Spectral Domain Optical Coherence Tomography Findings in Vision-Threatening Rhino-Orbital Cerebral Mucor Mycosis—A Prospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Demographic and Clinical Profile of Enrolled Patients
2.2. Image Acquisition
2.3. Image Evaluation
2.4. Histopathology
2.5. Statistical Analysis
3. Results
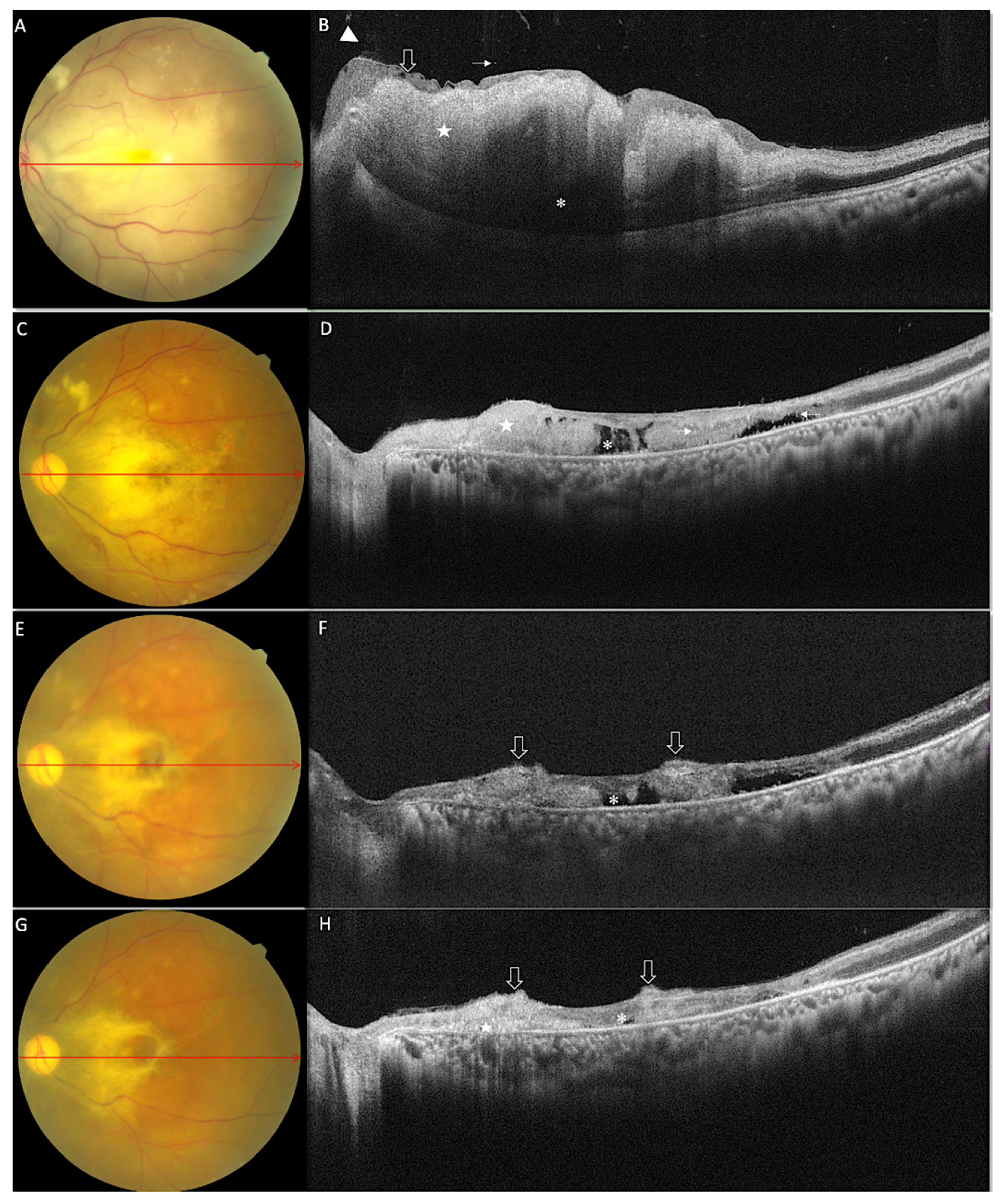
3.1. Morphological Features on OCT at Baseline
3.2. Morphological Features on OCT at One Month
3.3. Morphological Features on OCT at Last Follow-Up
3.4. Changes in Quantifiable Parameters over Time
3.5. Histopathology of Exenterated Eyes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wali, U.; Balkhair, A.; Al-Mujaini, A. Cerebro-rhino orbital mucormycosis: An update. J. Infect. Public Health 2012, 5, 116–126. [Google Scholar] [CrossRef]
- Lamoth, F.; Chung, J.; Damonti, L.; Alexander, B.D. Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clin. Infect. Dis. 2017, 64, 1619–1621. [Google Scholar] [CrossRef] [Green Version]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.-P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The emergence of COVID-19 associated mucormycosis: A review of cases from 18 countries. Lancet Microbe 2022, 3, e543–e552. [Google Scholar] [CrossRef]
- Karadeniz Uğurlu, Ş.; Selim, S.; Kopar, A.; Songu, M. Rhino-orbital Mucormycosis: Clinical Findings and Treatment Outcomes of Four Cases. Turk. J. Ophthalmol. 2015, 45, 169–174. [Google Scholar]
- Sponsler, T.A.; Sassani, J.W.; Johnson, L.N.; Towfighi, J. Ocular invasion in mucormycosis. Surv. Ophthalmol. 1992, 36, 345–350. [Google Scholar] [CrossRef]
- Dogra, M.; Bhutani, G.; Gupta, V. Mucormycosis Endophthalmitis in a Silicone Oil-Filled Eye of an Immunocompetent Patient. Ocul. Immunol. Inflamm. 2019, 27, 1293–1295. [Google Scholar] [CrossRef]
- Mohadjer, Y.; Smith, M.E.; Akduman, L. Mucormycosis Endophthalmitis after Cataract Surgery. Ocul. Immunol. Inflamm. 2007, 15, 117–120. [Google Scholar] [CrossRef]
- Gass, J.D.M. Ocular Manifestations of Acute Mucormycosis. Arch. Ophthalmol. 1961, 65, 226–237. [Google Scholar] [CrossRef]
- Hirano, Y.; Suzuki, N.; Tomiyasu, T.; Kurobe, R.; Yasuda, Y.; Esaki, Y.; Yasukawa, T.; Yoshida, M.; Ogura, Y. Multimodal Imaging of Microvascular Abnormalities in Retinal Vein Occlusion. J. Clin. Med. 2021, 10, 405. [Google Scholar] [CrossRef]
- Thomas, A.S.; Lin, P. Multimodal imaging in infectious and noninfectious intermediate, posterior and panuveitis. Curr. Opin. Ophthalmol. 2021, 32, 169–182. [Google Scholar] [CrossRef]
- Newman, R.M.; Kline, L.B. Evolution of fundus changes in mucormycosis. J. Neuro-Ophthalmol. 1997, 17, 51–52. [Google Scholar]
- Kim, I.T.; Shim, J.Y.; Jung, B.Y. Serous retinal detachment in a patient with rhino-orbital mucormycosis. Jpn. J. Ophthalmol. 2001, 45, 301–304. [Google Scholar]
- Song, Y.-M.; Shin, S.Y. Bilateral Ophthalmic Artery Occlusion in Rhino-Orbito-Cerebral Mucormycosis. Korean J. Ophthalmol. 2008, 22, 66–69. [Google Scholar] [CrossRef]
- Kadayifcilar, S.; Gedik, S.; Önerci, M.; Eldem, B. Chorioretinal alterations in mucormycosis. Eye 2001, 15 Pt 1, 99–102. [Google Scholar] [CrossRef]
- Luo, Q.L.; Orcutt, J.C.; Seifter, L.S. Orbital mucormycosis with retinal and ciliary artery occlusions. Br. J. Ophthalmol. 1989, 73, 680–683. [Google Scholar] [CrossRef]
- Ferry, A.P.; Abedi, S. Diagnosis and Management of Rhino-Orbitocerebral Mucormycosis (Phycomycosis). A report of 16 personally observed cases. Ophthalmology 1983, 90, 1096–1104. [Google Scholar] [CrossRef]
- Bhansali, A.; Bhadada, S.; Sharma, A.; Suresh, V.; Gupta, A.; Singh, P.; Chakarbarti, A.; Dash, R.J. Presentation and outcome of rhi-no-orbital-cerebral mucormycosis in patients with diabetes. Postgrad. Med. J. 2004, 80, 670–674. [Google Scholar]
- Yohai, R.A.; Bullock, J.D.; Aziz, A.A.; Markert, R.J. Survival factors in rhino-orbital-cerebral mucormycosis: Major review. Surv. Ophthalmol. 1994, 39, 3–22. [Google Scholar]
- Parfrey, N.A. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine 1986, 65, 113–123. [Google Scholar]
- Ho, H.C.; Liew, O.H.; Teh, S.S.; Hanizasurana, H.; Ibrahim, M.; Shatriah, I. Unilateral rhino–orbital–cerebral mucormycosis with contralateral endogenous fungal endophthalmitis. Clin. Ophthalmol. 2015, 9, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.P.; Patel, S.; Orlin, A.; Marlow, E.; Chee, R.I.; Nadelmann, J.; Chan, R.V.P.; D’Amico, D.J.; Kiss, S. Spectral domain optical co-herence tomography findings in macula-involving cytomegalovirus retinitis. Retina 2018, 38, 1000–1010. [Google Scholar]
- Ahn, S.J.; Woo, S.J.; Park, K.H.; Jung, C.; Hong, J.-H.; Han, M.-K. Retinal and Choroidal Changes and Visual Outcome in Central Retinal Artery Occlusion: An Optical Coherence Tomography Study. Am. J. Ophthalmol. 2015, 159, 667–676.e1. [Google Scholar] [CrossRef]
- Bawankar, P.; Lahane, S.; Pathak, P.; Gonde, P.; Singh, A. Central retinal artery occlusion as the presenting manifestation of invasive rhino-orbital-cerebral mucormycosis. Taiwan J. Ophthalmol. 2020, 10, 62–65. [Google Scholar] [CrossRef]
- Kaur, R.; Khan, B.; Sharma, A. Optical Coherence Tomography of Retinal Artery Occlusion Associated with Mucormycosis and COVID-19. JAMA Ophthalmol. 2021, 139, e214064. [Google Scholar] [CrossRef]
- Schmidt, D.; Schumacher, M. Stage-dependent efficacy of intra-arterial fibrinolysis in central retinal artery occlusion (CRAO). Neuro-Ophthalmology 1998, 20, 125–141. [Google Scholar] [CrossRef]
- Cornut, P.L.; Bieber, J.; Beccat, S.; Fortoul, V.; Poli, M.; Feldman, A.; Denis, P.; Burillon, C. Aspects évolutifs de la rétine en coupe OCT spectral domain lors des oblitérations artérielles rétiniennes. Spectral domain OCT in eyes with retinal artery occlusion. J. Fr. Ophtalmol. 2012, 35, 606–613. [Google Scholar]
- Gopalakrishnan, M.; Appukuttan, B.; Giridhar, A.; Sivaprasad, S. Normative spectral domain optical coherence tomography data on macular and retinal nerve fiber layer thickness in Indians. Indian J. Ophthalmol. 2014, 62, 316–321. [Google Scholar] [CrossRef]
- Park, S.W.; Woo, S.J.; Park, K.H.; Huh, J.W.; Jung, C.; Kwon, O.-K. Iatrogenic Retinal Artery Occlusion Caused by Cosmetic Facial Filler Injections. Am. J. Ophthalmol. 2012, 154, 653–662.e1. [Google Scholar] [CrossRef]
- Venkatesh, R.; Jayadev, C.; Sridharan, A.; Pereira, A.; Reddy, N.G.; Cherry, J.P.; Yadav, N.K.; Chhablani, J. Internal limiting membrane detachment in acute central retinal artery occlusion: A novel prognostic sign seen on OCT. Int. J. Retin. Vitr. 2021, 51, 7. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Qiu, Z.; Xiang, D.; Chen, W.; Shi, F.; Zheng, J.; Zhu, W.; Sonka, M. Quantitative analysis of retinal layers’ optical intensities on 3D optical coherence tomography for central retinal artery occlusion. Sci. Rep. 2015, 5, 09269. [Google Scholar] [CrossRef] [Green Version]
- Keane, P.A.; Karampelas, M.; Sim, D.A.; Sadda, S.R.; Tufail, A.; Sen, H.N.; Nussenblatt, R.B.; Dick, A.D.; Lee, R.W.; Murray, P.I.; et al. Objective measurement of vitreous inflammation using optical coherence tomography. Ophthalmology 2014, 121, 1706–1714. [Google Scholar]
- Nussenblatt, R.B.; Palestine, A.G.; Chan, C.C.; Roberge, F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985, 92, 467–471. [Google Scholar]
- Gallagher, M.J.; Yilmaz, T.; Cervantes-Castaneda, R.A.; Foster, C.S. The characteristic features of optical coherence tomography in posterior uveitis. Br. J. Ophthalmol. 2007, 91, 1680–1685. [Google Scholar] [CrossRef]
- Invernizzi, A.; Agarwal, A.; Ravera, V.; Oldani, M.; Staurenghi, G.; Viola, F. Optical coherence tomography findings in cytomegal-ovirus retinitis: A Longitudinal Study. Retina 2018, 38, 108–117. [Google Scholar]
- Bulkley, G.B. Free radical-mediated reperfusion injury: A selective review. Br. J. Cancer Suppl. 1987, 8, 66–73. [Google Scholar]
- Hayreh, S.S.; Zimmerman, M.B. Fundus Changes in Central Retinal Artery Occlusion. Retina 2007, 27, 276–289. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, Y.K.; Woo, S.J.; Kang, S.W.; Lee, W.K.; Choi, K.S.; Kwak, H.W.; Yoon, I.H.; Huh, K.; Kim, J.W.; et al. Iatrogenic occlusion of the ophthalmic artery after cosmetic facial filler injections: A national survey by the Korean Retina Society. JAMA Ophthalmol. 2014, 132, 714–723. [Google Scholar]
- Morales-Franco, B.; Nava-Villalba, M.; Medina-Guerrero, E.O.; Sánchez-Nuño, Y.A.; Davila-Villa, P.; Anaya-Ambriz, E.J.; Charles-Niño, C.L. Host-Pathogen Molecular Factors Contribute to the Pathogenesis of Rhizopus spp. in Diabetes Mellitus. Curr. Trop. Med. Rep. 2021, 8, 6–17. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]




| Patient No. | Age, Years | Gender | Laterality | Systemic Illness/ Condition | History of COVID-19 Infection | History of Steroid Intake | CNS Involvement at Presentation | Treatment Received |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | Unilateral | None | Yes | No | No | IV Amb + Debridement + LAMB |
| 2 | 42 | M | Unilateral | DM/HTN | Yes | No | No | IV Amb + Debridement + LAMB |
| 3 | 22 | M | Unilateral | DM Hepatomegaly Jaundice | No | No | No | IV Amb + Debridement + LAMB |
| 4 | 60 | M | Unilateral | DM | Yes | Yes | No | IV Amb + Debridement + LAMB |
| 5 | 39 | M | Unilateral | DM HTN | Yes | Yes | No | IV Amb + Debridement + LAMB |
| 6 | 63 | M | Unilateral | DM | Yes | No | No | IV Amb + Debridement + LAMB |
| 7 | 57 | F | Unilateral | DM | Yes | Yes | No | IV Amb + Debridement + LAMB |
| 8 | 46 | M | Unilateral | DM | Yes | No | No | IV Amb + Debridement + LAMB |
| 9 | 65 | M | Unilateral | DM | No | No | No | IV Amb + Debridement + LAMB |
| 10 | 48 | M | Unilateral | DM | No | No | No | IV Amb + Debridement + LAMB |
| 11 | 40 | M | Unilateral | DM | No | No | No | IV Amb + Debridement + LAMB |
| 12 | 66 | M | Unilateral | DM | Yes | Yes | No | IV Amb + Debridement + LAMB + Exenteration |
| 13 | 32 | M | Unilateral | DM | Yes | Yes | No | IV Amb + Debridement + LAMB + Exenteration |
| 14 | 38 | M | Unilateral | None | Yes | Yes | Yes | IV Amb + Debridement + LAMB + Exenteration |
| 15 | 42 | F | Unilateral * | DM | Yes | Yes | Yes | IV Amb + Debridement + LAMB + Exenteration |
| Patient No. | Eye | Extraocular Movements and Other Findings | Follow-Up | BCVA at Baseline | BCVA at Last Visit | p Value |
|---|---|---|---|---|---|---|
| 1 | LE | Restricted; Ptosis | 8 | 20/400 | 20/800 | 0.1362 |
| 2 | LE | Restricted; Ptosis; Proptosis | 10 | PL-ve | PL-ve | |
| 3 | RE | Restricted; Ptosis; Proptosis | 9 | PL-ve | PL-ve | |
| 4 | RE | Restricted; Ptosis; Proptosis | 8 | PL-ve | PL-ve | |
| 5 | LE | Restricted; Ptosis | 8 | 20/800 | PL-ve | |
| 6 | LE | Restricted; Ptosis; Proptosis | 8 | PL-ve | PL-ve | |
| 7 | RE | Restricted; Ptosis; Proptosis | 8 | PL-ve | PL-ve | |
| 8 | RE | Restricted; Ptosis; mild proptosis | 8 | 20/400 | 20/800 | |
| 9 | LE | Restricted; Ptosis | 8 | 20/800 | 20/800 | |
| 10 | LE | Restricted; Ptosis | 9 | 20/400 | 20/400 | |
| 11 | LE | Restricted; Ptosis; mild proptosis | 11 | HM | HM | |
| 12 | RE | Frozen globe; proptosis; Ptosis | 6 | PL-ve | PL-ve | |
| 13 | RE | Frozen globe; proptosis; Ptosis | 7 | PL-ve | PL-ve | |
| 14 | RE | Frozen globe; proptosis; Ptosis | 2 | PL-ve | PL-ve | |
| 15 | LE | Frozen globe; proptosis; Ptosis | 1 | PL-ve | PL-ve |
| Features | At Baseline n (%) (n = 15) | At One Month n (%) (n = 13) | At 2 Months n (%) (n = 11) |
|---|---|---|---|
| Vitreous haze/debris | 5 (33.3) | 4 (30.7) | 4 (36.3) |
| Vitreous cells | 10(66.6) | 9 (69.2) | 7 (63.6) |
| Posterior hyaloid thickening with partial detachment | 1 (6.6) | 3 (23.07) | 6 (54.54) |
| ILM detachment | 5 (33.3) | 0 | 0 |
| Increased ILM reflectivity | 7 (46.6) | 3(23.0) | 7(63.6) |
| Inner retinal thickening | 7 (100) ! | 0 * | 0 # |
| Outer retinal thickening | 4 (57) ! | 0 * | 0 # |
| Inner retinal hyperreflectivity | 15 (100) | 4 (66.6) * | 2 (50) # |
| Inner retinal loss of organised layer structure | 13 (86.6) | 6 (100) * | 4 (100) # |
| Ill-defined optically empty spaces (outer retinal tissue void) | 0 | 4 (30.7) | 3 (27.2) |
| Hyperreflective dots in outer nuclear layer | 0 | 4 (66.1) * | 1 (25) # |
| Outer nuclear layer cystic spaces | 0 | 2 (22.1) * | 3 (66.6) # |
| Disruption of external limiting membrane | 4 (40.0) ! | 6 (100) * | 4 (100) # |
| EZ layer disruptions without hyperreflective dots | 1 (10) ! | 6 (100) * | 4 (100) # |
| Full thickness loss of retinal architecture | 0 | 4 (30.7) | 4 (36.3) |
| Subretinal fluid at fovea | 1 (6.6) | 0 | 0 |
| Inner retinal thinning | 1 (6.6) | 6 (100) * | 4 (100) # |
| Retinal thinning | 1 (6.6) | 9 (69.2) | 10 (90.9) |
| Focal RPE disruption with choroidal thinning (scar) | 0 | 0 | 3 (27.7) |
| Central macular thickness (mean + SD) µm | 449.0 ± 308.7 | 194.5 ± 50.7 | 184.3 ± 52.1 |
| p-value = 0.0103 | |||
| Inner retinal thickness at 1 mm nasal to fovea (mean + SD) µm | 168.9 ± 43.0 ! | 135.4 ± 21.4 * | 77.34 ± 48.3 # |
| p-value = 0.0009 | |||
| Outer retinal thickness at 1 mm nasal to fovea (mean + SD) µm | 198.3 ± 37.9 ! | 183.01 ± 18.2 * | 154.87 ± 21.2 # |
| p-value = 0.0089 | |||
| Total retinal thickness at 1 mm nasal to fovea (mean + SD) µm | 498.8 ± 300.2 | 259.1 ± 72.4 | 221.8 ± 69.3 |
| p-value = 0.0090 | |||
| Central macular thickness of non-involved eye (mean + SD) µm | 247.26 ± 13.7 | 248.7 ± 13.6 | 245.0 ± 14.8 |
| ^ p-value = 0.0144 | ^ p-value = 0.0007 | ^ p-value = 0.0003 | |
| Inner retinal thickness at 1 mm nasal to fovea of non-involved eye (mean + SD) µm | 148.01 ± 9.6 ! | 149.56 ± 9.9 * | 151.23 ± 10.9 # |
| ^ p-value = 0.0636 | ^ p-value = 0.1718 | ^ p-value = 0.0036 | |
| Outer retinal thickness at 1 mm nasal to fovea of non-involved eye (mean + SD) µm | 182.95 ± 11.53 ! | 182.97 ± 12.01 * | 184.41 ± 13.03 # |
| ^ p-value = 0.3162 | ^ p-value = 0.4213 | ^ p-value = 0.0478 | |
| Total retinal thickness at 1 mm nasal to fovea of non-involved eye (mean + SD) µm | 330.4 ± 22 | 332.53 ± 22.0 | 331.72 ± 23.9 |
| ^ p-value = 0.0401 | ^ p-value = 0.0012 | ^ p-value < 0.0001 | |
| Patient No. | OCT Features | Histopathology Observations |
|---|---|---|
| 15 | Increased inner retinal thickness and hyperreflectivity, focal disruption of ELM and photoreceptor layer with focal areas of increased reflectivity of the outer nuclear layer. | Focal retinal necrosis with normal choroid and optic nerve. No sign of fungal elements in retina or choroid. |
| 14 | Increased inner retinal reflectivity with thickening, thinning of nasal retina at 1 mm from the fovea, minimal vitreous cells overlaying the disc and in posterior vitreous, disruption of the photoreceptor layer and ELM. | Retina: Acute neutrophilic infiltration; areas of focal necrosis; angio-invasion of retinal vessels with thrombosis of vessel. Choroid: Areas of necrosis, presence of fungal hyphae. Optic nerve: Branch of central retinal artery showing angioinvasion (fungal hyphae in wall). |
| 13 | Trace vitreous cells with haze over retinal vessel. Disruption of inner retinal layer with inner retinal hyperreflectivity, thinning of nasal retina at 1 mm from the fovea, disruption of the photoreceptor layer and ELM. | Retina: Acute neutrophilic infiltration with necrosis in all layers with fungal hyphae above RPE layer; angio-invasion of retinal vessels. Choroid: Areas of necrosis. Optic nerve: Central retinal artery and its 1st branch showing angioinvasion (fungal hyphae in wall). |
| 12 | Vitreous cells and haze with posterior hyaloid thickening. Disruption of all retinal layers. Optically empty spaces (necrotic spaces). Multiple hyperreflective foci (arrow) with disruption of the photoreceptor layer and external limiting membrane and areas of chorioretinal scarring. | Retina: Neutrophilic infiltration, Fungal hyphae seen in ganglion cell layer, disorganised retinal lamellar structure. Choroid: Fungal hyphae with necrosis and intense neutrophilic infiltrate. Optic nerve: Oedema and inflammation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Diwaker, P.; Agrawal, A.; Agarwal, A.; Rohatgi, J.; Singh, R.; Das, G.K.; Sahoo, P.K.; Arora, V.K. Spectral Domain Optical Coherence Tomography Findings in Vision-Threatening Rhino-Orbital Cerebral Mucor Mycosis—A Prospective Analysis. Diagnostics 2022, 12, 3098. https://doi.org/10.3390/diagnostics12123098
Singh A, Diwaker P, Agrawal A, Agarwal A, Rohatgi J, Singh R, Das GK, Sahoo PK, Arora VK. Spectral Domain Optical Coherence Tomography Findings in Vision-Threatening Rhino-Orbital Cerebral Mucor Mycosis—A Prospective Analysis. Diagnostics. 2022; 12(12):3098. https://doi.org/10.3390/diagnostics12123098
Chicago/Turabian StyleSingh, Ankur, Preeti Diwaker, Akanksha Agrawal, Aniruddha Agarwal, Jolly Rohatgi, Ramandeep Singh, Gopal Krushna Das, Pramod Kumar Sahoo, and Vinod Kumar Arora. 2022. "Spectral Domain Optical Coherence Tomography Findings in Vision-Threatening Rhino-Orbital Cerebral Mucor Mycosis—A Prospective Analysis" Diagnostics 12, no. 12: 3098. https://doi.org/10.3390/diagnostics12123098
APA StyleSingh, A., Diwaker, P., Agrawal, A., Agarwal, A., Rohatgi, J., Singh, R., Das, G. K., Sahoo, P. K., & Arora, V. K. (2022). Spectral Domain Optical Coherence Tomography Findings in Vision-Threatening Rhino-Orbital Cerebral Mucor Mycosis—A Prospective Analysis. Diagnostics, 12(12), 3098. https://doi.org/10.3390/diagnostics12123098





