Lesion Volume Quantification Using Two Convolutional Neural Networks in MRIs of Multiple Sclerosis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Sample and Image Data
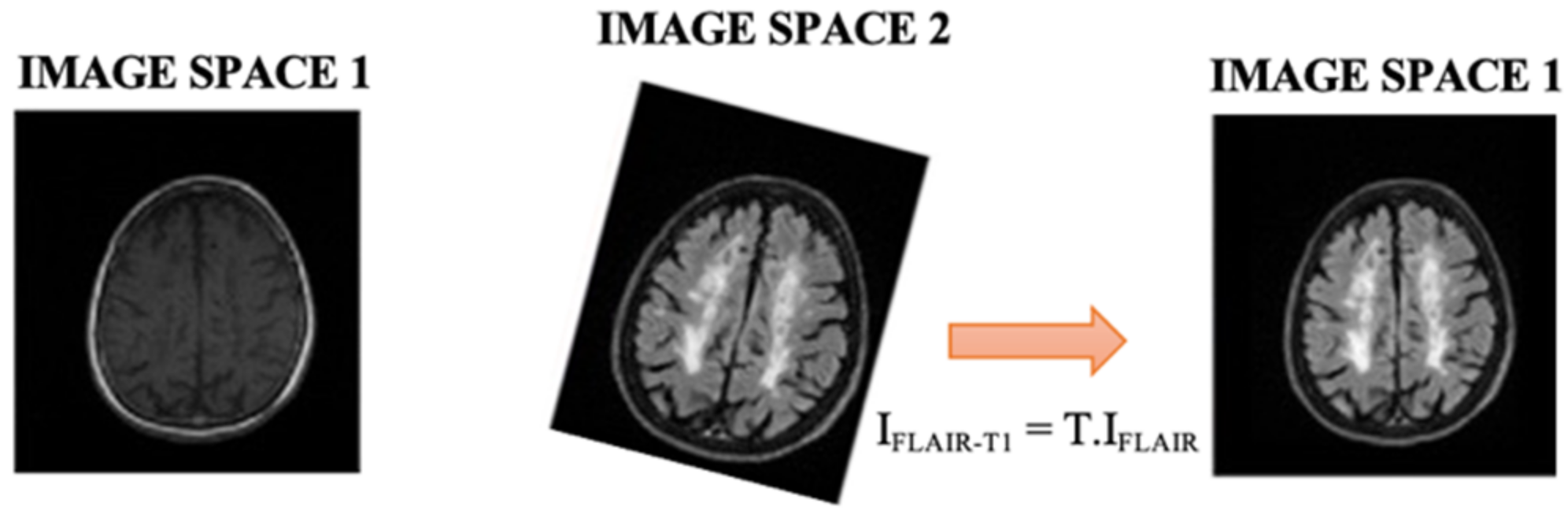
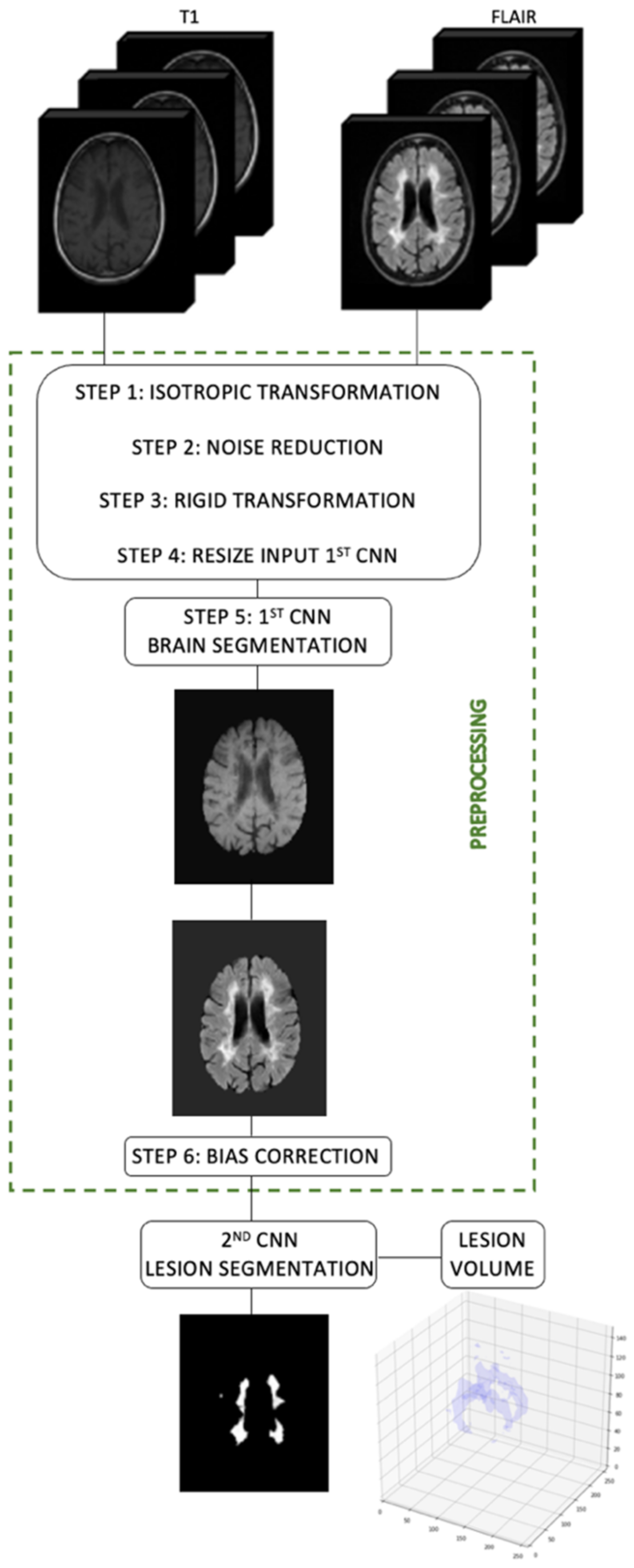
2.2. Image Preprocessing
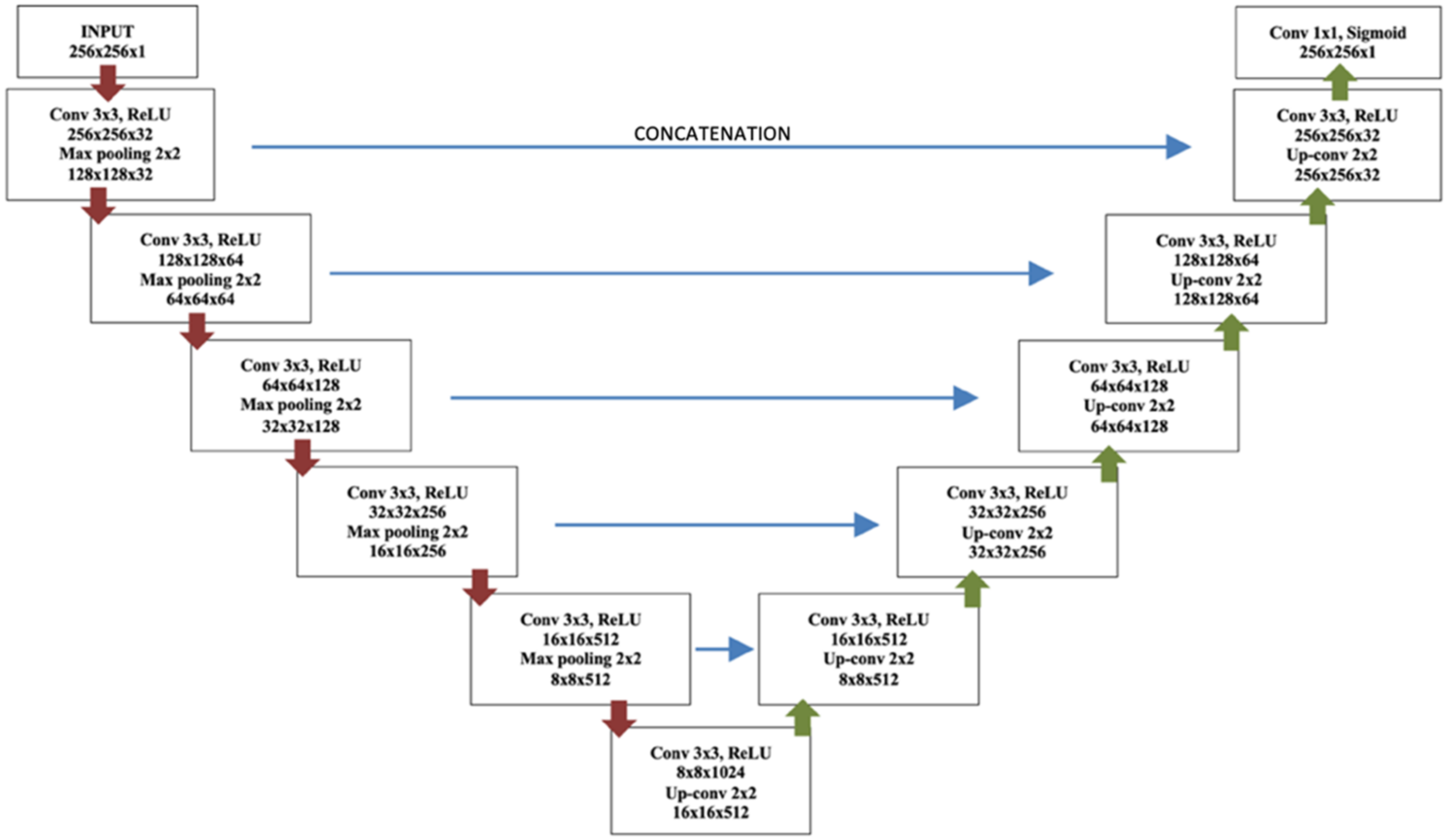
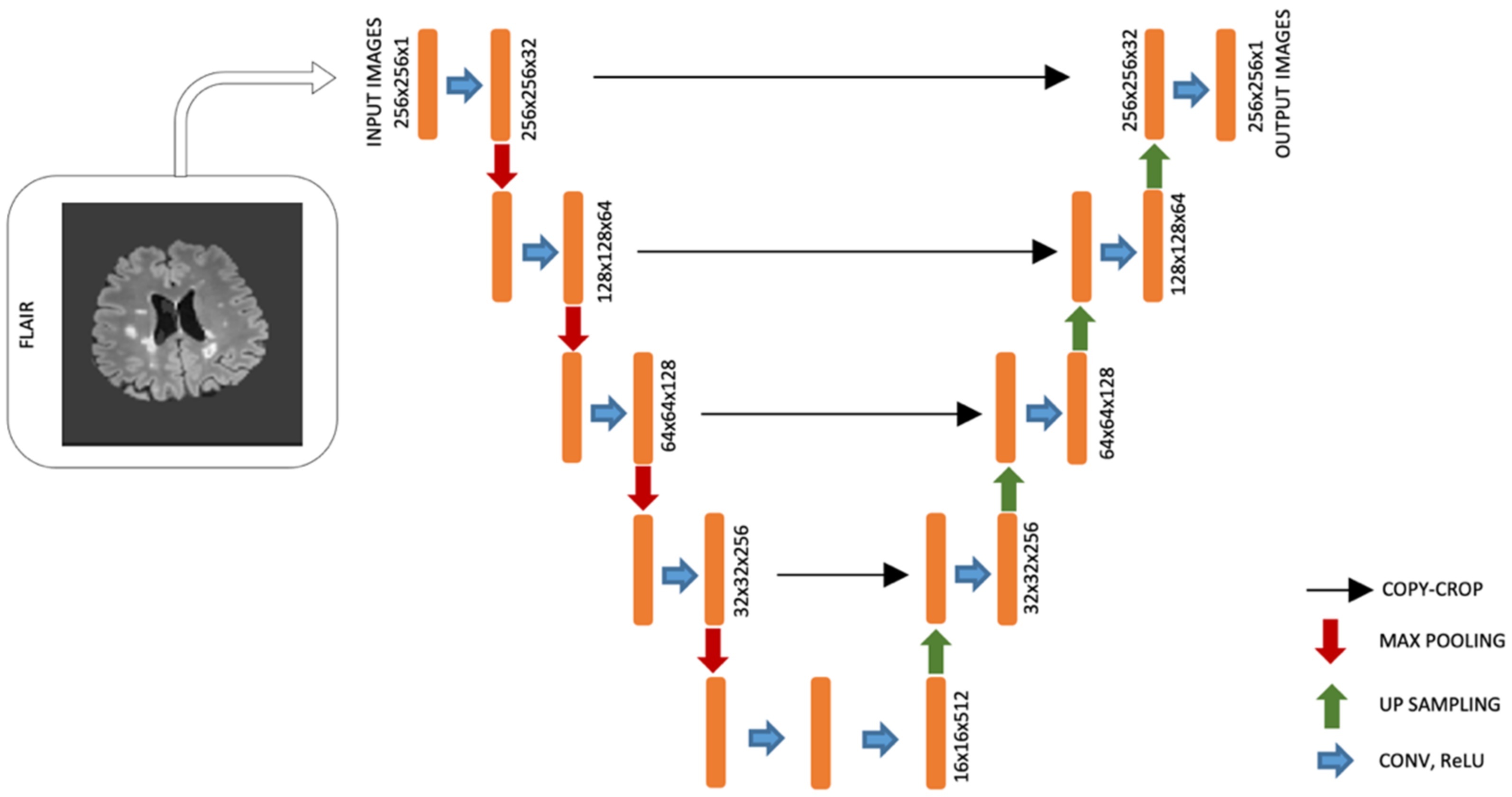
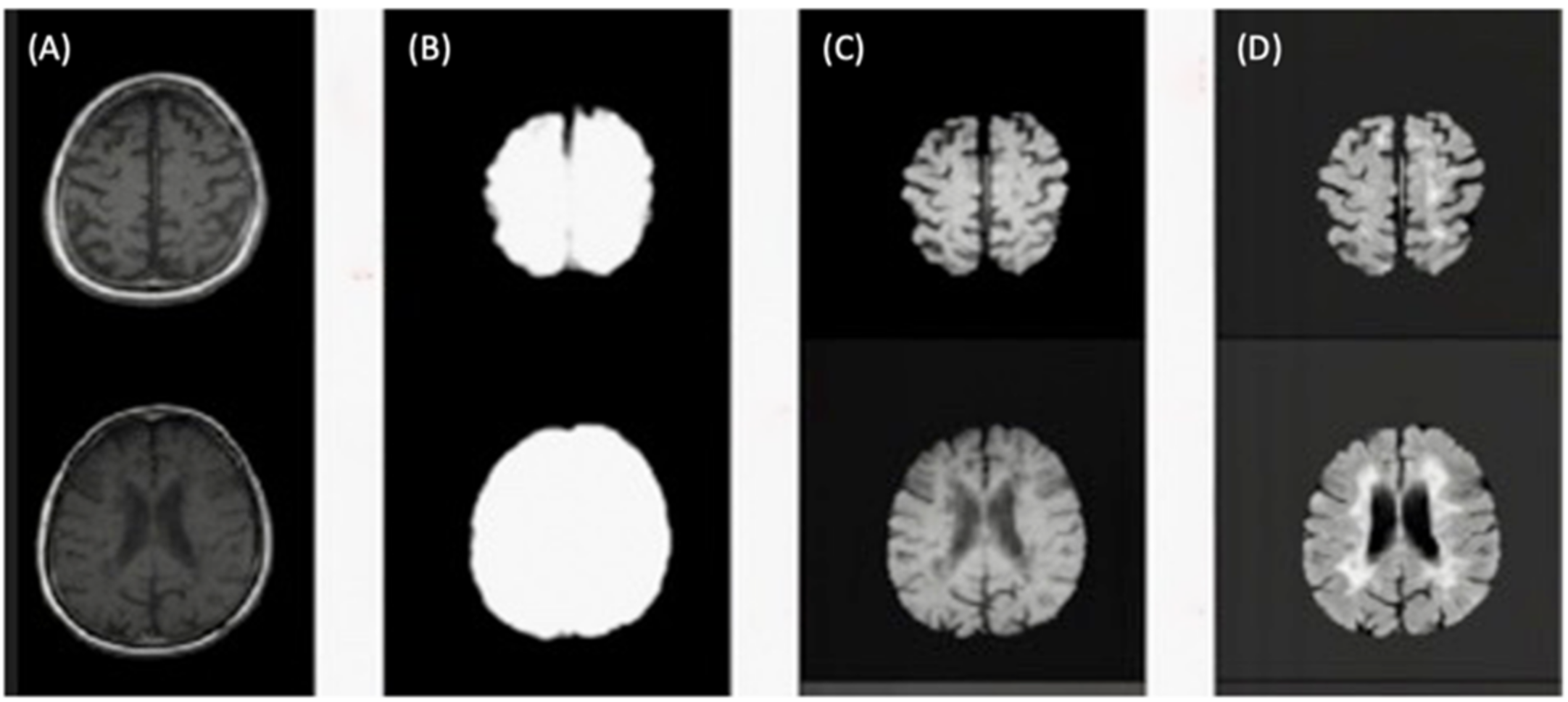
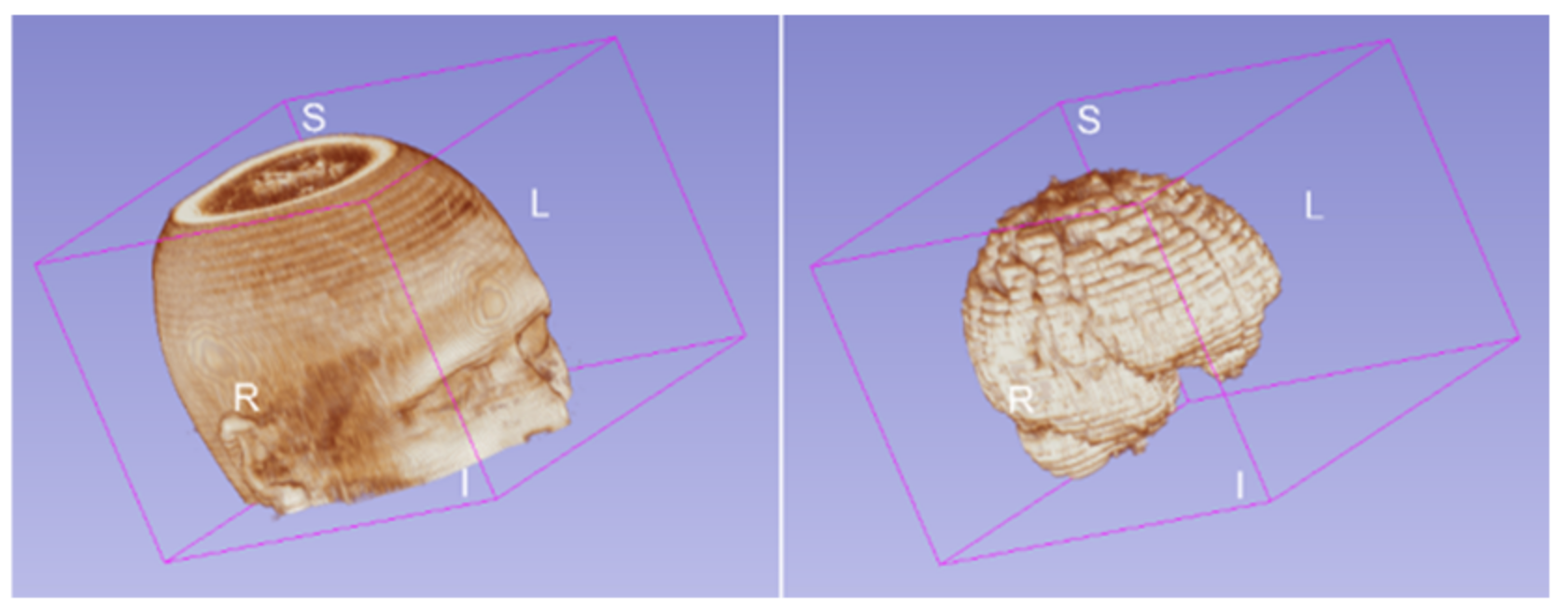
2.3. Brain Lesion Segmentation
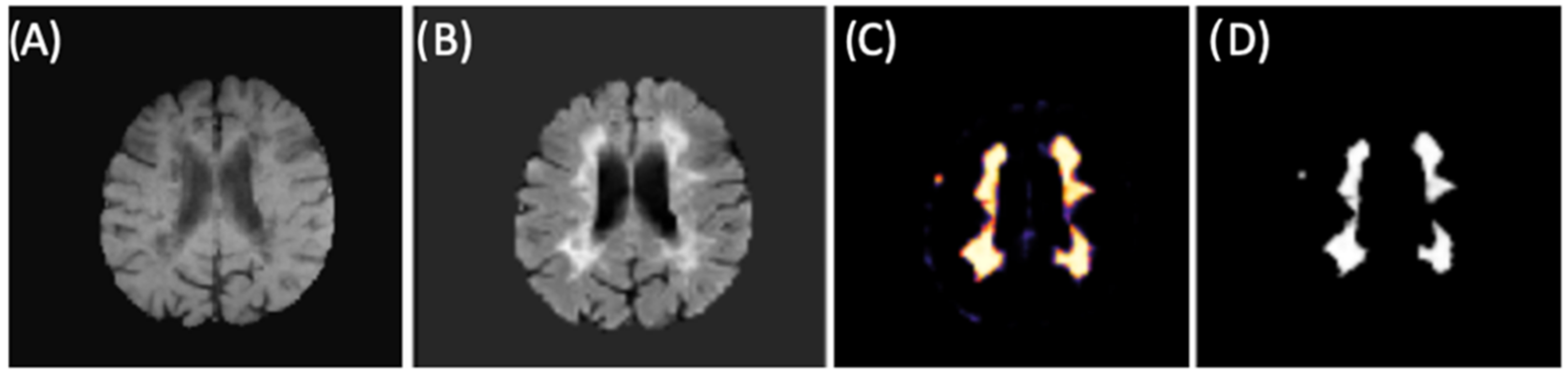
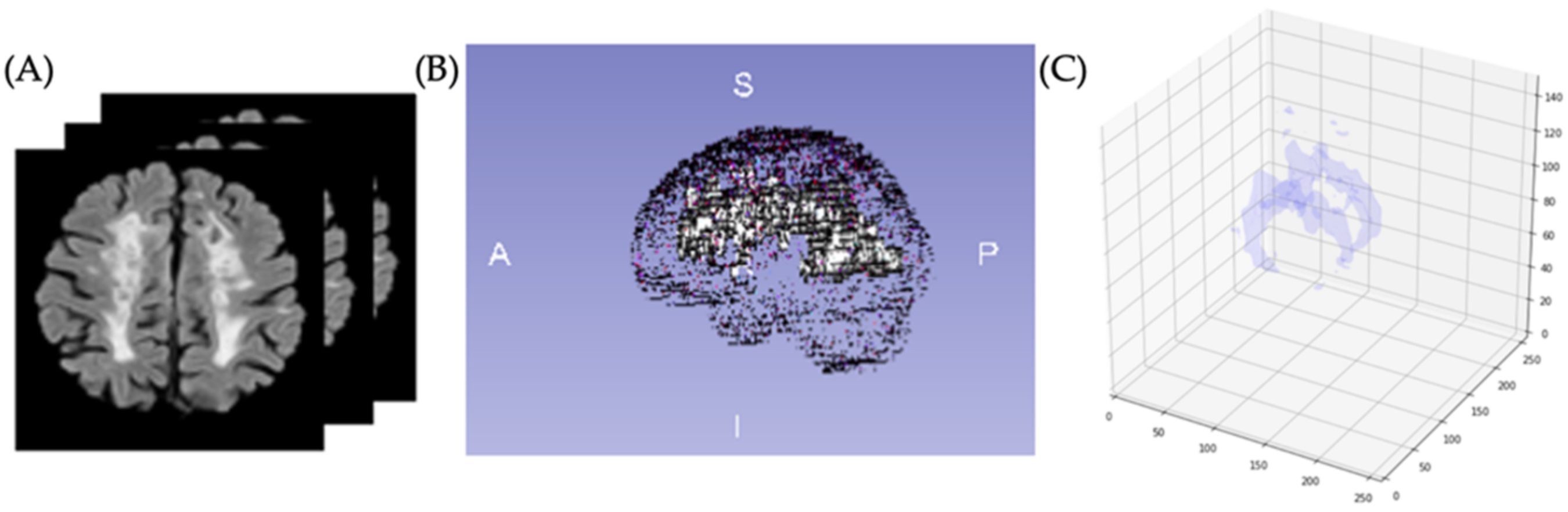
2.4. Brain Lesion Identification and Quantification from Test Group
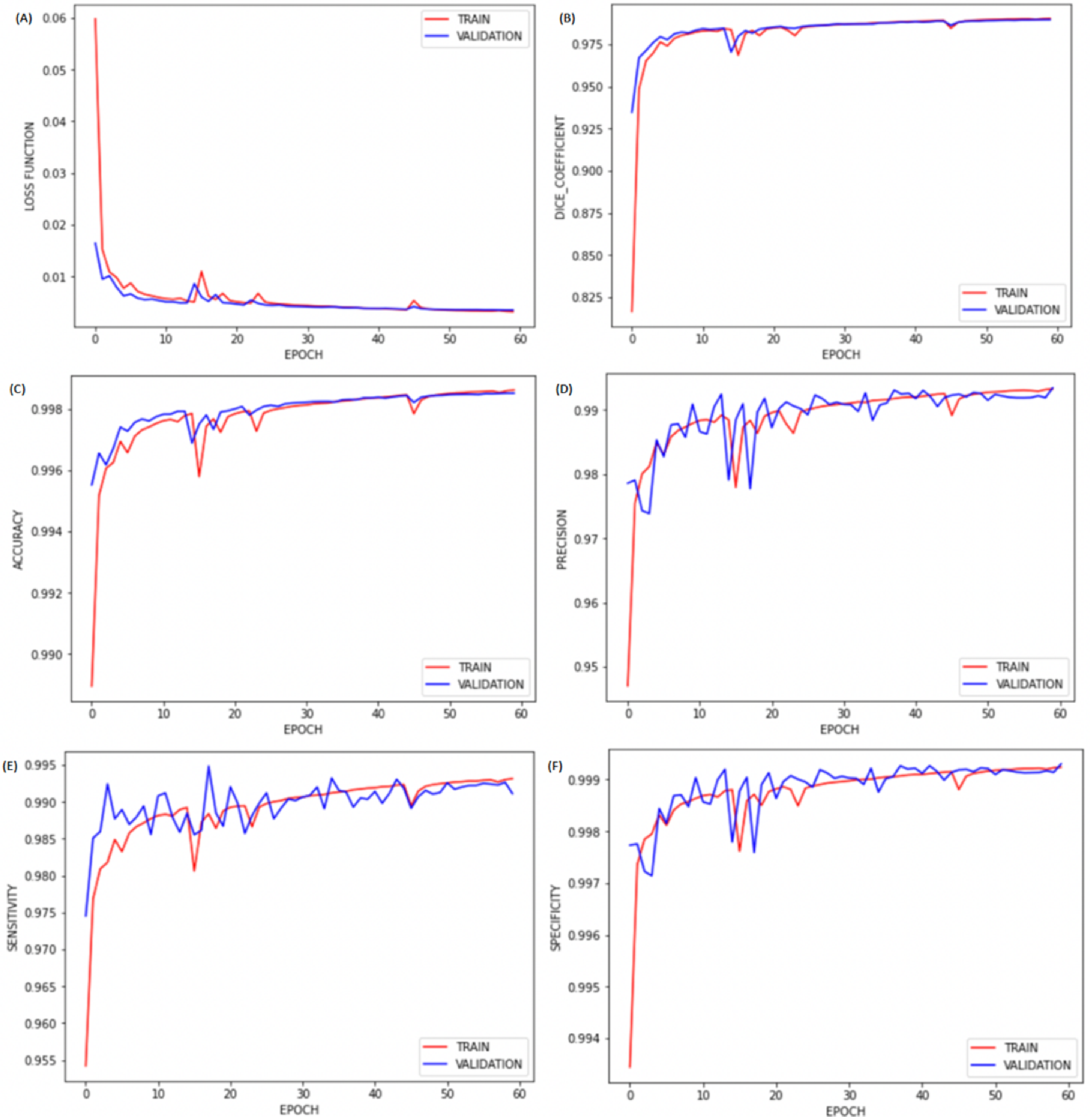
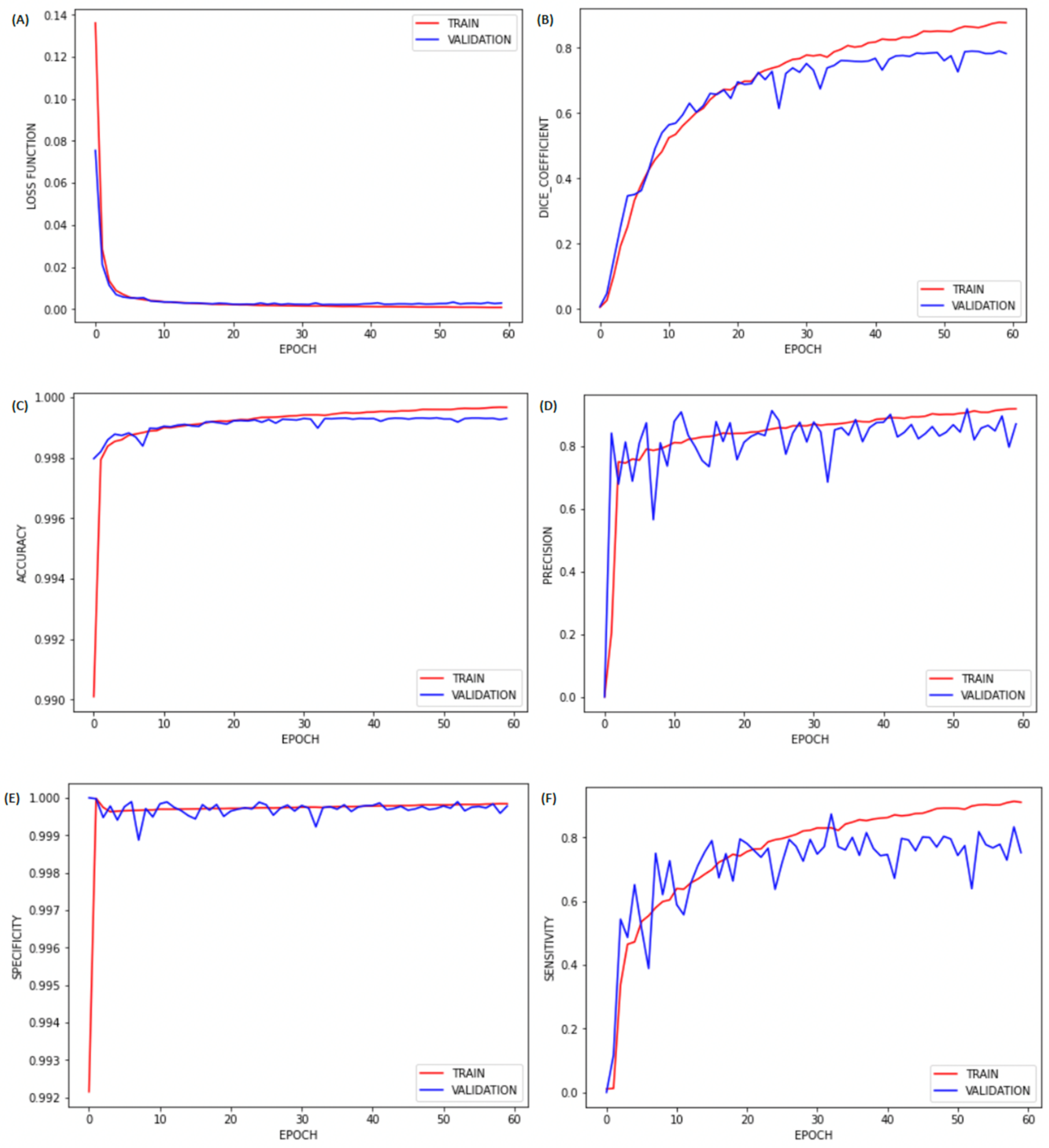
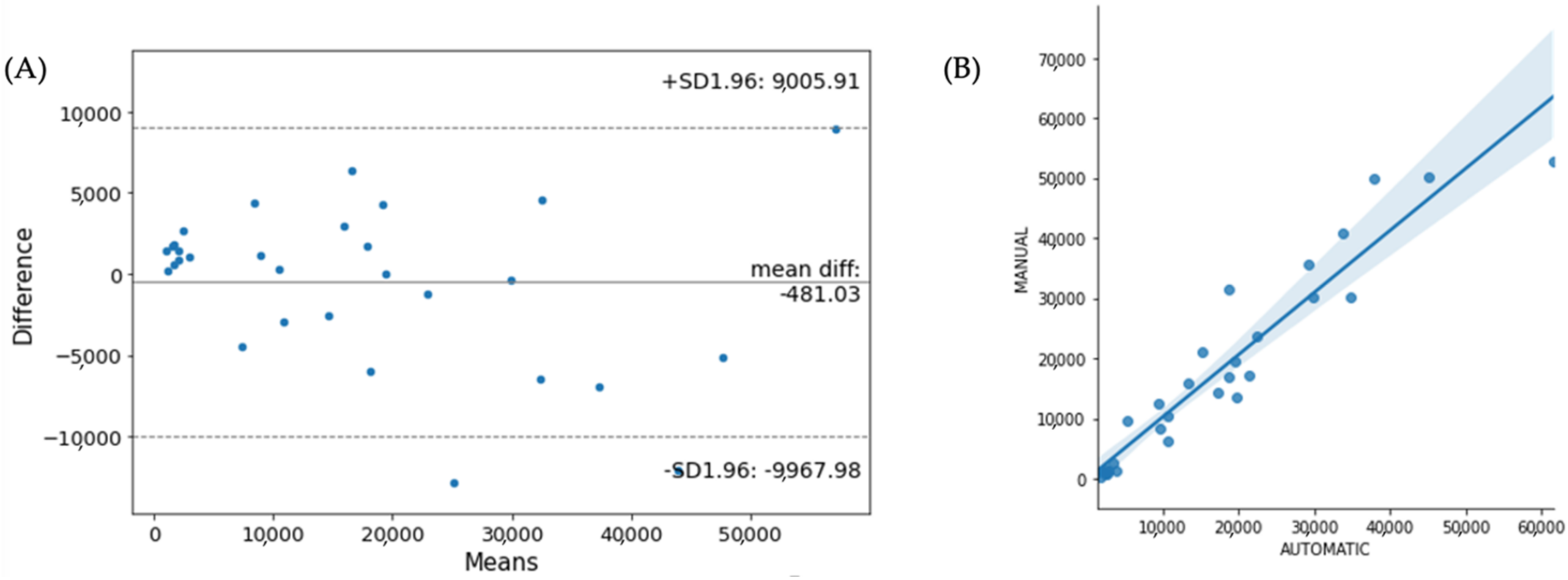
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Rosa, F.; Fartaria, M.J.; Kober, T.; Richiardi, J.; Granziera, C.; Thiran, J.-P.; Cuadra, M.B. Shallow vs. Deep Learning Architectures for White Matter Lesion Segmentation in the Early Stages of Multiple Sclerosis; Crimi, A., Bakas, S., Kuijf, H., Keyvan, F., Reyes, M., Van Walsum, T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 142–151. [Google Scholar] [CrossRef] [Green Version]
- Valverde, S.; Cabezas, M.; Roura, E.; González-Villà, S.; Pareto, D.; Vilanova, J.C.; Ramió-Torrentà, L.; Rovira, À.; Oliver, A.; Llado, X. Improving Automated Multiple Sclerosis Lesion Segmentation with a Cascaded 3D Convolutional Neural Network Approach. Neuroimage 2017, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Bakshi, R. Magnetic Resonance Imaging Advances in Multiple Sclerosis. J. Neuroimaging 2005, 15, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Dobson, R.; Meier, U.C.; Giovannoni, G. Multiple Sclerosis: Risk Factors, Prodromes, and Potential Causal Pathways. Lancet Neurol. 2010, 9, 727–739. [Google Scholar] [CrossRef]
- Bakshi, R.; Thompson, A.J.; Rocca, M.A.; Pelletier, D.; Dousset, V.; Barkhof, F.; Inglese, M.; Guttmann, C.R.; Horsfield, M.A.; Filippi, M. MRI in Multiple Sclerosis: Current Status and Future Prospects. Lancet Neurol. 2008, 7, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Lladó, X.; Ganiler, O.; Oliver, A.; Marti, R.; Freixenet, J.; Valls, L.; Vilanova, J.C.; Ramió-Torrentà, L.; Rovira, A. Automated Detection of Multiple Sclerosis Lesions in Serial Brain MRI. Neuroradiology 2012, 54, 787–807. [Google Scholar] [CrossRef]
- Meier, D.S.; Weiner, H.L.; Guttmann, C.R.G. MR Imaging Intensity Modeling of Damage and Repair In Multiple Sclerosis: Relationship of Short-Term Lesion Recovery to Progression and Disability. Am. J. Neuroradiol. 2007, 28, 1956–1963. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Preiningerova, J.; Cannistraci, C.J.; Vollmer, T.L.; Gore, J.C.; Anderson, A.W. Quantification of Multiple Sclerosis Lesion Load and Brain Tissue Volumetry Using Multiparameter MRI: Methodology and Reproducibility. Magn. Reson. Imaging 2005, 23, 445–452. [Google Scholar] [CrossRef]
- Lesjak, Ž.; Galimzianova, A.; Koren, A.; Lukin, M.; Pernuš, F.; Likar, B.; Špiclin, Ž. A Novel Public MR Image Dataset of Multiple Sclerosis Patients With Lesion Segmentations Based on Multi-Rater Consensus. Neuroinformatics 2018, 16, 51–63. [Google Scholar] [CrossRef]
- Carass, A.; Roy, S.; Jog, A.; Cuzzocreo, J.L.; Magrath, E.; Gherman, A.; Button, J.; Nguyen, J.; Prados, F.; Sudre, C.H.; et al. Longitudinal Multiple Sclerosis Lesion Segmentation: Resource and Challenge. Neuroimage 2017, 148, 77–102. [Google Scholar] [CrossRef] [Green Version]
- Khayati, R.; Vafadust, M.; Towhidkhah, F.; Nabavi, M. Fully Automatic Segmentation of Multiple Sclerosis Lesions in Brain MR FLAIR Images Using Adaptive Mixtures Method and Markov Random Field Model. Comput. Biol. Med. 2008, 38, 379–390. [Google Scholar] [CrossRef]
- Roy, S.; Butman, J.A.; Reich, D.S.; Calabresi, P.A.; Pham, D.L. Multiple Sclerosis Lesion Segmentation from Brain MRI via Fully Convolutional Neural Networks. arXiv 2018, arXiv:1803.09172. [Google Scholar]
- Gabr, R.E.; Coronado, I.; Robinson, M.; Sujit, S.J.; Datta, S.; Sun, X.; Allen, W.J.; Lublin, F.D.; Wolinsky, J.S.; Narayana, P.A. Brain and Lesion Segmentation in Multiple Sclerosis Using Fully Convolutional Neural Networks: A Large-Scale Study. Mult. Scler. J. 2020, 26, 1217–1226. [Google Scholar] [CrossRef]
- De Oliveira, M.; Santinelli, F.B.; Piacenti-Silva, M.; Rocha, F.C.G.; Barbieri, F.A.; Lisboa-Filho, P.N.; Santos, J.M.; Cardoso, J.D.S. Quantification of Brain Lesions in Multiple Sclerosis Patients Using Segmentation by Convolutional Neural Networks. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Korea, 16–19 December 2020; pp. 2045–2048. [Google Scholar]
- Akkus, Z.; Galimzianova, A.; Hoogi, A.; Rubin, D.L.; Erickson, B.J. Deep Learning for Brain MRI Segmentation: State of the Art and Future Directions. J. Digit. Imaging 2017, 30, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Isensee, F.; Schell, M.; Pflueger, I.; Brugnara, G.; Bonekamp, D.; Neuberger, U.; Wick, A.; Schlemmer, H.; Heiland, S.; Wick, W.; et al. Automated Brain Extraction of Multisequence MRI Using Artificial Neural Networks. Hum. Brain Mapp. 2019, 40, 4952–4964. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.; Piacenti-Silva, M.; Rocha, F.C.G.; Santos, J.M.; Cardoso, J.S.; Lisboa-Filho, P.N. Skull Extraction for Quantification of Brain Volume in Magnetic Resonance Imaging of Multiple Sclerosis Patients. Int. J. Biomed. Biol. Eng. 2020, 14, 2020. [Google Scholar]
- Smith, S.M. Fast Robust Automated Brain Extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Iglesias, J.E.; Liu, C.Y.; Thompson, P.M.; Tu, Z. Robust Brain Extraction across Datasets and Comparison with Publicly Available Methods. IEEE Trans. Med. Imaging 2011, 30, 1617–1634. [Google Scholar] [CrossRef]
- Bauer, S.; Nolte, L.-P.; Reyes, M. Skull-Stripping for Tumor-Bearing Brain Images. arXiv 2012, arXiv:1204.0357. [Google Scholar]
- Ségonne, F.; Dale, A.M.; Busa, E.; Glessner, M.; Salat, D.; Hahn, H.K.; Fischl, B. A Hybrid Approach to the Skull Stripping Problem in MRI. Neuroimage 2004, 22, 1060–1075. [Google Scholar] [CrossRef]
- Reuter, M.; Schmansky, N.J.; Rosas, H.D.; Fischl, B. Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis. Neuroimage 2012, 61, 1402–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puccio, B.; Pooley, J.P.; Pellman, J.S.; Taverna, E.C.; Craddock, R.C. The Preprocessed Connectomes Project Repository of Manually Corrected Skull-Stripped T1-Weighted Anatomical Mri Data. Gigascience 2016, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nooner, K.B.; Colcombe, S.J.; Tobe, R.H.; Mennes, M.; Benedict, M.M.; Moreno, A.L.; Panek, L.J.; Brown, S.; Zavitz, S.T.; Li, Q.; et al. The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Front. Neurosci. 2012, 6, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commowick, O.; Kain, M.; Casey, R.; Ameli, R.; Ferré, J.-C.; Kerbrat, A.; Tourdias, T.; Cervenansky, F.; Camarasu-Pop, S.; Glatard, T.; et al. Multiple Sclerosis Lesions Segmentation from Multiple Experts: The MICCAI 2016 Challenge Dataset. Neuroimage 2021, 244, 118589. [Google Scholar] [CrossRef]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.-P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended Diagnostic Criteria for Multiple Sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Perona, P.; Malik, J. Scale-Space and Edge Detection Using Anisotropic Diffusion. IEEE Trans. Pattern Anal. Mach. Intell. 1990, 12, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Gerig, G.; Kbler, O.; Kikinis, R.; Jolesz, F.A. Nonlinear Anisotropic Filtering of MRI Data. IEEE Trans. Med. Imaging 1992, 11, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Eskildsen, S.F.; Coupé, P.; Fonov, V.; Manjón, J.V.; Leung, K.K.; Guizard, N.; Wassef, S.N.; Østergaard, L.R.; Collins, D.L. BEaST: Brain Extraction Based on Nonlocal Segmentation Technique. Neuroimage 2012, 59, 2362–2373. [Google Scholar] [CrossRef]
- Nair, V.; Hinton, G. Rectified Linear Units Improve Restricted Boltzmann Machines; Omnipress: Madison, WI, USA, 2010; Volume 27. [Google Scholar]
- Hwang, H.; Rehman, H.Z.U.; Lee, S. 3D U-Net for Skull Stripping in Brain MRI. Appl. Sci. 2019, 9, 569. [Google Scholar] [CrossRef] [Green Version]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 Bias Correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, P.; Prasad, P.K.C.; Nandi, D. A Light Weighted Deep Learning Framework for Multiple Sclerosis Lesion Segmentation. In Proceedings of the 2019 Fifth International Conference on Image Information Processing (ICIIP), Shimla, India, 15–17 November 2019; Volume 2019, pp. 526–531. [Google Scholar]
- Kaunzner, U.W.; Gauthier, S.A. MRI in the Assessment and Monitoring of Multiple Sclerosis: An Update on Best Practice. Ther. Adv. Neurol. Disord. 2017, 10, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Buda, M.; Saha, A.; Mazurowski, M.A. Association of Genomic Subtypes of Lower-Grade Gliomas with Shape Features Automatically Extracted by a Deep Learning Algorithm. Comput. Biol. Med. 2019, 109, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Despotović, I.; Goossens, B.; Philips, W. MRI Segmentation of the Human Brain: Challenges, Methods, and Applications. Comput. Math. Methods Med. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Kamnitsas, K.; Ledig, C.; Newcombe, V.F.J.; Simpson, J.P.; Kane, A.D.; Menon, D.K.; Rueckert, D.; Glocker, B. Efficient Multi-Scale 3D CNN with Fully Connected CRF for Accurate Brain Lesion Segmentation. Med. Image Anal. 2017, 36, 61–78. [Google Scholar] [CrossRef]
- Tao, X.; Chang, M.-C. A Skull Stripping Method Using Deformable Surface and Tissue Classification. Med. Imaging 2010 Image Process. 2010, 7623, 76233L. [Google Scholar] [CrossRef]
- Moeskops, P.; Viergever, M.A.; Mendrik, A.M.; De Vries, L.S.; Benders, M.J.N.L.; Isgum, I. Automatic Segmentation of MR Brain Images with a Convolutional Neural Network. IEEE Trans. Med. Imaging 2016, 35, 1252–1261. [Google Scholar] [CrossRef] [Green Version]

| Scanner Model and Site | Sequence | Voxel Size (mm) | Echo Time (ms) | Repetition Time (ms) | Flip Angle (Degrees) | Inversion Time (ms) | |
|---|---|---|---|---|---|---|---|
| NFBS | SIEMENS MAGNETOM MR B17 (Nathan Kline Institute-Rockland Sample) | T1 | 1 × 1 × 1 | 3.02 | 2600 | 8 | 900 |
| MICCAI 2016 | Siemens Verio 3T (University Hospital of Rennes) | FL | 0.5 × 0.5 × 1.1 | 400 | 5000 | 120 | 1800 |
| T1 | 1 × 1 × 1 | 2.26 | 1900 | 9 | NA | ||
| Siemens Aera 1.5T (University Hospital of Lyon) | FL | 1.03 × 1.03 × 1.25 | 336 | 5000 | 120 | 1800 | |
| T1 | 1.08 × 1.08 × 0.9 | 3.37 | 1860 | 15 | NA | ||
| Philips Ingenia 3T (University Hospital of Lyon) | FL | 0.74 × 0.74 × 0.7 | 360 | 5400 | 90 | 1800 | |
| T1 | 0.74 × 0.74 × 0.85 | 4.3 | 9.4 | 8 | NA | ||
| IBSI 2015 | Philips Medical Systems 3T | FL | 0.82 × 0.82 × 2.2 | 68 | NA | NA | 835 |
| T1 | 0.82 × 0.82 × 1.17 | 6 | 10.3 | 8 | NA | ||
| HC-FMB | Siemens Verio 3T (Hospital of Clinics—Botucatu Medical School, São Paulo State University | FL | 0.43 × 0.43 × 4.6 | 80 | 9000 | 150 | 2500 |
| T1 | 0.47 × 0.47 × 4.6 | 9 | 465 | 69 | NA |
| Brain Segmentation—1st CNN | Lesion Segmentation—2nd CNN | |
|---|---|---|
| Dice Coefficient | 0.9786 | 0.8893 |
| Accuracy | 0.9969 | 0.9996 |
| Precision | 0.9851 | 0.9376 |
| Sensitivity | 0.9851 | 0.8609 |
| Specificity | 0.9985 | 0.9999 |
| Dice Coefficient | Precision | Sensitivity | Accuracy | Specificity | |
|---|---|---|---|---|---|
| Buda et al., 2019 [37] | 0.8752 | 0.9274 | 0.9030 | 0.9995 | 0.9998 |
| Gabr et al., 2020 [14] | 0.7839 | 0.8956 | 0.7981 | 0.9994 | 0.9998 |
| Ghosal et al., 2019 [35] | 0.8701 | 0.9213 | 0.8911 | 0.9996 | 0.9998 |
| Our CNN | 0.8893 | 0.9376 | 0.8609 | 0.9996 | 0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, M.; Piacenti-Silva, M.; da Rocha, F.C.G.; Santos, J.M.; Cardoso, J.d.S.; Lisboa-Filho, P.N. Lesion Volume Quantification Using Two Convolutional Neural Networks in MRIs of Multiple Sclerosis Patients. Diagnostics 2022, 12, 230. https://doi.org/10.3390/diagnostics12020230
de Oliveira M, Piacenti-Silva M, da Rocha FCG, Santos JM, Cardoso JdS, Lisboa-Filho PN. Lesion Volume Quantification Using Two Convolutional Neural Networks in MRIs of Multiple Sclerosis Patients. Diagnostics. 2022; 12(2):230. https://doi.org/10.3390/diagnostics12020230
Chicago/Turabian Stylede Oliveira, Marcela, Marina Piacenti-Silva, Fernando Coronetti Gomes da Rocha, Jorge Manuel Santos, Jaime dos Santos Cardoso, and Paulo Noronha Lisboa-Filho. 2022. "Lesion Volume Quantification Using Two Convolutional Neural Networks in MRIs of Multiple Sclerosis Patients" Diagnostics 12, no. 2: 230. https://doi.org/10.3390/diagnostics12020230
APA Stylede Oliveira, M., Piacenti-Silva, M., da Rocha, F. C. G., Santos, J. M., Cardoso, J. d. S., & Lisboa-Filho, P. N. (2022). Lesion Volume Quantification Using Two Convolutional Neural Networks in MRIs of Multiple Sclerosis Patients. Diagnostics, 12(2), 230. https://doi.org/10.3390/diagnostics12020230







