A Multimodal Deep Learning Approach to Predicting Systemic Diseases from Oral Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
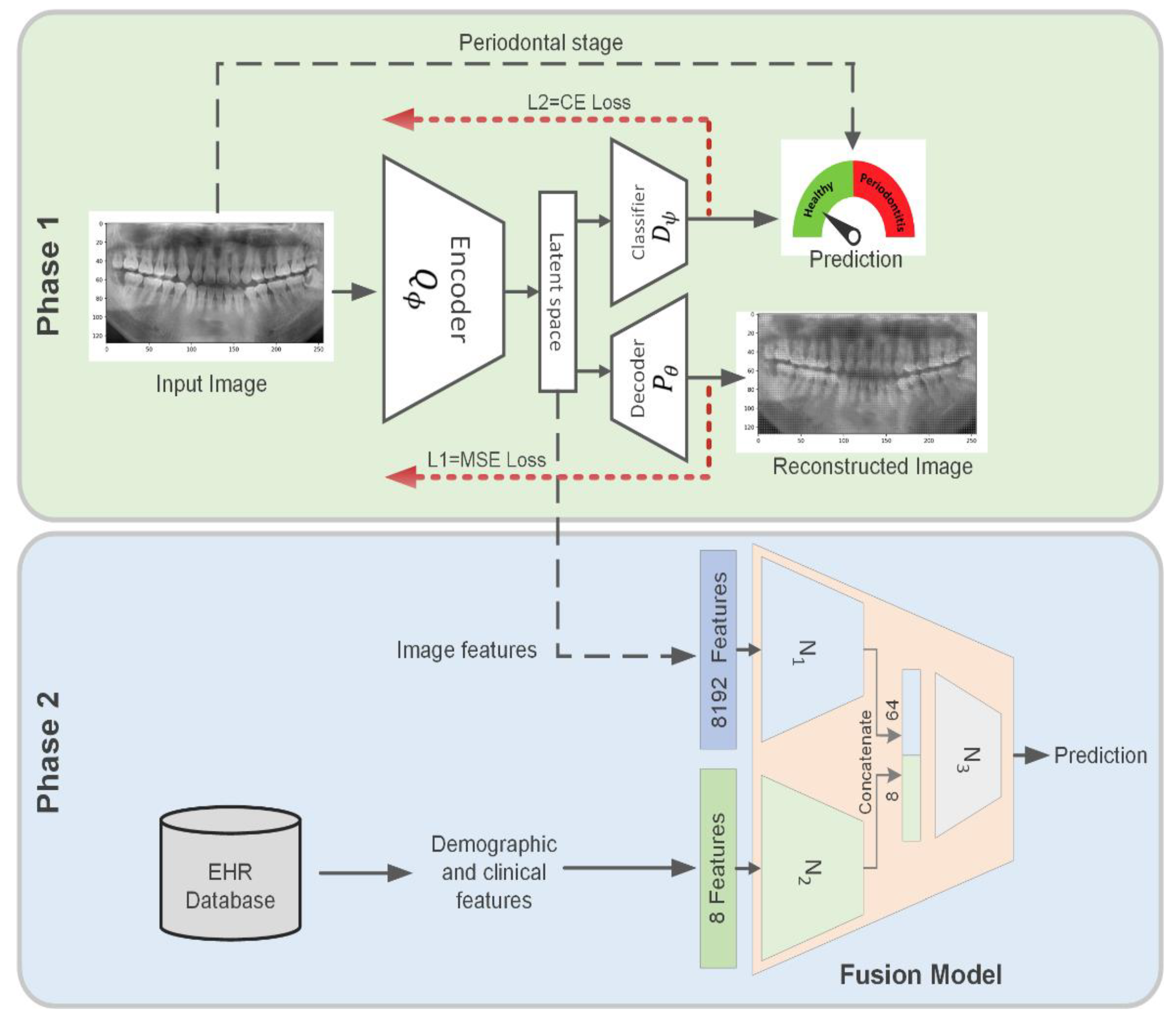
2.2. Dual-Loss Autoencoder to Extract Periodontal-Related Features from OPG
3. Results
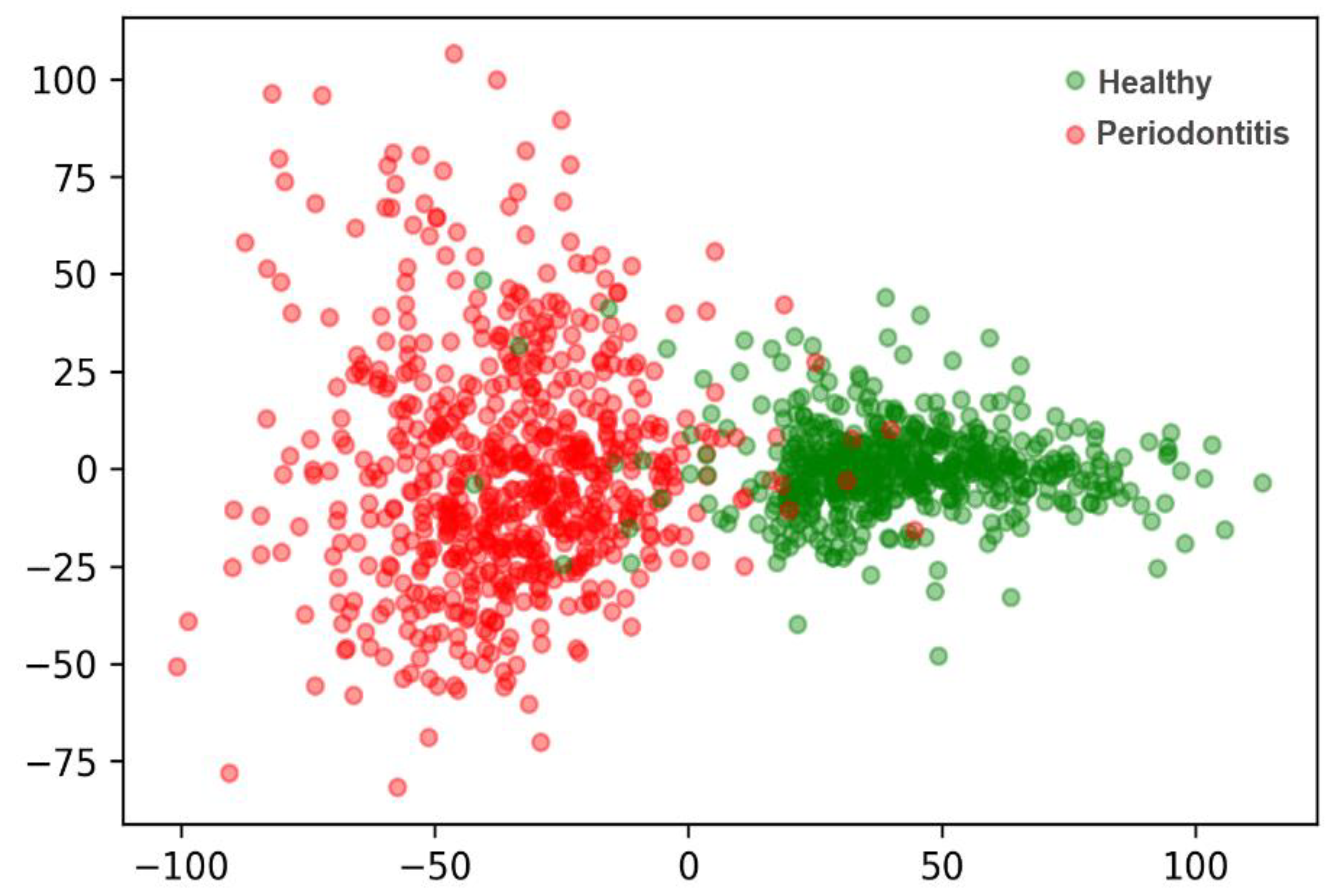
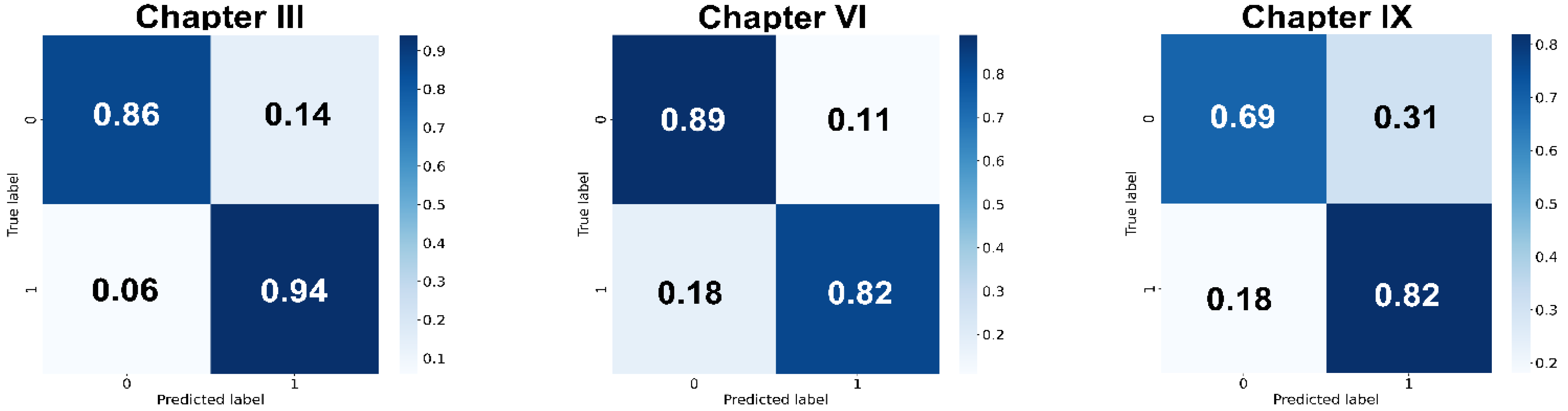
3.1. Extract Periodontal-Related Features from OPG
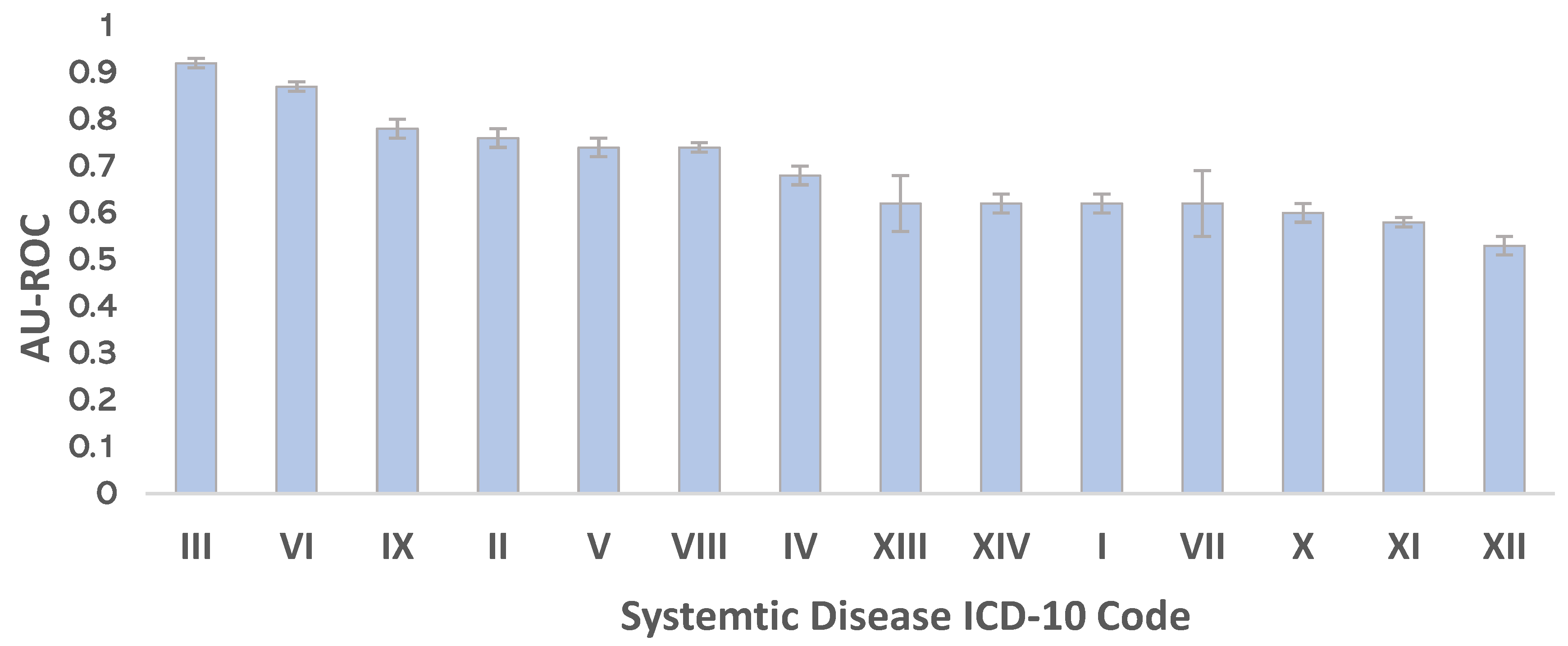
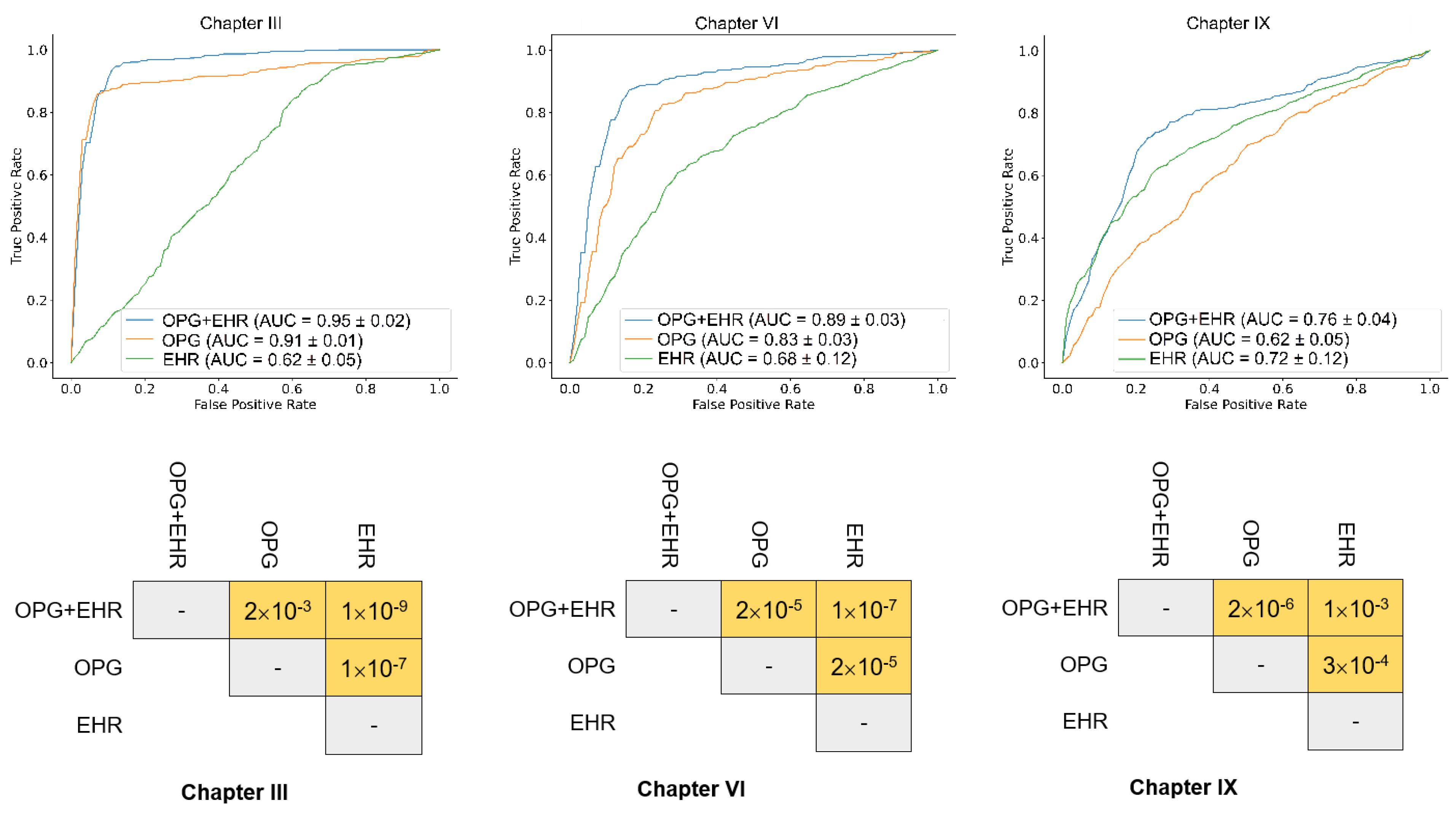
3.2. Predict Systemic Disease Using a Fusion Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonetti, M.S.; Bottenberg, P.; Conrads, G.; Eickholz, P.; Heasman, P.; Huysmans, M.C.; Lopez, R.; Madianos, P.; Müller, F.; Needleman, I.; et al. Dental caries and periodontal diseases in the ageing population: Call to action to protect and enhance oral health and well-being as an essential component of healthy ageing—Consensus report of group 4 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S135–S144. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health. 2022. Available online: https://www.who.int/health-topics/oral-health/#tab=tab_1 (accessed on 25 February 2022).
- Page, R.C.; Kornman, K.S. The pathogenesis of human periodontitis: An introduction. Periodontology 2000 1997, 14, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Monsarrat, P.; Blaizot, A.; Kémoun, P.; Ravaud, P.; Nabet, C.; Sixou, M.; Vergnes, J.-N. Clinical research activity in periodontal medicine: A systematic mapping of trial registers. J. Clin. Periodontol. 2016, 43, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.K.; Liu, X.; Neelakandan, S.; Prakash, M. Modeling of human action recognition using hyperparameter tuned deep learning model. J. Electron. Imaging 2022, 32, 011211. [Google Scholar]
- Zhang, C.; Lu, Y. Study on artificial intelligence: The state of the art and future prospects. J. Ind. Inf. Integr. 2021, 23, 100224. [Google Scholar] [CrossRef]
- Liao, K.-M.; Liu, C.-F.; Chen, C.-J.; Shen, Y.-T. Machine Learning Approaches for Predicting Acute Respiratory Failure, Ventilator Dependence, and Mortality in Chronic Obstructive Pulmonary Disease. Diagnostics 2021, 11, 2396. [Google Scholar] [CrossRef]
- Aich, S.; Youn, J.; Chakraborty, S.; Pradhan, P.M.; Park, J.-H.; Park, S.; Park, J. A Supervised Machine Learning Approach to Detect the On/Off State in Parkinson’s Disease Using Wearable Based Gait Signals. Diagnostics 2020, 10, 421. [Google Scholar] [CrossRef]
- Heidari, A.; Navimipour, N.J.; Unal, M.; Toumaj, S. The COVID-19 epidemic analysis and diagnosis using deep learning: A systematic literature review and future directions. Comput. Biol. Med. 2021, 141, 105141. [Google Scholar] [CrossRef]
- Heidari, A.; Toumaj, S.; Navimipour, N.J.; Unal, M. A privacy-aware method for COVID-19 detection in chest CT images using lightweight deep conventional neural network and blockchain. Comput. Biol. Med. 2022, 145, 105461. [Google Scholar] [CrossRef]
- Heidari, A.; Navimipour, N.J.; Unal, M.; Toumaj, S. Machine learning applications for COVID-19 outbreak management. Neural Comput. Appl. 2022, 34, 15313–15348. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, C.J.P.; Jiang, B.; Chen, J.; Song, J.; Liu, Z.; He, Z.; Krittanawong, C.; Fang, P.-H.; Ming, W.-K. Artificial Intelligence Versus Clinicians in Disease Diagnosis: Systematic Review. JMIR Public Health Surveill. 2019, 7, e10010. [Google Scholar] [CrossRef] [PubMed]
- Leslie, A.; Jones, A.J.; Goddard, P.R. The influence of clinical information on the reporting of CT by radiologists. Br. J. Radiol. 2000, 73, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-C.; Pareek, A.; Seyyedi, S.; Banerjee, I.; Lungren, M.P. Fusion of medical imaging and electronic health records using deep learning: A systematic review and implementation guidelines. NPJ Digit. Med. 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berisha, V.; Krantsevich, C.; Hahn, P.R.; Hahn, S.; Dasarathy, G.; Turaga, P.; Liss, J. Digital medicine and the curse of dimensionality. npj Digit. Med. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Available online: https://icd.who.int/browse10/2016/en (accessed on 10 July 2022).
- Mathieu, M.; Couprie, C.; LeCun, Y. Deep multi-scale video prediction beyond mean square error. arXiv 2015, arXiv:1511.05440. [Google Scholar]
- Zhang, Z.; Sabuncu, M. Generalized cross entropy loss for training deep neural networks with noisy labels. Adv. Neural Inf. Process. Syst. 2018, 31, 8792–8802. [Google Scholar]
- Azad, R.; Asadi-Aghbolaghi, M.; Fathy, M.; Escalera, S. Bi-directional ConvLSTM U-Net with densley connected convolutions. In Proceedings of the 2019 IEEE/CVF International Conference on Computer Vision Workshop (ICCVW), Seoul, Republic of Korea, 27–28 October 2019. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-cam: Visual explanations from deep networks via gradient-based localization. In Proceedings of the 2017 IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood J. Am. Soc. Hematol. 2019, 133, 40–50. [Google Scholar] [CrossRef]
- Spoto, B.; Kakkar, R.; Lo, L.; Devalaraja, M.; Pizzini, P.; Torino, C.; Leonardis, D.; Cutrupi, S.; Tripepi, G.; Mallamaci, F.; et al. Serum Erythroferrone Levels Associate with Mortality and Cardiovascular Events in Hemodialysis and in CKD Patients: A Two Cohorts Study. J. Clin. Med. 2019, 8, 523. [Google Scholar] [CrossRef]
- Ganz, T.; Jung, G.; Naeim, A.; Ginzburg, Y.; Pakbaz, Z.; Walter, P.B.; Kautz, L.; Nemeth, E. Immunoassay for human serum erythroferrone. Blood J. Am. Soc. Hematol. 2017, 130, 1243–1246. [Google Scholar] [CrossRef]
- Schümann, K.; Solomons, N.W. Perspective: What Makes It So Difficult to Mitigate Worldwide Anemia Prevalence? Adv. Nutr. Int. Rev. J. 2017, 8, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lin, Z.; Zhang, S.; Cao, F.; Liang, D.; Zhou, X. Decreased Hemoglobin Concentration and Iron Metabolism Disorder in Periodontitis: Systematic Review and Meta-Analysis. Front. Physiol. 2020, 10, 1620. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Khademi, A.; Fakheran, O. Association between periodontal disease and non-apnea sleep disorder: A systematic review. Clin. Oral Investig. 2020, 24, 3335–3345. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Beukers, N.G.F.M.; van der Heijden, G.J.M.G.; van Wijk, A.J.; Loos, B.G. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J. Epidemiol. Community Health 2016, 71, 37–42. [Google Scholar] [CrossRef]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of Periodontitis and Endothelial Function. New Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.N.; Zhen, Z.; Yiu, K.H.; Tse, H.F.; Pelekos, G.; Tonetti, M.; Jin, L. Periodontal treatment modulates gene expression of endothelial progenitor cells in diabetic patients. J. Clin. Periodontol. 2017, 44, 1253–1263. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.N.; Zhen, Z.; Pelekos, G.; Wu, M.Z.; Chen, Y.; Tonetti, M.; Tse, H.F.; Yiu, K.H.; Jin, L. A randomized controlled trial of the effects of non-surgical periodontal therapy on cardiac function assessed by echocardiography in type 2 diabetic patients. J. Clin. Periodontol. 2020, 47, 726–736. [Google Scholar] [CrossRef]
- Stelzel, M.; Conrads, G.; Pankuweit, S.; Maisch, B.; Vogt, S.; Moosdorf, R.; Flores-De-Jacoby, L. Detection of Porphyromonas gingivalis DNA in Aortic Tissue by PCR. J. Periodontol. 2002, 73, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, M.; Górska, R.; Suwalski, P.; Kowalski, J. Evaluation of the Incidence of Periodontitis-Associated Bacteria in the Atherosclerotic Plaque of Coronary Blood Vessels. J. Periodontol. 2007, 78, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Figuero, E.; Lindahl, C.; Marin, M.J.; Renvert, S.; Herrera, D.; Ohlsson, O.; Wetterling, T.; Sanz, M. Quantification of Periodontal Pathogens in Vascular, Blood, and Subgingival Samples From Patients With Peripheral Arterial Disease or Abdominal Aortic Aneurysms. J. Periodontol. 2014, 85, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Papapanou, P.N.; Philips, K.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Listl, S.; Galloway, J.; Mossey, P.; Marcenes, W. Global Economic Impact of Dental Diseases. J. Dent. Res. 2015, 94, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-Da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Grossi, S.G.; Genco, R.J. Periodontal Disease and Diabetes Mellitus: A Two-Way Relationship. Ann. Periodontol. 1998, 3, 51–61. [Google Scholar] [CrossRef]
- Mattila, K.J.; Nieminen, M.S.; Valtonen, V.V.; Rasi, V.P.; Kesaniemi, Y.A.; Syrjala, S.L.; Jungell, P.S.; Isoluoma, M.; Hietaniemi, K.; Jokinen, M.J. Association between dental health and acute myocardial infarction. BMJ 1989, 298, 779–781. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, M.-Z.; Yu, S.Y.; Pelekos, G.; Yiu, K.H.; Jin, L. Periodontitis links to concurrent systemic comorbidities among ‘self-perceived health’ individuals. J. Periodontal Res. 2022, 57, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Khawaja, A.; Jin, L.; Li, K.Y.; Tonetti, M.; Pelekos, G. The directional and non-directional associations of periodontitis with chronic kidney disease: A systematic review and meta-analysis of observational studies. J. Periodontal Res. 2018, 53, 682–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, H.; Pan, Y.; Jin, L.; Hu, R.; Lu, Y.; Deng, W.; Sun, W.; Chen, C.; Shen, X.; et al. Periodontal disease increases the host susceptibility to COVID-19 and its severity: A Mendelian randomization study. J. Transl. Med. 2021, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case–control study. J. Clin. Periodontol. 2021, 48, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhen, Z.; Pelekos, G.; Yiu, K.H.; Jin, L. Periodontal disease increases the risk for onset of systemic comorbidities in dental hospital attendees: An 18-year retrospective cohort study. J. Periodontol. 2018, 90, 225–233. [Google Scholar] [CrossRef] [PubMed]

| EHR feature | Description |
|---|---|
| Age | Continuous |
| Gender | Binary |
| Income | Continuous |
| Number of teeth | Discrete |
| Periodontal stage | Continuous |
| Extent bone loss | Continuous |
| Bone loss max | Continuous |
| Age-adjusted bone loss | Continuous |
| Chapter Code | Accuracy | Sensitivity | Specificity | Precision | F1 Score |
|---|---|---|---|---|---|
| III | 0.88 ± 0.01 | 0.93 ± 0.02 | 0.83 ± 0.03 | 0.85 ± 0.02 | 0.89 ± 0.01 |
| VI | 0.82 ± 0.02 | 0.84 ± 0.02 | 0.80 ± 0.06 | 0.81 ± 0.04 | 0.83 ± 0.01 |
| IX | 0.72 ± 0.02 | 0.77 ± 0.05 | 0.68 ± 0.04 | 0.71 ± 0.02 | 0.74 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Homayounfar, M.; Zhen, Z.; Wu, M.-Z.; Yu, S.Y.; Yiu, K.-H.; Vardhanabhuti, V.; Pelekos, G.; Jin, L.; Koohi-Moghadam, M. A Multimodal Deep Learning Approach to Predicting Systemic Diseases from Oral Conditions. Diagnostics 2022, 12, 3192. https://doi.org/10.3390/diagnostics12123192
Zhao D, Homayounfar M, Zhen Z, Wu M-Z, Yu SY, Yiu K-H, Vardhanabhuti V, Pelekos G, Jin L, Koohi-Moghadam M. A Multimodal Deep Learning Approach to Predicting Systemic Diseases from Oral Conditions. Diagnostics. 2022; 12(12):3192. https://doi.org/10.3390/diagnostics12123192
Chicago/Turabian StyleZhao, Dan, Morteza Homayounfar, Zhe Zhen, Mei-Zhen Wu, Shuk Yin Yu, Kai-Hang Yiu, Varut Vardhanabhuti, George Pelekos, Lijian Jin, and Mohamad Koohi-Moghadam. 2022. "A Multimodal Deep Learning Approach to Predicting Systemic Diseases from Oral Conditions" Diagnostics 12, no. 12: 3192. https://doi.org/10.3390/diagnostics12123192
APA StyleZhao, D., Homayounfar, M., Zhen, Z., Wu, M.-Z., Yu, S. Y., Yiu, K.-H., Vardhanabhuti, V., Pelekos, G., Jin, L., & Koohi-Moghadam, M. (2022). A Multimodal Deep Learning Approach to Predicting Systemic Diseases from Oral Conditions. Diagnostics, 12(12), 3192. https://doi.org/10.3390/diagnostics12123192






