Texture Parameters Measured by UHF-MRI and CT Scan Provide Information on Bone Quality in Addition to BMD: A Biomechanical Ex Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Femoral Specimens
2.2. CT Measurements
2.3. DXA Measurements
2.4. MRI Measurements
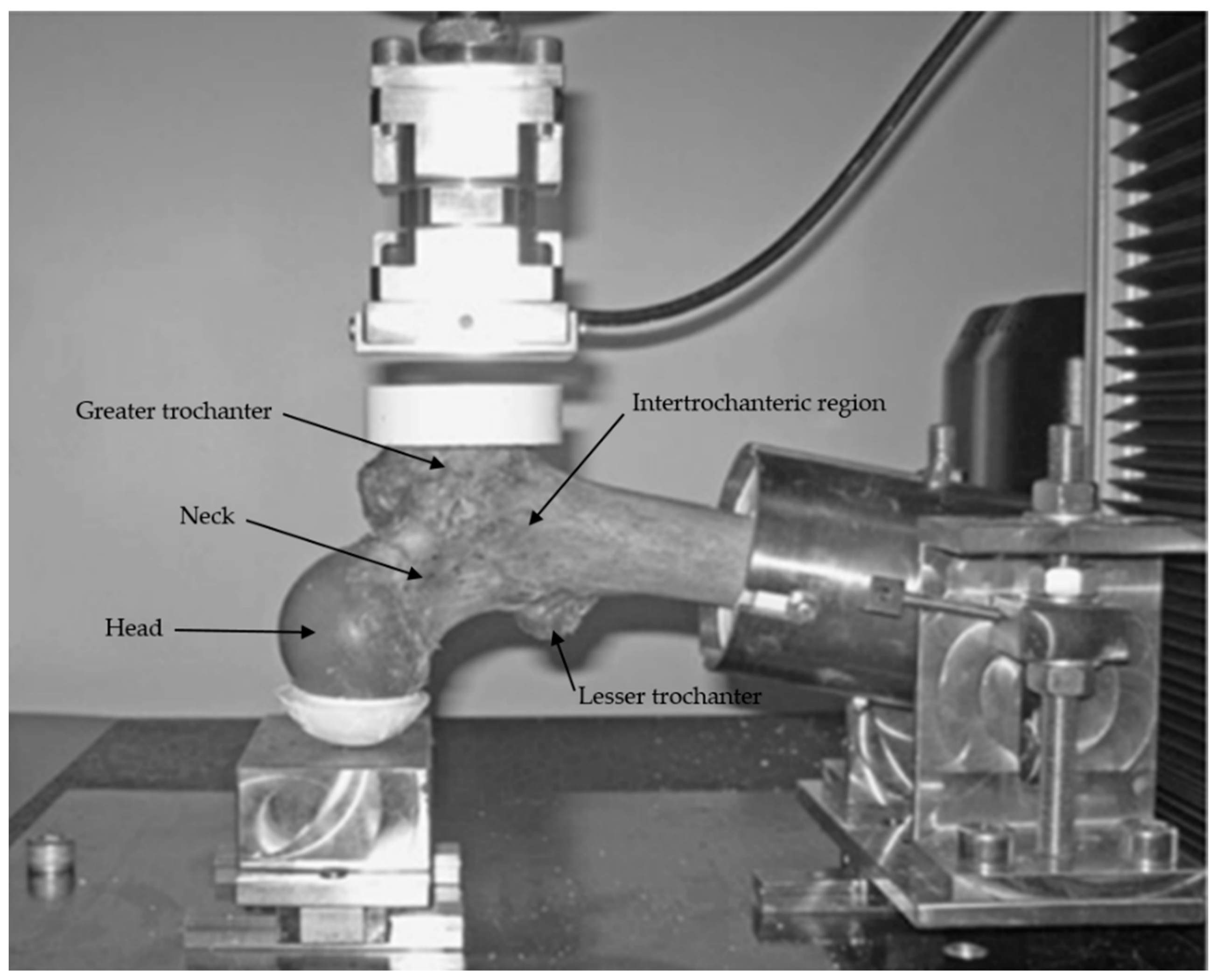
2.5. Mechanical Testing
2.6. Textural Analysis
- −
- Neck: on the coronal plane, a VOI was placed in the middle of a line passing through the neck axis. This line joined the femoral head physis and a perpendicular line passing through the upper extremity of the greater trochanter physis. The other planes allowed for avoiding cortical bone. The same VOI was used for each specimen.
- −
- Intertrochanteric: on the coronal plane, a VOI was placed at the crossing of the neck and diaphysis axis. The other planes allowed for avoiding cortical bone.
- −
- Greater trochanter: on the axial plane, a VOI was placed in the middle of a line joining the external cortical bone and the physis; on the sagittal plane the middle of a line joining the anterior and posterior cortical bone; on the coronal plane the VOI was placed to avoid cortical bone and physis (Figure 2).
- −
- Neck: the ROI was placed in the middle of a line passing through the neck axis. This line joined the femoral head physis and a perpendicular line passing through the upper extremity of the great trochanter physis.
- −
- Intertrochanteric: the ROI was placed at the crossing of the neck and diaphysis axis.
- −
- Greater trochanter: the ROI was placed in the middle of a line joining the upper and lower extremities of the greater trochanter (vertical axis).
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Schuit, S.C.; van der Klift, M.; Weel, A.E.; de Laet, C.E.; Burger, H.; Seeman, E.; Hofman, A.; Uitterlinden, A.G.; van Leeuwen, J.P.; Pols, H.A. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone 2004, 34, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bousson, V.; Le Bras, A.; Roqueplan, F.; Kang, Y.; Mitton, D.; Kolta, S.; Bergot, C.; Skalli, W.; Vicaut, E.; Kalender, W.; et al. Volumetric quantitative computed tomography of the proximal femur: Relationships linking geometric and densitometric variables to bone strength. Role for compact bone. Osteoporos. Int. 2006, 17, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, H.; Moreira-Gonçalves, D.; Coriolano, H.J.; Duarte, J.A. Bone quality: The determinants of bone strength and fragility. Sports Med. 2014, 44, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, M.; Le Corroller, T.; Blanc, G.; Parratte, S.; Champsaur, P.; Chabrand, P.; Argenson, J.N. Radiographic bone texture analysis is correlated with 3D microarchitecture in the femoral head, and improves the estimation of the femoral neck fracture risk when combined with bone mineral density. Eur. J. Radiol. 2013, 82, 1494–1498. [Google Scholar] [CrossRef]
- Chang, G.; Honig, S.; Liu, Y.; Chen, C.; Chu, K.K.; Rajapakse, C.S.; Egol, K.; Xia, D.; Saha, P.K.; Regatte, R.R. 7 Tesla MRI of bone microarchitecture discriminates between women without and with fragility fractures who do not differ by bone mineral density. J. Bone Miner. Metab. 2015, 33, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Bousson, V.D.; Adams, J.; Engelke, K.; Aout, M.; Cohen-Solal, M.; Bergot, C.; Haguenauer, D.; Goldberg, D.; Champion, K.; Aksouh, R.; et al. In vivo discrimination of hip fracture with quantitative computed tomography: Results from the prospective European Femur Fracture Study (EFFECT). J. Bone Miner. Res. 2011, 26, 881–893. [Google Scholar] [CrossRef]
- Museyko, O.; Bousson, V.; Adams, J.; Laredo, J.D.; Engelke, K. QCT of the proximal femur—Which parameters should be measured to discriminate hip fracture? Osteoporos. Int. 2016, 27, 1137–1147. [Google Scholar] [CrossRef]
- Lespessailles, E.; Chappard, C.; Bonnet, N.; Benhamou, C.L. Imaging techniques for evaluating bone microarchitecture. Jt. Bone Spine 2006, 73, 254–261. [Google Scholar] [CrossRef]
- Kawashima, Y.; Fujita, A.; Buch, K.; Li, B.; Qureshi, M.M.; Chapman, M.N.; Sakai, O. Using texture analysis of head CT images to differentiate osteoporosis from normal bone density. Eur. J. Radiol. 2019, 116, 212–218. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Apostol, L.; Boudousq, V.; Basset, O.; Odet, C.; Yot, S.; Tabary, J.; Dinten, J.M.; Boiler, E.; Kotzki, P.O.; Peyrin, F. Relevance of 2D radiographic texture analysis for the assessment of 3D bone micro-architecture. Med. Phys. 2006, 33, 3546–3556. [Google Scholar] [CrossRef]
- Ranjanomennahary, P.; Ghalila, S.S.; Malouche, D.; Marchadier, A.; Rachidi, M.; Benhamou, C.; Chappard, C. Comparison of radiograph-based texture analysis and bone mineral density with three-dimensional microarchitecture of trabecular bone. Med. Phys. 2011, 38, 420–428. [Google Scholar] [CrossRef]
- Shirvaikar, M.; Huang, N.; Dong, X.N. The measurement of bone quality using gray level co-occurrence matrix textural features. J. Med. Imaging Health Inform. 2016, 6, 1357–1362. [Google Scholar] [CrossRef] [Green Version]
- Le Corroller, T.; Pithioux, M.; Chaari, F.; Rosa, B.; Parratte, S.; Maurel, B.; Argenson, J.N.; Champsaur, P.; Chabrand, P. Bone texture analysis is correlated with three-dimensional microarchitecture and mechanical properties of trabecular bone in osteoporotic femurs. J. Bone Miner. Metab. 2013, 31, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Guenoun, D.; Pithioux, M.; Souplet, J.C.; Guis, S.; Le Corroller, T.; Fouré, A.; Pauly, V.; Mattei, J.P.; Bernard, M.; Guye, M.; et al. Assessment of proximal femur microarchitecture using ultra-high field MRI at 7 Tesla. Diagn. Interv. Imaging 2020, 101, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Galloway, M.M. Texture Analysis Using Gray Level Run Lengths. Comput. Graph. Image Process. 1975, 4, 172–179. [Google Scholar] [CrossRef]
- Chu, A.; Sehgal, C.M.; Greenleaf, J.F. Use of gray value distribution of run lengths for texture analysis. Pattern Recognit. Lett. 1990, 11, 415–419. [Google Scholar] [CrossRef]
- Soldati, E.; Vicente, J.; Guenoun, D.; Bendahan, D.; Pithioux, M. Validation and Optimization of Proximal Femurs Microstructure Analysis Using High Field and Ultra-High Field MRI. Diagnostics 2021, 11, 1603. [Google Scholar] [CrossRef]
- Pulkkinen, P.; Jämsä, T.; Lochmüller, E.M.; Kuhn, V.; Nieminen, M.T.; Eckstein, F. Experimental hip fracture load can be predicted from plain radiography by combined analysis of trabecular bone structure and bone geometry. Osteoporos. Int. 2008, 19, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Dragomir-Daescu, D.; Rossman, T.L.; Rezaei, A.; Carlson, K.D.; Kallmes, D.F.; Skinner, J.A.; Khosla, S.; Amin, S. Factors associated with proximal femur fracture determined in a large cadaveric cohort. Bone 2018, 116, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.L.; Seeley, D.G.; Lui, L.Y.; Cauley, J.A.; Ensrud, K.; Browner, W.S.; Nevitt, M.C.; Cummings, S.R.; Osteoporotic Fractures Research Group. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2003, 18, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Rajapakse, C.S.; Chen, C.; Welbeck, A.; Egol, K.; Regatte, R.R.; Saha, P.K.; Honig, S. Imaging of Proximal Femur Microarchitecture in Subjects with and without Fragility Fracture and Nonosteoporotic Proximal Femur Bone Mineral Density. Radiology 2018, 287, 608–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Corroller, T.; Halgrin, J.; Pithioux, M.; Guenoun, D.; Chabrand, P.; Champsaur, P. Combination of texture analysis and bone mineral density improves the prediction of fracture load in human femurs. Osteoporos. Int. 2012, 23, 163–169. [Google Scholar] [CrossRef]
- Rajapakse, C.S.; Kobe, E.A.; Batzdorf, A.S.; Hast, M.W.; Wehrli, F.W. Accuracy of MRI-based finite element assessment of distal tibia compared to mechanical testing. Bone 2018, 108, 71–78. [Google Scholar] [CrossRef]
- Eckstein, F.; Matsuura, M.; Kuhn, V.; Priemel, M.; Müller, R.; Link, T.M.; Lochmüller, E.M. Sex differences of human trabecular bone microstructure in aging are site-dependent. J. Bone Miner. Res. 2007, 22, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Thevenot, J.; Hirvasniemi, J.; Pulkkinen, P.; Määttä, M.; Korpelainen, R.; Saarakkala, S.; Jämsä, T. Assessment of risk of femoral neck fracture with radiographic texture parameters: A retrospective study. Radiology 2014, 272, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Chappard, C.; Bousson, V.; Bergot, C.; Mitton, D.; Marchadier, A.; Moser, T.; Benhamou, C.L.; Laredo, J.D. Prediction of femoral fracture load: Cross-sectional study of texture analysis and geometric measurements on plain radiographs versus bone mineral density. Radiology 2010, 255, 536–543. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]


| Sex | Age | Femur Side | Total Femur BMD | Failure Load (N) | Failure Stress (MPa) |
|---|---|---|---|---|---|
| Women | 81 | Right | 0.849 | 2075.36 | 2.39 |
| Left | 0.861 | 2113.96 | 2.38 | ||
| Women | 83 | Right | 0.701 | 1293.2 | 2.13 |
| Left | 0.714 | 1477.2 | 2.51 | ||
| Women | 83 | Right | 0.722 | 2318.69 | 2.31 |
| Left | 0.651 | 1524.42 | 1.55 | ||
| Women | 86 | Right | 0.735 | 866.9 | 1.14 |
| Left | 0.701 | excluded | excluded | ||
| Women | 89 | Right | 0.508 | 743 | 1.1 |
| Left | 0.615 | 973 | 1.5 | ||
| Men | 62 | Right | 0.861 | 1114 | 1.33 |
| Left | 0.842 | 1494 | 1.86 | ||
| Men | 80 | Right | 0.884 | 1760.04 | 2.12 |
| Left | 0.849 | 2148.1 | 2.73 | ||
| Women | 91 | Right | 0.773 | 710.58 | 1.16 |
| Left | 0.731 | 876.28 | 1.36 |
| Textural Parameters | r | p Value |
|---|---|---|
| First Order | ||
| Energy | −0.489 | (0.066) |
| Entropy | −0.821 | (0.0002) |
| Mean | −0.532 | (0.043) |
| Median | −0.625 | (0.014) |
| GLCM | ||
| Contrast | −0.282 | (0.307) |
| Correlation | −0.182 | (0.515) |
| Joint Energy | 0.75 | (0.0019) |
| Joint Entropy | −0.7 | (0.0048) |
| Inverse Difference Moment | 0.228 | (0.411) |
| Maximum Probability | 0.489 | (0.066) |
| Sum Average | −0.864 | (<0.0001) |
| Sum of Squares | −0.753 | (0.0017) |
| GLRM | ||
| Short Run Emphasis | −0.26 | (0.346) |
| Long Run Emphasis | 0.214 | (0.442) |
| Gray Level Non Uniformity | 0.589 | (0.023) |
| Run Length Non Uniformity | −0.010 | (0.974) |
| Run Percentage | −0.214 | (0.442) |
| Low Gray Level Run Emphasis | 0.846 | (<0.0001) |
| High Gray Level Run Emphasis | −0.896 | (<0.0001) |
| MRI GT Neck | CT GT Neck | |||||||
|---|---|---|---|---|---|---|---|---|
| Textural Parameters | r | p Value | r | p Value | r | p Value | r | p Value |
| First Order | ||||||||
| Energy | −0.137 | (0.624) | 0.086 | (0.761) | 0.191 | (0.477) | −0.160 | (0.552) |
| Entropy | −0.631 | (0.011) | −0.259 | (0.35) | 0.091 | (0.737) | −0.368 | (0.160) |
| Mean | −0.116 | (0.679) | 0.07 | (0.805) | 0.263 | (0.324) | −0.720 | (0.0016) |
| Median | −0.134 | (0.633) | 0.086 | (0.761) | 0.295 | (0.266) | −0.546 | (0.028) |
| GLCM | ||||||||
| Contrast | −0.135 | (0.629) | −0.263 | (0.344) | 0.059 | (0.828) | 0.108 | (0.687) |
| Correlation | −0.250 | (0.367) | 0.114 | (0.684) | 0.258 | (0.335) | −0.764 | (0.0005) |
| Joint Energy | 0.599 | (0.018) | 0.288 | 0.298) | −0.081 | (0.765) | 0.362 | (0.167) |
| Joint Entropy | −0.583 | (0.022) | −0.327 | (0.233) | 0.043 | (0.875) | −0.288 | (0.278) |
| Inverse Difference Moment | 0.180 | (0.519) | 0.164 | (0.558) | −0.103 | (0.704) | 0.116 | (0.667) |
| Maximum Probability | 0.567 | (0.027) | 0.182 | (0.515) | −0.277 | (0.299) | 0.288 | (0.278) |
| Sum Average | −0.533 | (0.04) | −0.161 | (0.566) | 0.187 | (0.488) | −0.319 | (0.227) |
| Sum of Squares | −0.556 | (0.031) | −0.218 | (0.434) | 0.122 | (0.652) | −0.379 | (0.146) |
| GLRM | ||||||||
| Short Run Emphasis | −0.191 | (0.494) | −0.213 | (0.446) | 0.109 | (0.688) | 0.081 | (0.765) |
| Long Run Emphasis | 0.135 | (0.629) | 0.164 | (0.558) | −0.128 | (0.636) | −0.108 | (0.687) |
| Gray Level Non Uniformity | 0.332 | (0.225) | −0.172 | (0.54) | 0.085 | (0.753) | 0.474 | (0.063) |
| Run Length Non Uniformity | −0.071 | (0.799) | −0.268 | (0.333) | 0.182 | (0.498) | 0.307 | (0.246) |
| Run Percentage | −0.135 | (0.629) | −0.172 | (0.54) | 0.125 | (0.644) | 0.081 | (0.765) |
| Low Gray Level Run Emphasis | 0.533 | (0.040) | 0.086 | (0.761) | −0.11 | (0.684) | 0.522 | (0.037) |
| High Gray Level Run Emphasis | −0.529 | (0.042) | −0.181 | (0.519) | 0.215 | (0.424) | −0.343 | (0.193) |
| Textural Parameters | r | p-Value |
|---|---|---|
| First Order | ||
| Energy | −0.312 | (0.257) |
| Entropy | −0.692 | (0.004) |
| Mean | −0.455 | (0.088) |
| Median | −0.475 | (0.073) |
| GLCM | ||
| Contrast | −0.194 | (0.487) |
| Correlation | −0.445 | (0.096) |
| Joint Energy | 0.593 | (0.019) |
| Joint Entropy | −0.617 | (0.014) |
| Inverse Difference Moment | 0.122 | (0.663) |
| Maximum Probability | 0.668 | (0.006) |
| Sum Average | −0.707 | (0.003) |
| Sum of Squares | −0.681 | (0.005) |
| GLRM | ||
| Short Run Emphasis | −0.079 | (0.778) |
| Long Run Emphasis | 0.084 | (0.764) |
| Gray Level Non Uniformity | 0.371 | (0.172) |
| Run Length Non Uniformity | −0.22 | (0.429) |
| Run Percentage | −0.083 | (0.767) |
| Low Gray Level Run Emphasis | 0.725 | (0.002) |
| High Gray Level Run Emphasis | −0.718 | (0.002) |
| R² | Adjusted R² | p-Value | |
|---|---|---|---|
| aBMD Alone | 0.2066 | ||
| aBMD + MRI Textural parameters | |||
| First Order Entropy | 0.569 | (0.0012) | |
| GLCM Joint Energy | 0.431 | (0.0128) | |
| GLCM Joint Entropy | 0.48 | (0.006) | |
| GLCM Sum Average | 0.783 | (<0.0001) | |
| GLCM Sum of Squares | 0.53 | (0.0025) | |
| GLRM Gray Level Non Uniformity | 0.348 | (0.0412) | |
| GLRM Low Gray Level Run Emphasis | 0.761 | (<0.0001) | |
| GLRM High Gray Level Run Emphasis | 0.782 | (<0.0001) |
| R² | Adjusted R² | p-Value | |
|---|---|---|---|
| aBMD Alone | 0.1530 | ||
| aBMD + MRI Textural parameters | |||
| First Order Entropy | 0.407 | (0.0103) | |
| GLCM Joint Entropy | 0.299 | (0.0444) | |
| GLCM Sum Average | 0.433 | (0.0071) | |
| GLCM Sum of Squares | 0.401 | (0.0113) | |
| GLRM Low Gray Level Run Emphasis | 0.46 | (0.0047) | |
| GLRM High Gray Level Run Emphasis | 0.452 | (0.0054) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoepflin, P.; Pithioux, M.; Bendahan, D.; Poullain, F.; Le Corroller, T.; Fabre, C.; Pauly, V.; Creze, M.; Soldati, E.; Champsaur, P.; et al. Texture Parameters Measured by UHF-MRI and CT Scan Provide Information on Bone Quality in Addition to BMD: A Biomechanical Ex Vivo Study. Diagnostics 2022, 12, 3143. https://doi.org/10.3390/diagnostics12123143
Knoepflin P, Pithioux M, Bendahan D, Poullain F, Le Corroller T, Fabre C, Pauly V, Creze M, Soldati E, Champsaur P, et al. Texture Parameters Measured by UHF-MRI and CT Scan Provide Information on Bone Quality in Addition to BMD: A Biomechanical Ex Vivo Study. Diagnostics. 2022; 12(12):3143. https://doi.org/10.3390/diagnostics12123143
Chicago/Turabian StyleKnoepflin, Paul, Martine Pithioux, David Bendahan, François Poullain, Thomas Le Corroller, Cyprien Fabre, Vanessa Pauly, Maud Creze, Enrico Soldati, Pierre Champsaur, and et al. 2022. "Texture Parameters Measured by UHF-MRI and CT Scan Provide Information on Bone Quality in Addition to BMD: A Biomechanical Ex Vivo Study" Diagnostics 12, no. 12: 3143. https://doi.org/10.3390/diagnostics12123143
APA StyleKnoepflin, P., Pithioux, M., Bendahan, D., Poullain, F., Le Corroller, T., Fabre, C., Pauly, V., Creze, M., Soldati, E., Champsaur, P., & Guenoun, D. (2022). Texture Parameters Measured by UHF-MRI and CT Scan Provide Information on Bone Quality in Addition to BMD: A Biomechanical Ex Vivo Study. Diagnostics, 12(12), 3143. https://doi.org/10.3390/diagnostics12123143








