The Benign Side of the Abdominal Wall: A Pictorial Review of Non-Neoplastic Diseases
Abstract
1. Introduction
2. Infective Pathologies
2.1. Abscess
2.2. Necrotizing Fasciitis
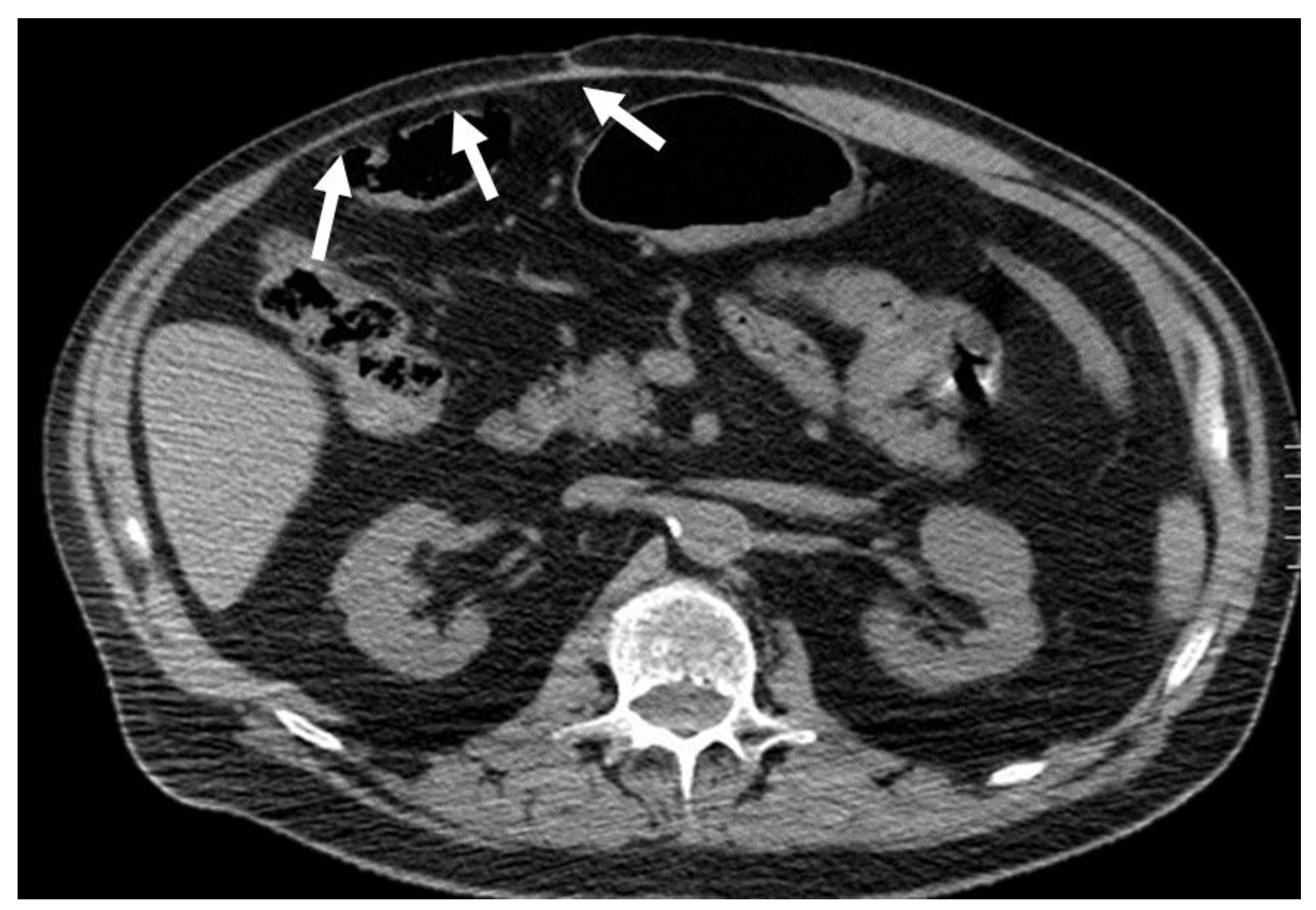
2.3. Foreign Body Retention: Granuloma and Gossypiboma
2.4. Fistulae
3. Congenital Conditions
3.1. Urachal Anomalies
3.2. Congenital Defects of the Abdominal Wall
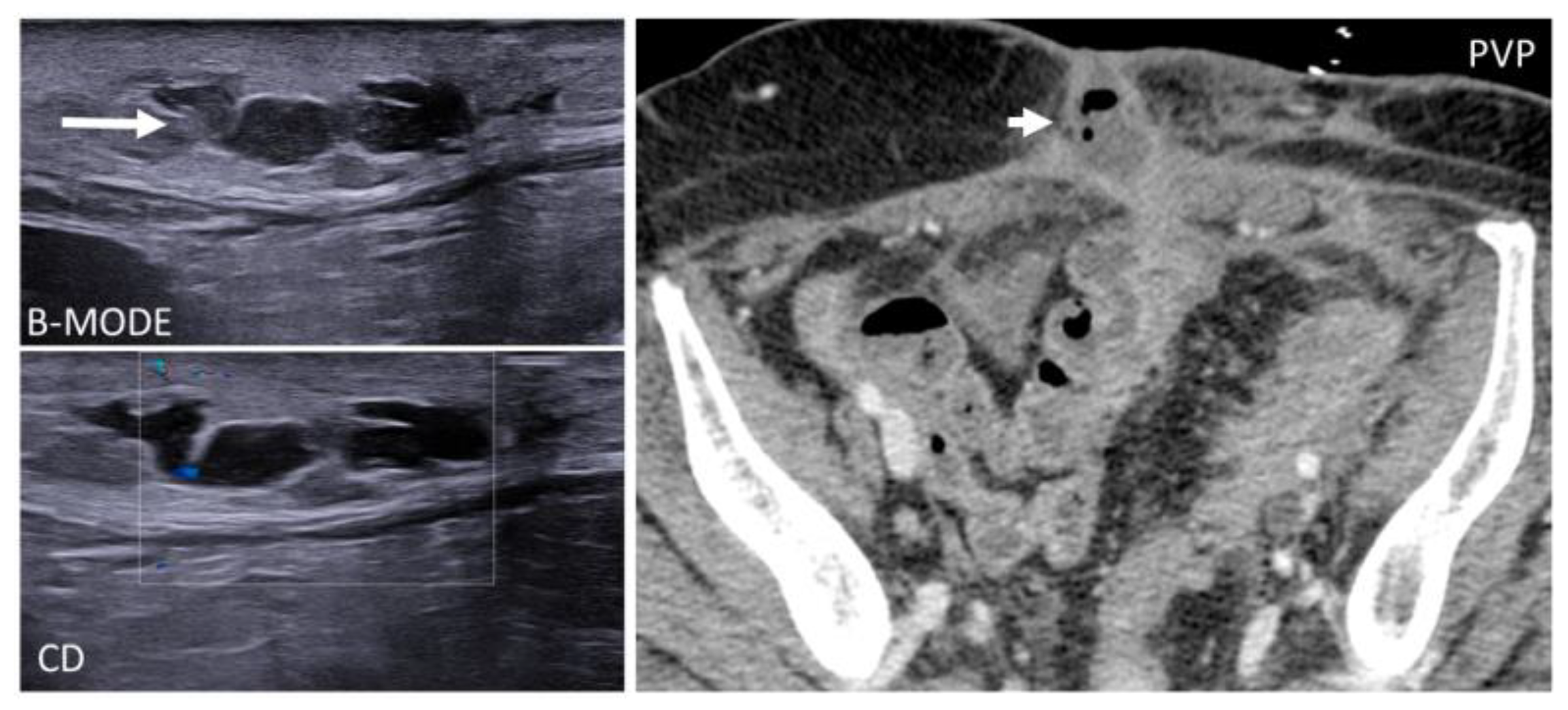
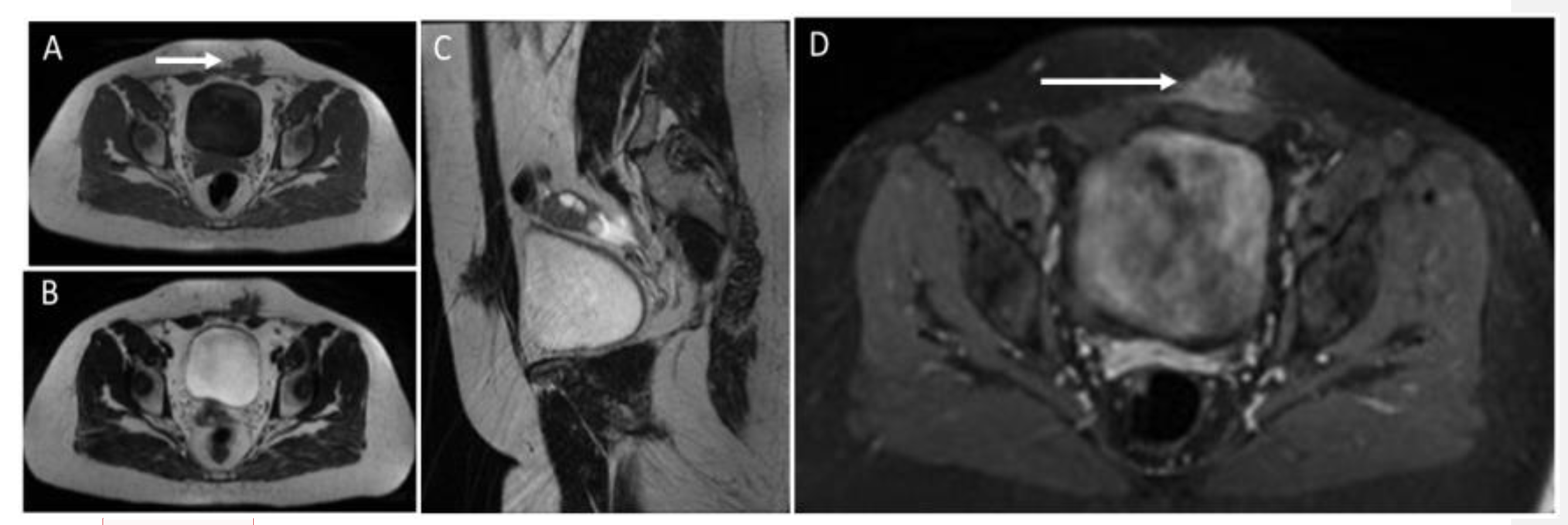
3.3. Arteriovenous Malformations
4. Acquired Vascular Conditions
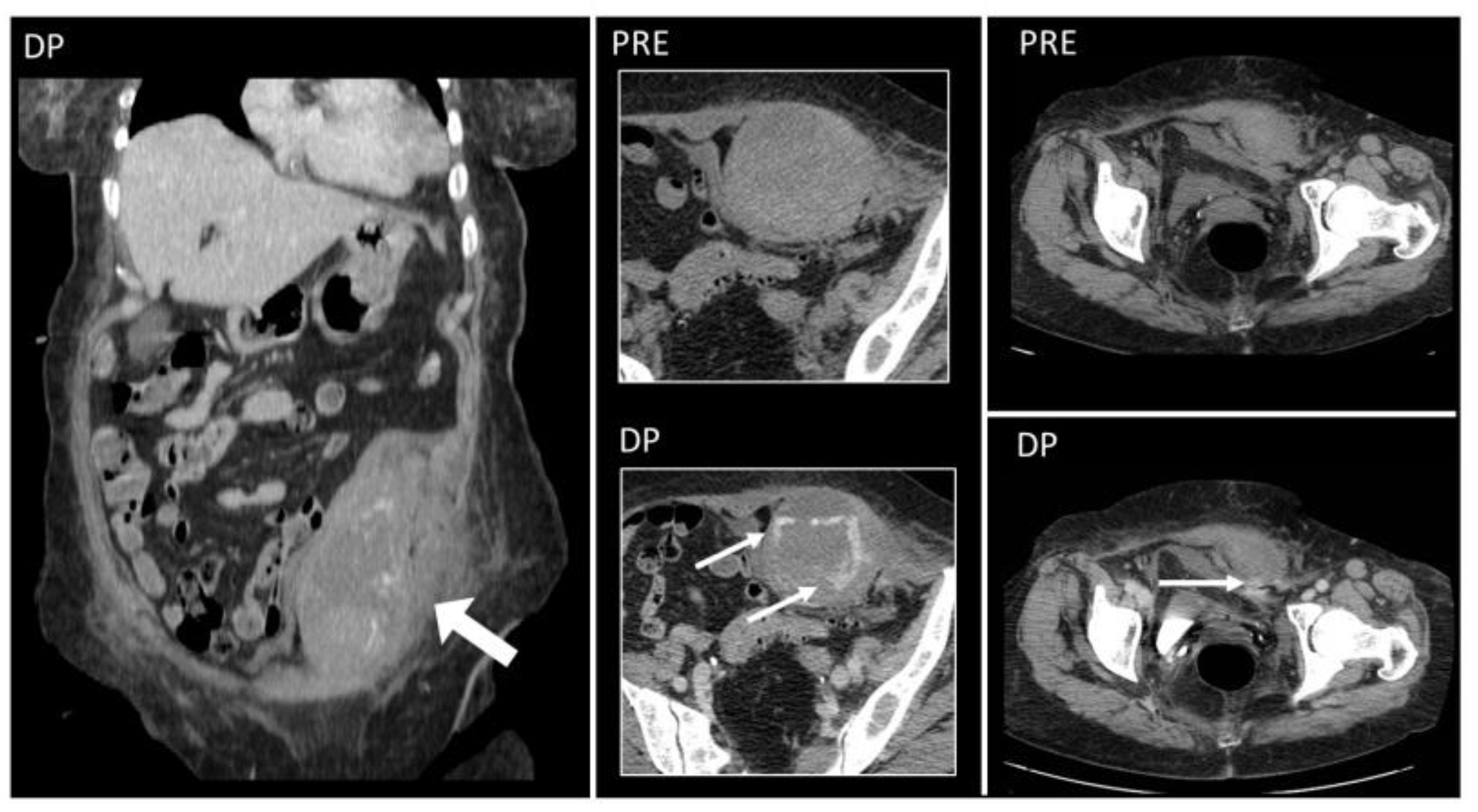
4.1. Shunts
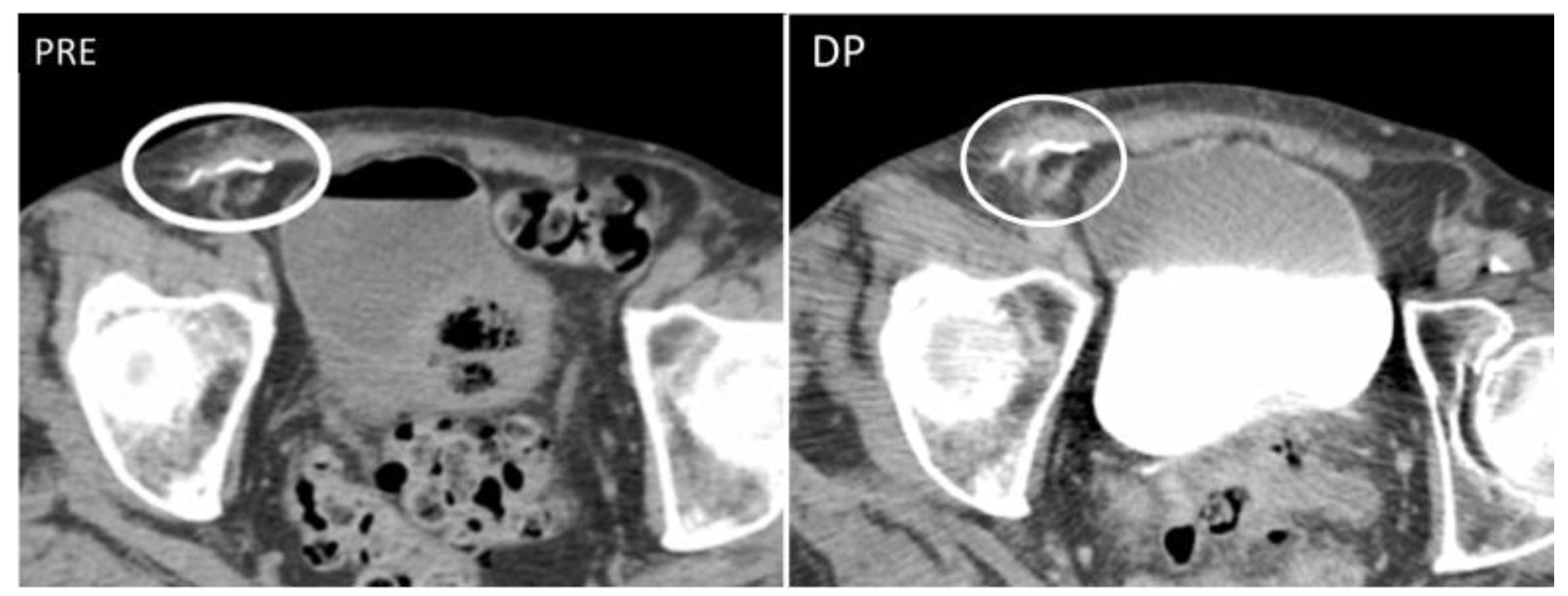
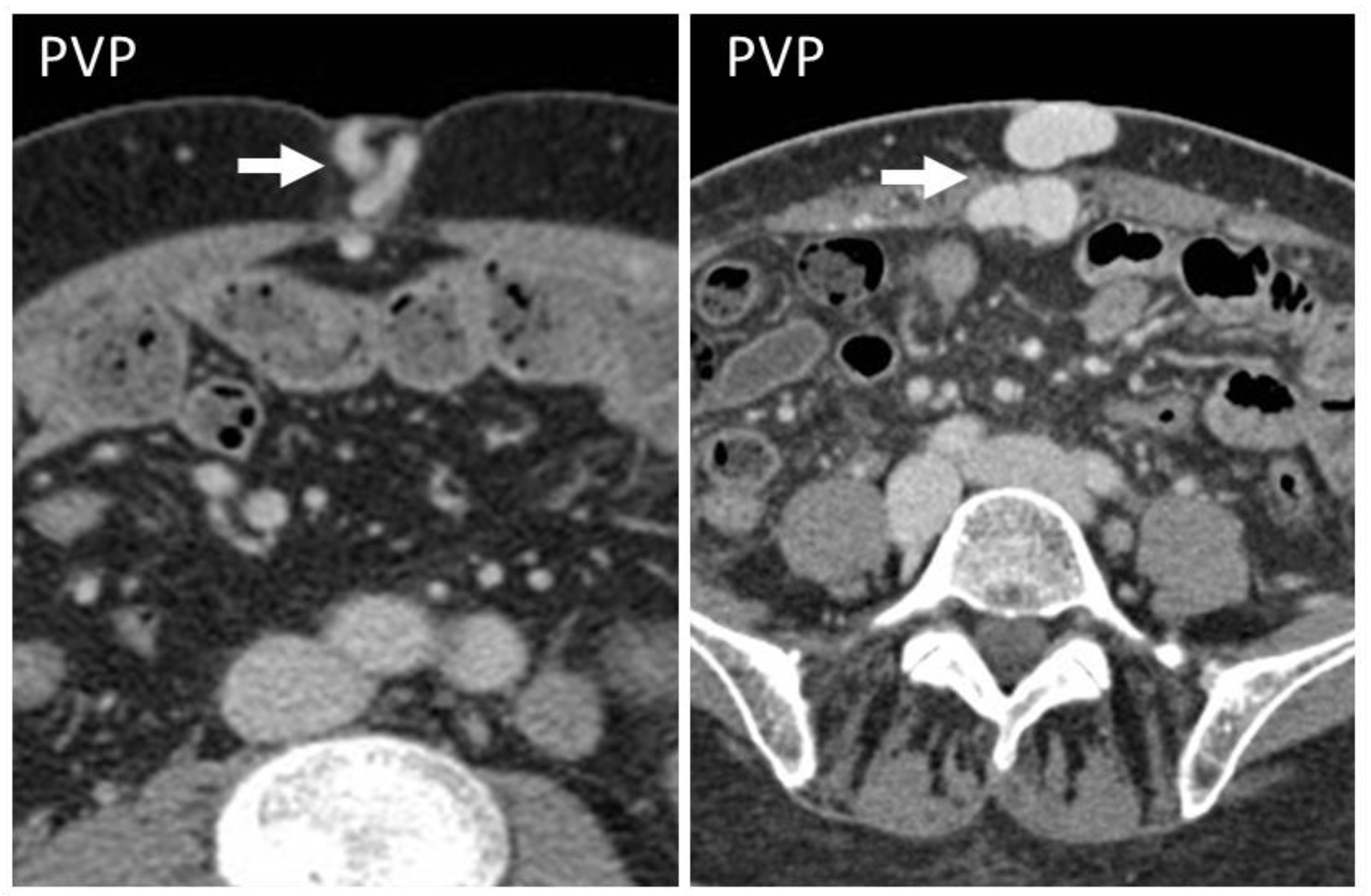
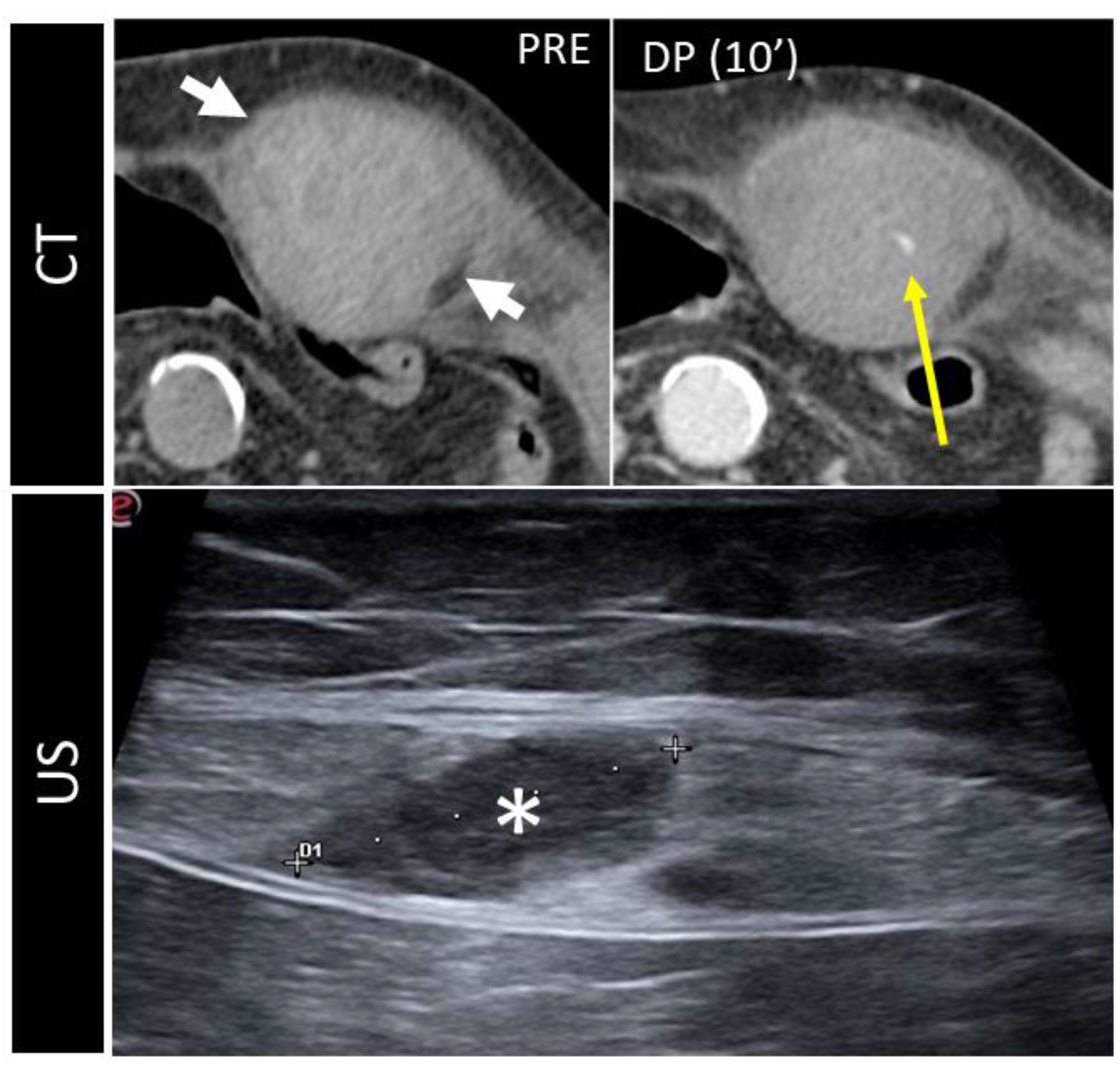
4.2. Pseudoaneurysm
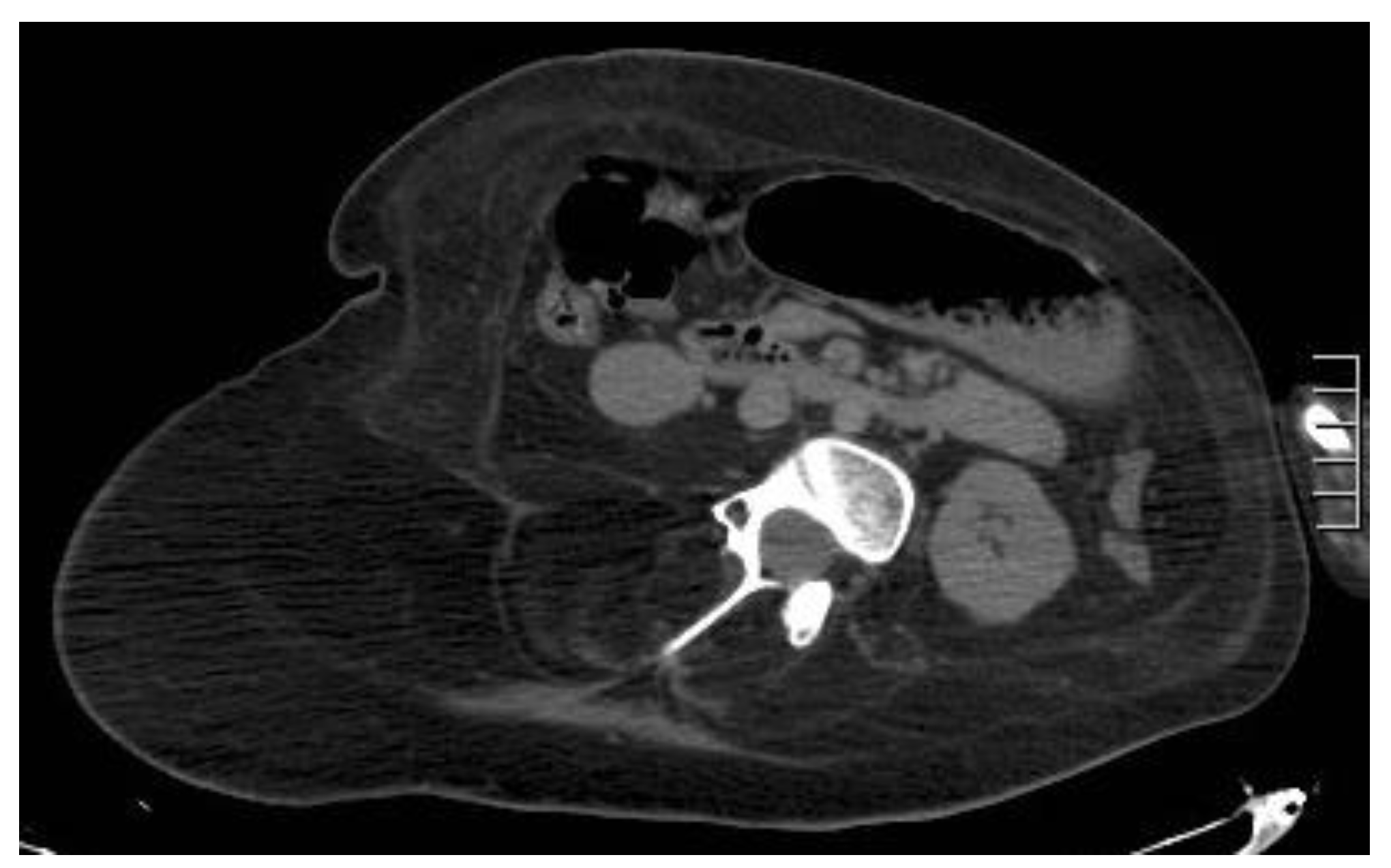
4.3. Hematoma
5. Storing and Miscellaneous Diseases
5.1. Injection Granulomas
5.2. Silicone and Paraffin
5.3. Subcutaneous Drugs
5.4. Calcinosis and Collagen Diseases
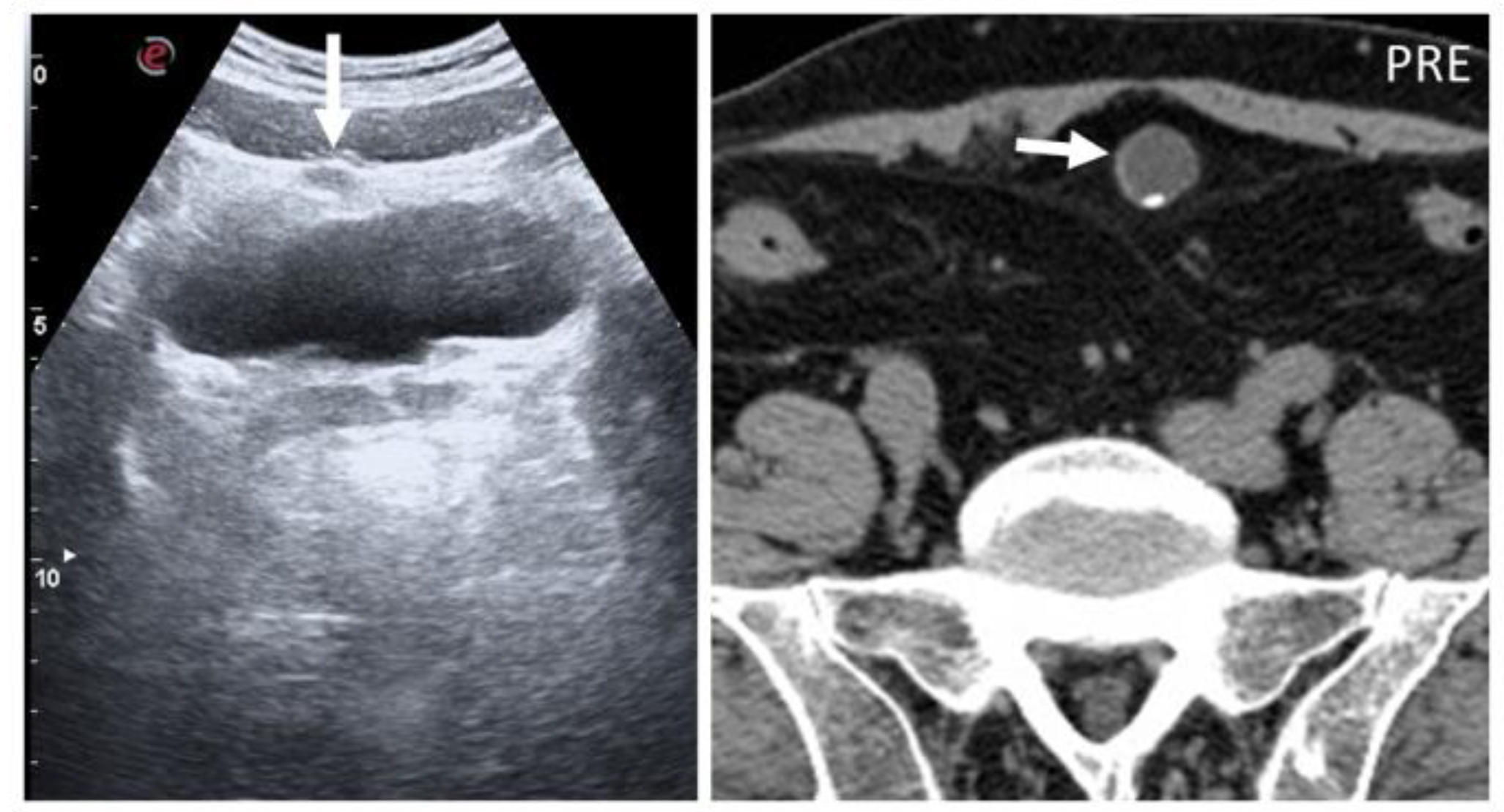
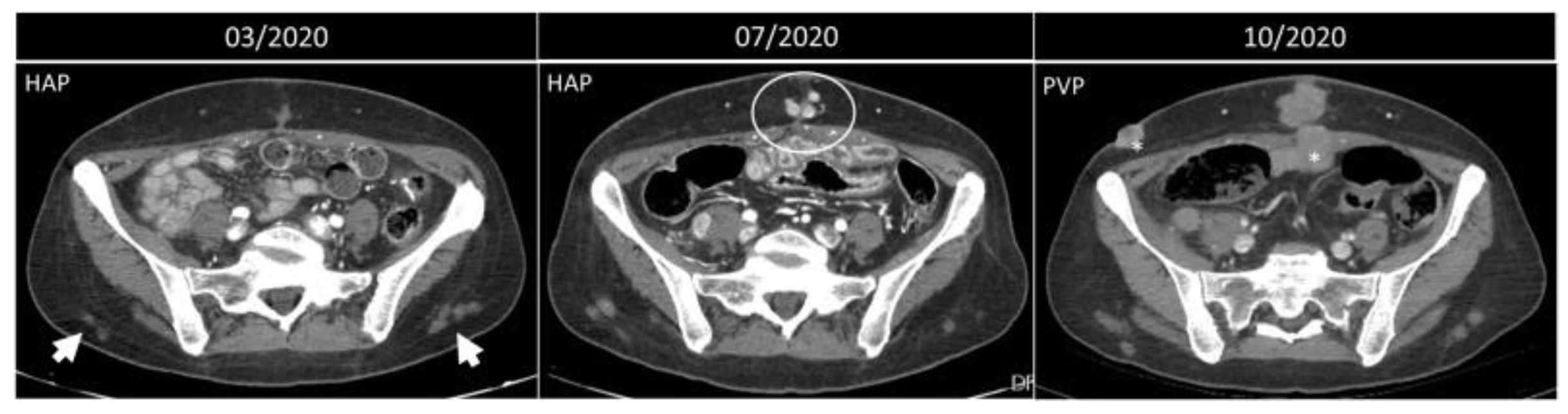
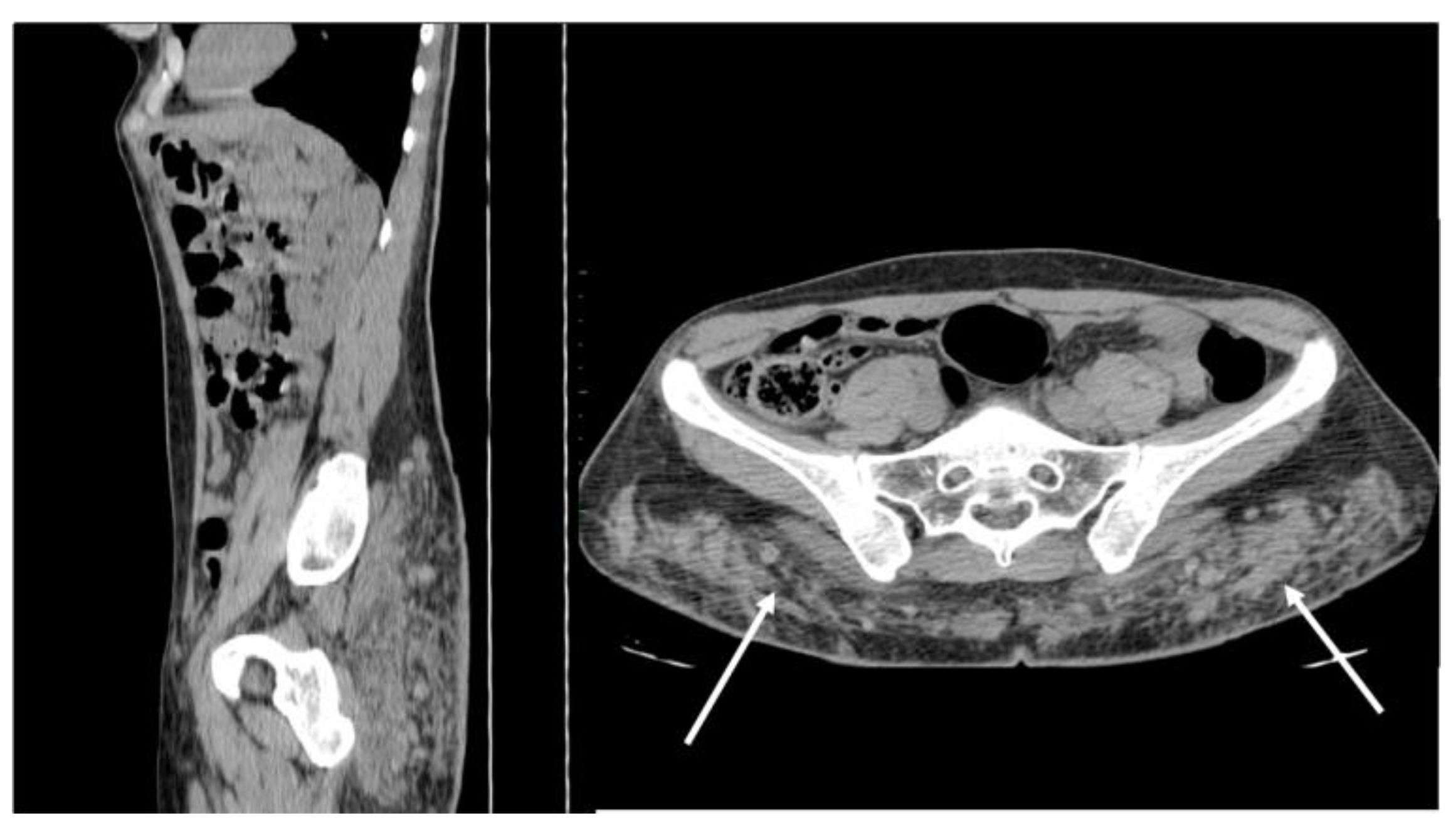
5.5. Splenosis
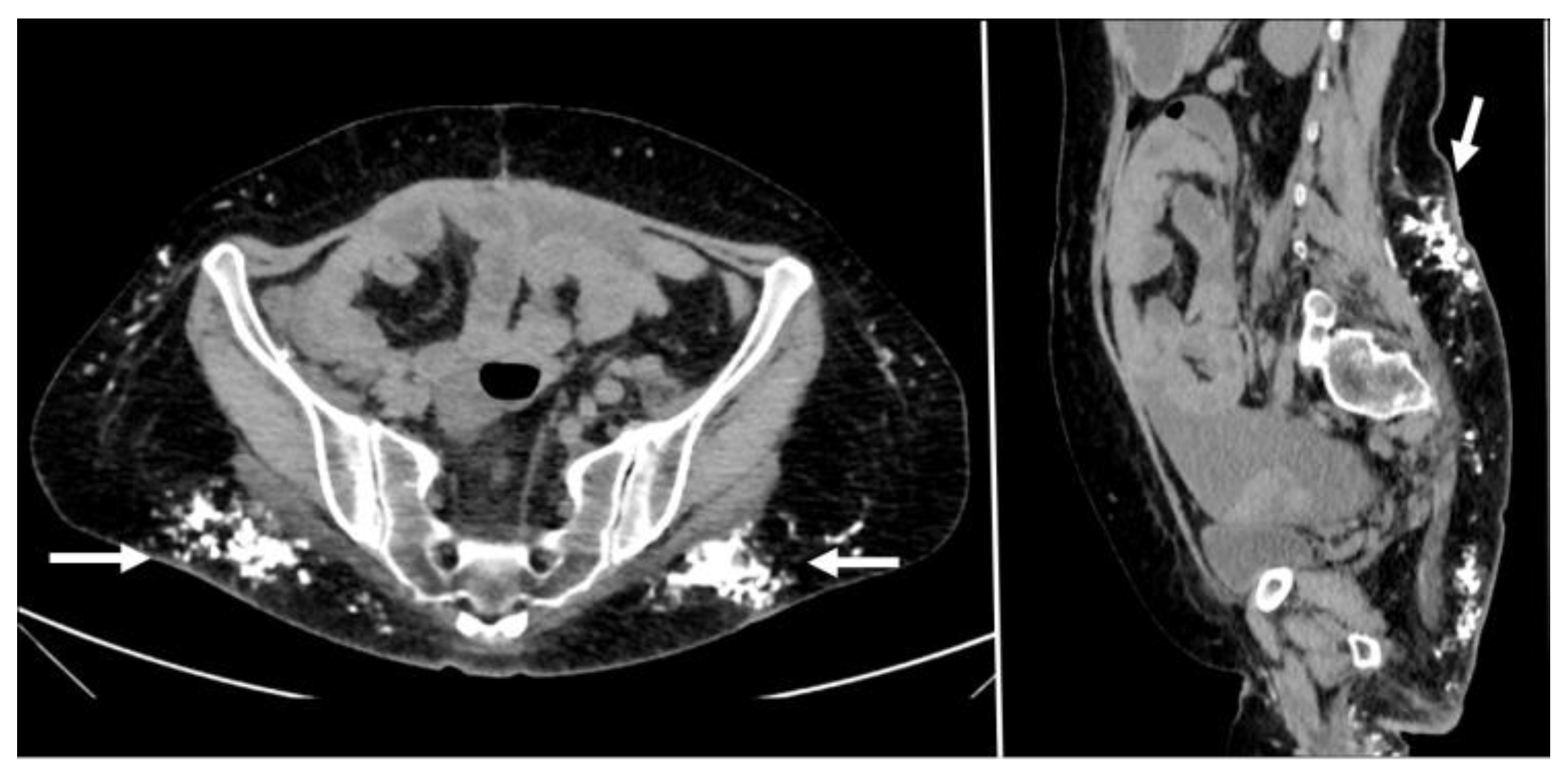
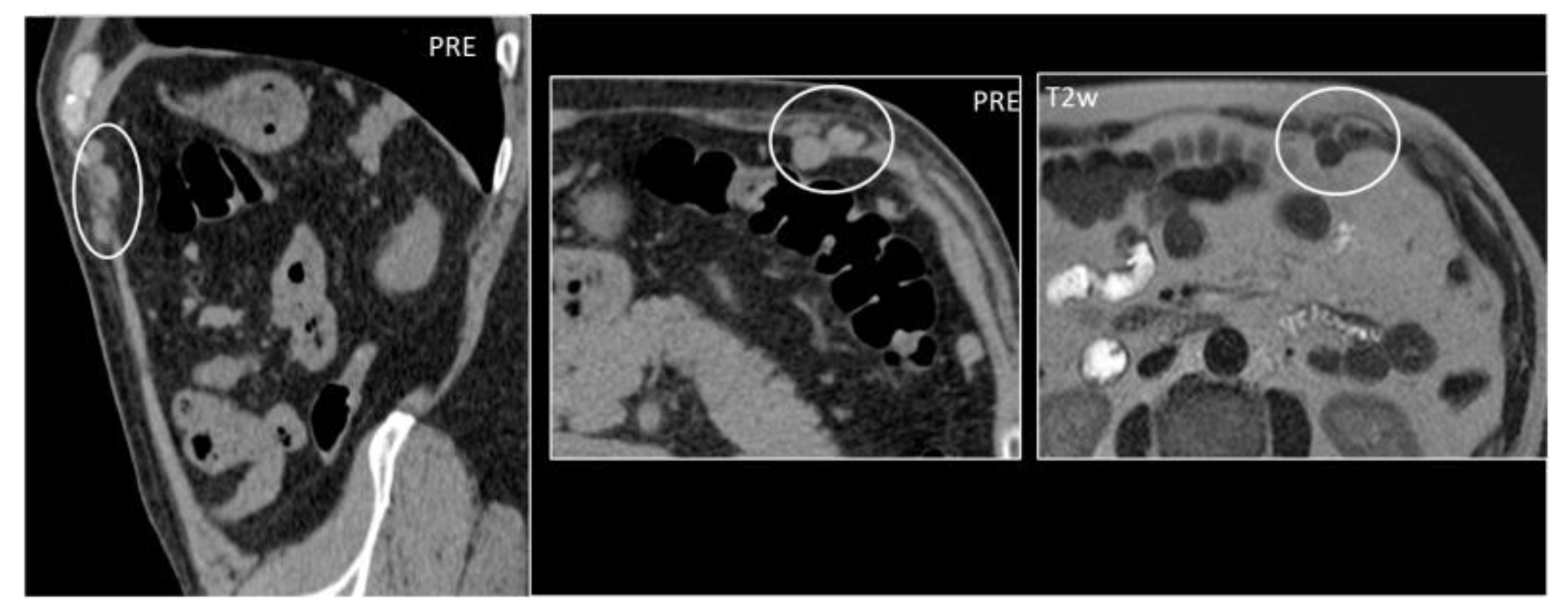
5.6. Endometriosis
5.7. Abdominal Wall Adhesions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanhoenacker; Filip, M.; Parizel, P.M.; Gielen, J.L. Imaging of Soft Tissue Tumors, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- van Rijswijk, C.S.; Geirnaerdt, M.J.; Hogendoorn, P.C.; Taminiau, A.H.; van Coevorden, F.; Zwinderman, A.H.; Pope, T.L.; Bloem, J.L. Soft-tissue tumors: Value of static and dynamic gadopentetate dimeglumine-enhanced MR imaging in prediction of malignancy. Radiology 2004, 233, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Goyal, N.; Mukherjee, K.; Kamath, S. Ultrasound of the abdominal wall: What lies beneath? Clin. Radiol. 2013, 68, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.M.D.E.S.; Barbuto, T.M.; Freitas, F.A.; Vianna, N.F.; Zanchetta, C.M.C.; Forsait, S.; Borba, C.; Azambuja, A.M.P.D.; Cristofani, L.M.; Odone, V. An unusual abdominal wall mass in a child. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e16. [Google Scholar] [CrossRef] [PubMed]
- Virmani, V.; Sethi, V.; Fasih, N.; Ryan, J.; Kielar, A. The abdominal wall lumps and bumps: Cross-sectional imaging spectrum. Can. Assoc. Radiol. J. 2014, 65, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, K.; Mokuno, Y.; Matsubara, H.; Kaneko, H.; Shamoto, M.; Iyomasa, S. Development of an abdominal wall abscess caused by fish bone ingestion: A case report. J. Med. Case Rep. 2019, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.; Zaghi, C.; Manenti, A.; Luppi, D.; Ugoletti, L.; Bonilauri, S. Abdominal wall abscess secondary to spontaneous rupture of pyogenic liver abscess. Int. J. Surg. Case Rep. 2016, 25, 110–113. [Google Scholar] [CrossRef]
- Bashir, U.; Moskovic, E.; Strauss, D.; Hayes, A.; Thway, K.; Pope, R.; Messiou, C. Soft-tissue masses in the abdominal wall. Clin. Radiol. 2014, 69, e422–e431. [Google Scholar] [CrossRef]
- Stein, L.; Elsayes, K.M.; Wagner-Bartak, N. Subcutaneous Abdominal Wall Masses: Radiological Reasoning. Am. J. Roentgenol. 2012, 198, W146–W151. [Google Scholar] [CrossRef]
- Yoon, M.A.; Chung, H.W.; Yeo, Y.; Yoo, H.J.; Kang, Y.; Chee, C.G.; Lee, M.H.; Lee, S.H.; Shin, M.J. Distinguishing necrotizing from non-necrotizing fasciitis: A new predictive scoring integrating MRI in the LRINEC score. Eur. Radiol. 2019, 29, 3414–3423. [Google Scholar] [CrossRef]
- Carbonetti, F.; Cremona, A.; Carusi, V.; Guidi, M.; Iannicelli, E.; Di Girolamo, M.; Sergi, D.; Clarioni, A.; Baio, G.; Antonelli, G.; et al. The role of contrast enhanced computed tomography in the diagnosis of necrotizing fasciitis and comparison with the laboratory risk indicator for necrotizing fasciitis (LRINEC). Radiol. Med. 2016, 121, 106–121. [Google Scholar] [CrossRef]
- Tso, D.K.; Singh, A.K. Necrotizing fasciitis of the lower extremity: Imaging pearls and pitfalls. Br. J. Radiol. 2018, 91, 20180093. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H.; Mazaheri, P.; Oppenheimer, D.C.; Lubner, M.G.; Menias, C.O.; Pickhardt, P.J.; Middleton, W.D.; Mellnick, V.M. Imaging of Abdominal Wall Masses, Masslike Lesions, and Diffuse Processes. RadioGraphics 2020, 40, 684–706. [Google Scholar] [CrossRef] [PubMed]
- Huston, T.L.; Grant, R.T. Abdominal wall gossypiboma. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, e463–e464. [Google Scholar] [CrossRef]
- Sozutek, A.; Colak, T.; Reyhan, E.; Turkmenoglu, O.; Akpınar, E. Intra-abdominal Gossypiboma Revisited: Various Clinical Presentations and Treatments of this Potential Complication. Indian J. Surg. 2015, 77 (Suppl. 3), 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Stein, S.L. Radiographic and endoscopic diagnosis and treatment of enterocutaneous fistulas. Clin. Colon Rectal Surg. 2010, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Parada Villavicencio, C.; Adam, S.Z.; Nikolaidis, P.; Yaghmai, V.; Miller, F.H. Imaging of the Urachus: Anomalies, Complications, and Mimics. Radiographics 2016, 36, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lin, J.; Du, L.; Wu, R.; Li, Z. Ultrasound findings of urachal anomalies. A series of interesting cases. Med. Ultrason. 2019, 21, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Tublin, M.; Nelson, J.; Borhani, A.; Furlan, A.; Heller, M.T.; Squires, J. Urachal Anomalies, Imaging in Urology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 204–205. ISBN 9780323548090. [Google Scholar] [CrossRef]
- Yang, J.D.; Hwang, H.P.; Kim, J.H.; Rodríguez-Vázquez, J.F.; Abe, S.; Murakami, G.; Cho, B.H. Development of the rectus abdominis and its sheath in the human foetus. Yonsei Med. J. 2012, 53, 1028–1035. [Google Scholar] [CrossRef]
- Lopes, R.I.; Tavares, A.; Srougi, M.; Dénes, F.T. 27 years of experience with the comprehensive surgical treatment of prune belly syndrome. J. Pediatr. Urol. 2015, 11, 276-e1. [Google Scholar] [CrossRef] [PubMed]
- Jiddane, M.; Gastaut, J.L.; Pellissier, J.F.; Pouget, J.; Serratrice, G.; Salamon, G. CT of primary muscle diseases. AJNR Am. J. Neuroradiol. 1983, 4, 773–776. [Google Scholar]
- Kim, H.K.; Laor, T.; Horn, P.S.; Racadio, J.M.; Wong, B.; Dardzinski, B.J. T2 mapping in Duchenne muscular dystrophy: Distribution of disease activity and correlation with clinical assessments. Radiology 2010, 255, 899–908, Erratum in Radiology 2010, 256, 1016. [Google Scholar] [CrossRef] [PubMed]
- Gregorio-Hernández, R.; Sanz-López, E.; Hoyo, A.A.; Manrique-Martín, G.; De-Agustín, J.C.; Sánchez-Luna, M. A Rare Complex Case of Congenital Umbilical Arteriovenous Malformation and Review of Literature. AJP Rep. 2016, 6, e216–e221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aymard, A.; Bisdorf, A.; Saint-Maurice, J.P.; Labeyrie, M.A.; Houdart, E. Malformations arterio-veineuses de l’abdomen et du pelvis: Diagnostic et indications thérapeutiques [Abdomino-pelvic arteriovenous malformations: Clinical presentation and management]. Presse Med. 2019, 48, 411–418. (In French) [Google Scholar] [CrossRef] [PubMed]
- Aagaard, J.; Jensen, L.I.; Sørensen, T.I.; Christensen, U.; Burcharth, F. Recanalized umbilical vein in portal hypertension. AJR Am. J. Roentgenol. 1982, 139, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Giambelluca, D.; Caruana, G.; Cannella, R.; Picone, D.; Midiri, M. The “caput medusae” sign in portal hypertension. Abdom. Radiol. 2018, 43, 2535–2536. [Google Scholar] [CrossRef]
- Adachi, Y.; Yano, S. Caput Medusae-like Venous Dilatation in Lung Cancer. Intern. Med. 2019, 58, 3341–3342. [Google Scholar] [CrossRef]
- Philips, C.A.; Arora, A.; Shetty, R.; Kasana, V. A Comprehensive Review of Portosystemic Collaterals in Cirrhosis: Historical Aspects, Anatomy, and Classifications. Int. J. Hepatol. 2016, 2016, 6170243. [Google Scholar] [CrossRef]
- Hasslinger, C.I.; Allison, C.F.; Barker, J.J.; Muns, E.E.; Mackenzie, D.C. Woman With a Groin Mass. Ann. Emerg. Med. 2018, 72, e107–e108. [Google Scholar] [CrossRef]
- Madhusudhan, K.S.; Venkatesh, H.A.; Gamanagatti, S.; Garg, P.; Srivastava, D.N. Interventional Radiology in the Management of Visceral Artery Pseudoaneurysms: A Review of Techniques and Embolic Materials. Korean J. Radiol. 2016, 17, 351–363. [Google Scholar] [CrossRef]
- Park, S.Y.; Ahn, S.K.; Kim, H.Y.; Shin, J.Y.; Min, S. Referred pain in right arm from abdominal wall pseudoaneurysm. Korean J. Pain 2013, 26, 191–194. [Google Scholar] [CrossRef]
- Chae, S.Y.; Park, C.; Kim, J.K.; Kim, H.O.; Lee, B.C. Ultrasound-Guided Percutaneous Thrombin Injection of Femoral Artery Pseudoaneurysms Caused by Vascular Access. Taehan Yongsang Uihakhoe Chi. 2021, 82, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Stillman, K.; Kellar, J. Rectus sheath hematoma: An unfortunate consequence of novel anticoagulants. West. J. Emerg. Med. 2015, 16, 420–421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Contrella, B.N.; Park, A.W.; Wilkins, L.R.; Sheeran, D.; Hassinger, T.E.; Angle, J.F. Spontaneous Rectus Sheath Hematoma: Factors Predictive of Conservative Management Failure. J. Vasc. Interv. Radiol. 2020, 31, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Shikhman, A.; Tuma, F. Abdominal Hematoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519551/ (accessed on 22 November 2022).
- Leyva, A.; Tran, T.; Cibulas, A.T.; Warden, D., IV; Danger, F.J.; Scherer, K.; Wasyliw, C. Filler Migration and Granuloma Formation After Gluteal Augmentation with Free-silicone Injections. Cureus 2018, 10, e3294. [Google Scholar] [CrossRef]
- Wosnitzer, B.; Mirtcheva, R. Silicone granulomas following free silicone gluteal augmentation. Radiol. Case Rep. 2015, 6, 491. [Google Scholar] [CrossRef]
- Wong, K.T.; Lee, P.S.; Chan, Y.L.; Chow, L.T. Paraffinoma in anterior abdominal wall mimicking liposarcoma. Br. J. Radiol. 2003, 76, 264–267. [Google Scholar] [CrossRef]
- Pasternack, F.R.; Fox, L.P.; Engler, D.E. Silicone granulomas treated with etanercept. Arch. Dermatol. 2005, 141, 13–15. [Google Scholar] [CrossRef][Green Version]
- Kamaya, A.; Federle, M.P.; Desser, T.S. Imaging manifestations of abdominal fat necrosis and its mimics. Radiographics 2011, 31, 2021–2034. [Google Scholar] [CrossRef]
- Shiba, M.; Kitazawa, T. Progressive insulin-derived amyloidosis in a patient with type 2 diabetes. Case Rep. Plast. Surg. Hand Surg. 2016, 3, 73–76. [Google Scholar] [CrossRef]
- Iwaya, K.; Zako, T.; Fukunaga, J.; Sörgjerd, K.M.; Ogata, K.; Kogure, K.; Kosano, H.; Noritake, M.; Maeda, M.; Ando, Y.; et al. Toxicity of insulin-derived amyloidosis: A case report. BMC Endocr. Disord. 2019, 19, 61. [Google Scholar] [CrossRef]
- Valenzuela, A.; Chung, L.; Casciola-Rosen, L.; Fiorentino, D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol. 2014, 150, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Traineau, H.; Aggarwal, R.; Monfort, J.B.; Senet, P.; Oddis, C.V.; Chizzolini, C.; Barbaud, A.; Francès, C.; Arnaud, L.; Chasset, F. Treatment of calcinosis cutis in systemic sclerosis and dermatomyositis: A review of the literature. J. Am. Acad. Dermatol. 2020, 82, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Brancatelli, G.; Vilgrain, V.; Zappa, M.; Lagalla, R. Case 80: Splenosis. Radiology 2005, 234, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Vernuccio, F.; Dimarco, M.; Porrello, G.; Cannella, R.; Cusmà, S.; Midiri, M.; Brancatelli, G. Abdominal splenosis and its differential diagnoses: What the radiologist needs to know. Curr. Probl. Diagn. Radiol. 2021, 50, 229–235. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Kalles, V.; Papapanagiotou, I.; Piperos, T.; Karakaxas, D.; Bonatsos, V.; Tsoumakas, K.; Orfanos, F.; Mariolis-Sapsakos, T. Subcutaneous splenosis of the abdominal wall: Report of a case and review of the literature. Case Rep. Surg. 2013, 2013, 454321. [Google Scholar] [CrossRef]
- Rizzo, S.; Monfardini, L.; Belmonte, M.; Rocco, B.; Belloni, M. Benign splenosis mimicking peritoneal seeding in a bladder cancer patient: A case report. Cases J. 2009, 2, 8982. [Google Scholar] [CrossRef]
- Hensen, J.-H.J.; Van Breda Vriesman, A.C.; Puylaert, J.B.C.M. Abdominal Wall Endometriosis: Clinical Presentation and Imaging Features with Emphasis on Sonography. Am. J. Roentgenol. 2006, 186, 616–620. [Google Scholar] [CrossRef]
- Khamechian, T.; Alizargar, J.; Mazoochi, T. 5-Year data analysis of patients following abdominal wall endometrioma surgery. BMC Women’s Health 2014, 14, 151. [Google Scholar] [CrossRef][Green Version]
- Zhang, P.; Sun, Y.; Zhang, C.; Yang, Y.; Zhang, L.; Wang, N.; Xu, H. Cesarean scar endometriosis: Presentation of 198 cases and literature review. BMC Women’s Health 2019, 19, 14. [Google Scholar] [CrossRef]
- Chamié, L.P.; Ribeiro, D.M.F.R.; Tiferes, D.A.; de Macedo Neto, A.C.; Serafini, P.C. Atypical Sites of Deeply Infiltrative Endometriosis: Clinical Characteristics and Imaging Findings. RadioGraphics 2018, 38, 309–328. [Google Scholar] [CrossRef]
- Ahn, S.E.; Park, S.J.; Moon, S.K.; Lee, D.H.; Lim, J.W. Sonography of Abdominal Wall Masses and Masslike Lesions: Correlation With Computed Tomography and Magnetic Resonance Imaging. J. Ultrasound Med. 2016, 35, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Yarmish, G.; Sala, E.; Goldman, D.A.; Lakhman, Y.; Soslow, R.A.; Hricak, H.; Gardner, G.J.; Vargas, H.A. Abdominal wall endometriosis: Differentiation from other masses using CT features. Abdom. Radiol. 2017, 42, 1517–1523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Busard, M.P.H.; Mijatovic, V.; van Kuijk, C.; Hompes, P.G.A.; van Waesberghe, J.H.T.M. Appearance of abdominal wall endometriosis on MR imaging. Eur. Radiol. 2010, 20, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Terzea, D.C.; Valea, A.; Gheorghisan-Galateanu, A.A. Abdominal wall endometriosis (a narrative review). Int. J. Med. Sci. 2020, 17, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Rindos, N.B.; Mansuria, S. Diagnosis and Management of Abdominal Wall Endometriosis: A Systematic Review and Clinical Recommendations. Obstet. Gynecol. Surv. 2017, 72, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Limperg, T.; Chaves, K.; Jesse, N.; Zhao, Z.; Yunker, A. Ultrasound Visceral Slide Assessment to Evaluate for Intra-abdominal Adhesions in Patients Undergoing Abdominal Surgery—A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2021, 28, 1993–2003.e10. [Google Scholar] [CrossRef]
- Gerner-Rasmussen, J.; Donatsky, A.M.; Bjerrum, F. The role of non-invasive imaging techniques in detecting intra-abdominal adhesions: A systematic review. Langenbeck’s Arch. Surg. 2019, 404, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Buhmann-Kirchhoff, S.; Lang, R.; Kirchhoff, C.; Steitz, H.O.; Jauch, K.W.; Reiser, M.; Lienemann, A. Functional cine MR imaging for the detection and mapping of intraabdominal adhesions: Method and surgical correlation. Eur. Radiol. 2008, 18, 1215–1223. [Google Scholar] [CrossRef]
- Ten Broek, R.P.; Issa, Y.; van Santbrink, E.J.; Bouvy, N.D.; Kruitwagen, R.F.; Jeekel, J.; Bakkum, E.A.; Rovers, M.M.; van Goor, H. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ (Clin. Res. Ed.) 2013, 347, f5588. [Google Scholar] [CrossRef]
- Loftus, T.J.; Morrow, M.L.; Lottenberg, L.; Rosenthal, M.D.; Croft, C.A.; Smith, R.S.; Moore, F.A.; Brakenridge, S.C.; Borrego, R.; Efron, P.A.; et al. The Impact of Prior Laparotomy and Intra-abdominal Adhesions on Bowel and Mesenteric Injury Following Blunt Abdominal Trauma. World J. Surg. 2019, 43, 457–465. [Google Scholar] [CrossRef]
- Stuparich, M.A.; Ecker, A.M.; Lee, T.T. Lysis of Anterior Abdominal Wall Adhesions: A Systematic Approach. J. Minim. Invasive Gynecol. 2015, 22, S3. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porrello, G.; Vernuccio, F.; Alvarez-Hornia Pérez, E.; Brancatelli, G.; Cannella, R. The Benign Side of the Abdominal Wall: A Pictorial Review of Non-Neoplastic Diseases. Diagnostics 2022, 12, 3211. https://doi.org/10.3390/diagnostics12123211
Porrello G, Vernuccio F, Alvarez-Hornia Pérez E, Brancatelli G, Cannella R. The Benign Side of the Abdominal Wall: A Pictorial Review of Non-Neoplastic Diseases. Diagnostics. 2022; 12(12):3211. https://doi.org/10.3390/diagnostics12123211
Chicago/Turabian StylePorrello, Giorgia, Federica Vernuccio, Eduardo Alvarez-Hornia Pérez, Giuseppe Brancatelli, and Roberto Cannella. 2022. "The Benign Side of the Abdominal Wall: A Pictorial Review of Non-Neoplastic Diseases" Diagnostics 12, no. 12: 3211. https://doi.org/10.3390/diagnostics12123211
APA StylePorrello, G., Vernuccio, F., Alvarez-Hornia Pérez, E., Brancatelli, G., & Cannella, R. (2022). The Benign Side of the Abdominal Wall: A Pictorial Review of Non-Neoplastic Diseases. Diagnostics, 12(12), 3211. https://doi.org/10.3390/diagnostics12123211








