. Murine models of the human porphyrias: Contributions toward understanding disease pathogenesis and the development of new therapies. Mol. Genet. Metab. 2019, 128, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Ajioka, R.S.; Phillips, J.D.; Weiss, R.B.; Dunn, D.M.; Smit, M.W.; Proll, S.C.; Katze, M.G.; Kushner, J.P. Down-regulation of hepcidin in porphyria cutanea tarda. Blood 2008, 112, 4723–4728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panton, N.A.; Strickland, N.J.; Hift, R.J.; Warnich, L.; Zaahl, M.G. Iron homeostasis in porphyria cutanea tarda: Mutation analysis of promoter regions of CP, CYBRD1, HAMP and SLC40A1. J. Clin. Pathol. 2013, 66, 160–161. [Google Scholar] [CrossRef]
- Corradini, E.; Buzzetti, E.; Dongiovanni, P.; Scarlini, S.; Caleffi, A.; Pelusi, S.; Bernardis, I.; Ventura, P.; Rametta, R.; Tenedini, E.; et al. Ceruloplasmin gene variants are associated with hyperferritinemia and increased liver iron in patients with NAFLD. J. Hepatol. 2021, 75, 506–513. [Google Scholar] [CrossRef] [PubMed]
- To-Figueras, J. Association between hepatitis C virus and porphyria cutanea tarda. Mol. Genet. Metab. 2019, 128, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Nishina, S.; Hino, K.; Korenaga, M.; Vecchi, C.; Pietrangelo, A.; Mizukami, Y.; Furutani, T.; Sakai, A.; Okuda, M.; Hidaka, I.; et al. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology 2008, 134, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, P.R.; Gorman, N.; Trask, H.W.; Bement, W.J.; Szakacs, J.G.; Elder, G.H.; Balestra, D.; Sinclair, J.F.; Gerhard, G.S. Uroporphyria caused by ethanol in Hfe(−/−) mice as a model for porphyria cutanea tarda. Hepatology 2003, 37, 351–358. [Google Scholar] [CrossRef]
- Petagine, L.; Zariwala, M.G.; Patel, V.B. Alcoholic liver disease: Current insights into cellular mechanisms. World J. Biol. Chem. 2021, 12, 87–103. [Google Scholar] [CrossRef]
- Harrison-Findik, D.D.; Schafer, D.; Klein, E.; Timchenko, N.A.; Kulaksiz, H.; Clemens, D.; Fein, E.; Andriopoulos, B.; Pantopoulos, K.; Gollan, J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 2006, 281, 22974–22982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkovsky, H.L.; Dixon, N.; Rudnick, S. Pathogenesis and clinical features of the acute hepatic porphyrias (AHPs). Mol. Genet. Metab. 2019, 128, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Bjersing, L.; Lithner, F. The epidemiology of hepatocellular carcinoma in patients with acute intermittent porphyria. J. Intern. Med. 1996, 240, 195–201. [Google Scholar] [CrossRef]
- Willandt, B.; Langendonk, J.G.; Biermann, K.; Meersseman, W.; D’Heygere, F.; George, C.; Verslype, C.; Monbaliu, D.; Cassiman, D. Liver Fibrosis Associated with Iron Accumulation Due to Long-Term Heme-Arginate Treatment in Acute Intermittent Porphyria: A Case Series. JIMD Rep. 2016, 25, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Pietrangelo, A. Iron and the liver. Liver Int. 2016, 36 (Suppl. 1), 116–123. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, C.; Montosi, G.; Garuti, C.; Corradini, E.; Sabelli, M.; Canali, S.; Pietrangelo, A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 2014, 146, 1060–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troadec, M.B.; Warner, D.; Wallace, J.; Thomas, K.; Spangrude, G.J.; Phillips, J.; Khalimonchuk, O.; Paw, B.H.; Ward, D.M.; Kaplan, J. Targeted deletion of the mouse Mitoferrin1 gene: From anemia to protoporphyria. Blood 2011, 117, 5494–5502. [Google Scholar] [CrossRef]
- Chung, J.; Anderson, S.A.; Gwynn, B.; Deck, K.M.; Chen, M.J.; Langer, N.B.; Shaw, G.C.; Huston, N.C.; Boyer, L.F.; Datta, S.; et al. Iron regulatory protein-1 protects against mitoferrin-1-deficient porphyria. J. Biol. Chem. 2014, 289, 7835–7843. [Google Scholar] [CrossRef] [Green Version]
- Cooperman, S.S.; Meyron-Holtz, E.G.; Olivierre-Wilson, H.; Ghosh, M.C.; McConnell, J.P.; Rouault, T.A. Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood 2005, 106, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzzetti, E.; Ventura, P.; Corradini, E. Iron in Porphyrias: Friend or Foe? Diagnostics 2022, 12, 272. https://doi.org/10.3390/diagnostics12020272
Buzzetti E, Ventura P, Corradini E. Iron in Porphyrias: Friend or Foe? Diagnostics. 2022; 12(2):272. https://doi.org/10.3390/diagnostics12020272
Chicago/Turabian StyleBuzzetti, Elena, Paolo Ventura, and Elena Corradini. 2022. "Iron in Porphyrias: Friend or Foe?" Diagnostics 12, no. 2: 272. https://doi.org/10.3390/diagnostics12020272
APA StyleBuzzetti, E., Ventura, P., & Corradini, E. (2022). Iron in Porphyrias: Friend or Foe? Diagnostics, 12(2), 272. https://doi.org/10.3390/diagnostics12020272





