Bull’s Eye Maculopathy in Near-Infrared Reflectance as An Early Sign of Hydroxychloroquine Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. HCQ Screening Programme
2.4. NIR Imaging
2.5. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data
3.2. Results of routine HCQ Toxicity Screening
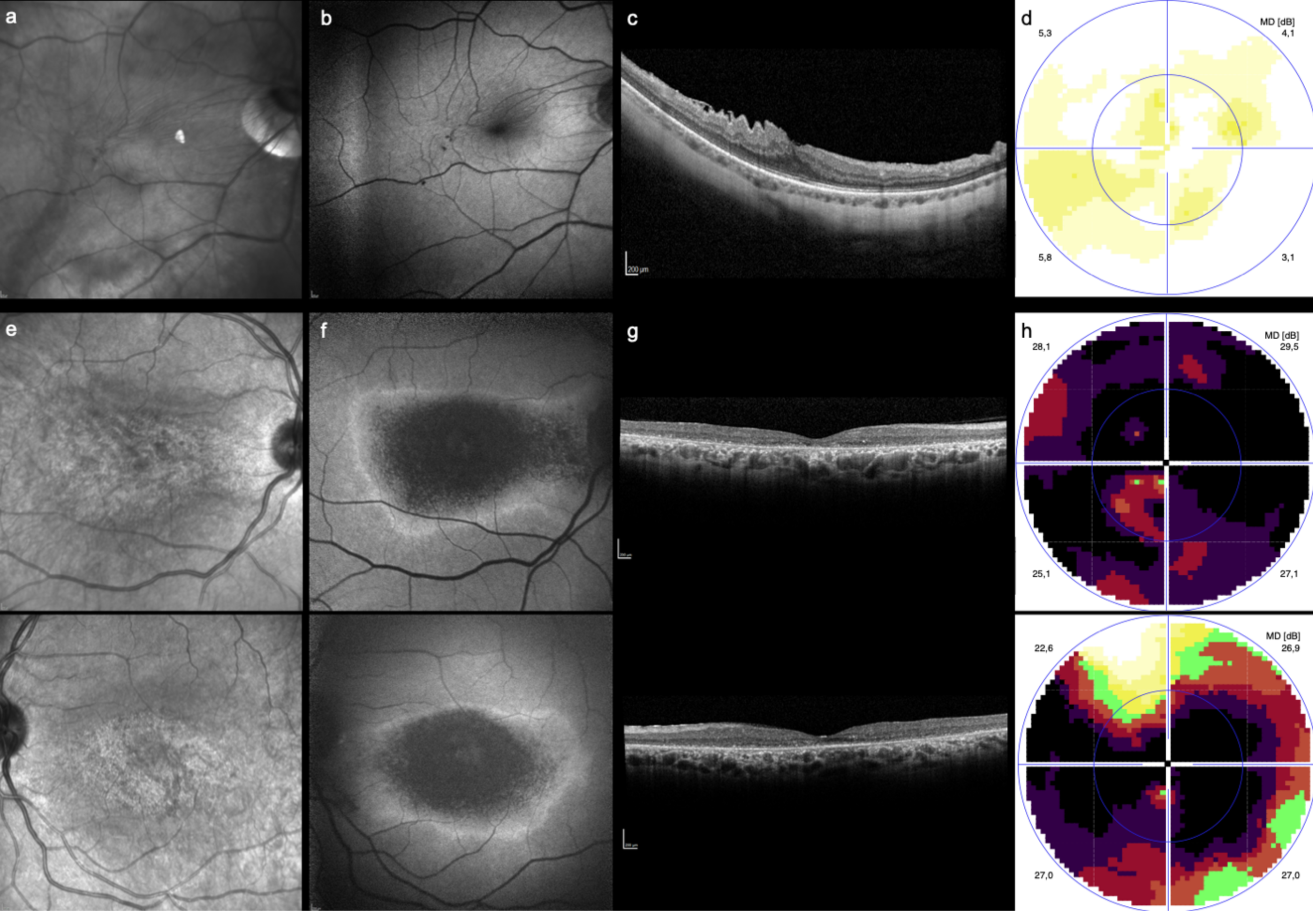
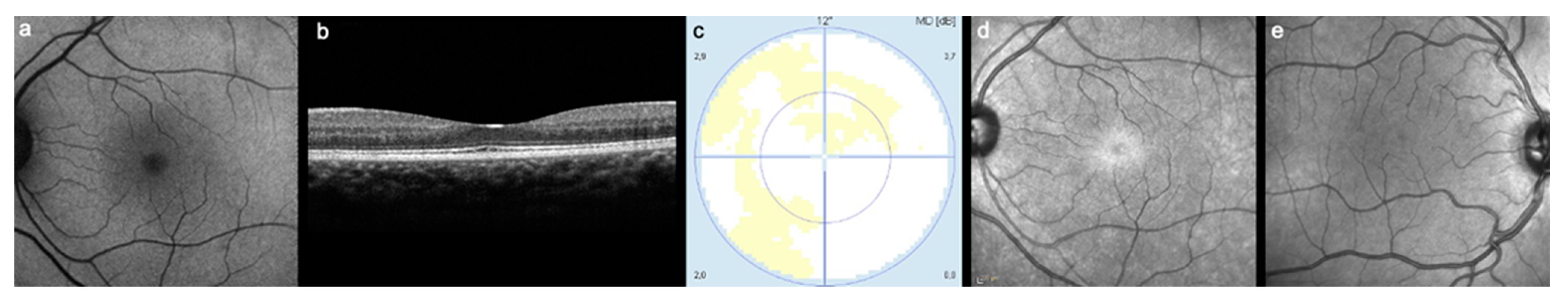
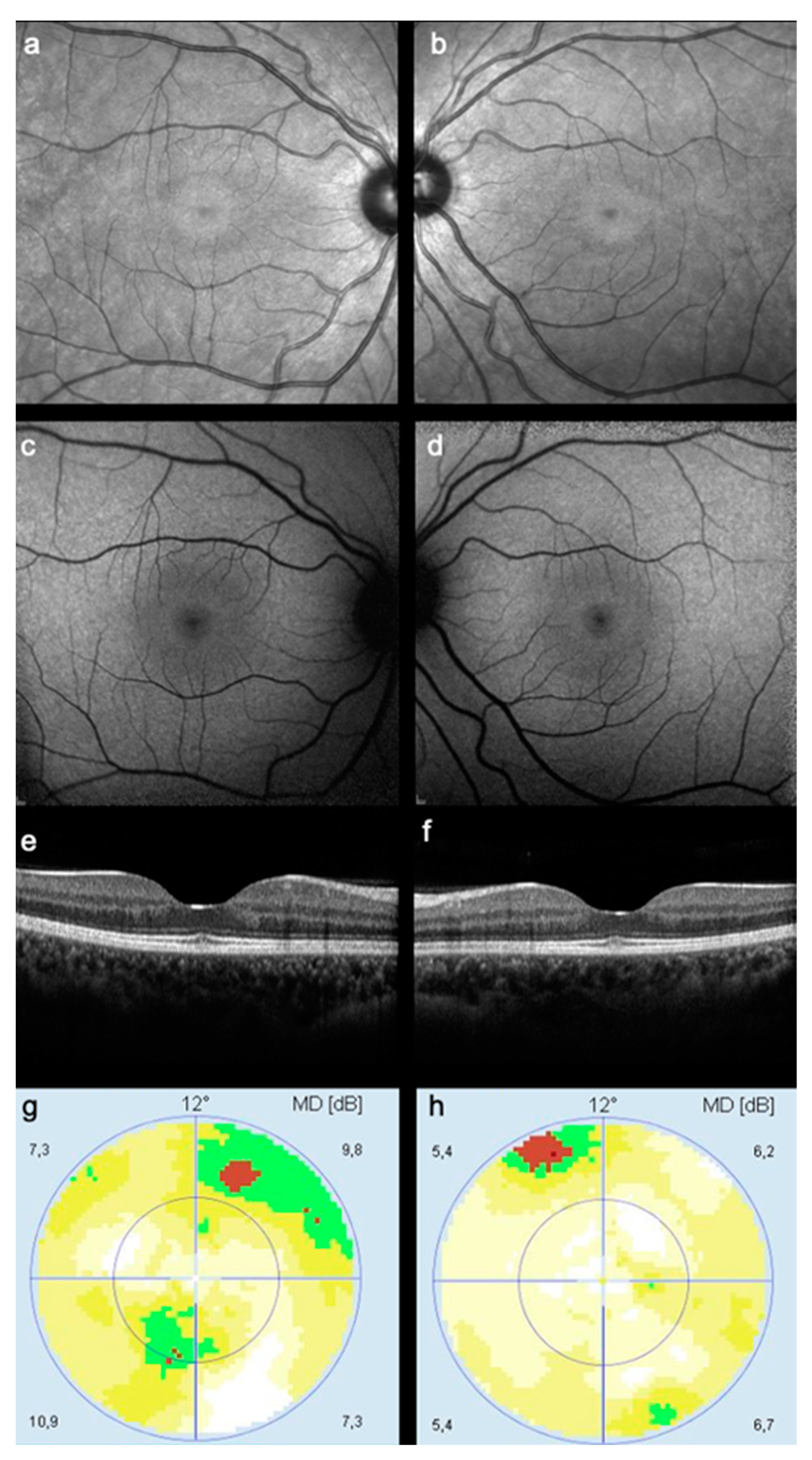
3.3. Near Infrared Imaging Results
3.4. Accuracy of NIR Imaging in Detecting HCQ Toxicity
3.5. Intergrader Agreement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, G.R.V. Hydroxychloroquine: An Update. Lupus 2018, 27, 1402–1403. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, F.H.; Rennie, C.A.; Drinkwater, A.K.; Sahu, D.; Akyol, E.; Lotery, A.J. How to Set up a Hydroxychloroquine Retinopathy Screening Service. Eye 2019, 33, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Abdulaziz, N.; Shah, A.R.; McCune, W.J. Hydroxychloroquine: Balancing the Need to Maintain Therapeutic Levels with Ocular Safety: An Update. Curr. Opin. Rheumatol. 2018, 30, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.H.; Marmor, M.F. Sequential Changes in Hydroxychloroquine Retinopathy up to 20 Years after Stopping the Drug. Retina 2019, 39, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.Y.; Melles, R.B.; Mieler, W.F.; Lum, F. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Ophthalmologists. Hydroxychloroquine and Chloroquine Retinopathy: Recommendations on Monitoring (Clinical Guideline); RCOphth; The Royal College of Ophthalmologists: London, UK, 2020. [Google Scholar]
- Browning, D.J. The Prevalence of Hydroxychloroquine Retinopathy and Toxic Dosing, and the Role of the Ophthalmologist in Reducing Both. Am. J. Ophthalmol. 2016, 166, ix–xi. [Google Scholar] [CrossRef] [PubMed]
- Melles, R.B.; Marmor, M.F. The Prevalence of Hydroxychloroquine Retinopathy and Toxic Dosing, and the Role of the Ophthalmologist in Reducing Both. Am. J. Ophthalmol. 2016, 170, 240. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, I.H.; Sharma, S.; Luqmani, R.; Downes, S.M. Hydroxychloroquine retinopathy. Eye 2017, 31, 828–845. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Pautler, S.E.; Browning, D.J. Near-Infrared Reflectance Bull’s Eye Maculopathy as an Early Indication of Hydroxychloroquine Toxicity. Clin. Ophthalmol. 2015, 9, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, K.; Roy, R.; Thomas, N.; Chowdhury, M. Multimodal Imaging Characteristics of Hydroxychloroquine Retinopathy. Indian J. Ophthalmol. 2018, 66, 324. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.L.; Sampson, D.M.; Chelva, E.; Khan, J.C.; Chen, F.K. Perifoveal Interdigitation Zone Loss in Hydroxychloroquine Toxicity Leads to Subclinical Bull’s Eye Lesion Appearance on near-Infrared Reflectance Imaging. Doc. Ophthalmol. 2018, 136, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Paques, M.; Krivosic, V.; Dupas, B.; Couturier, A.; Kulcsar, C.; Tadayoni, R.; Massin, P.; Gaudric, A. Meaning of Visualizing Retinal Cone Mosaic on Adaptive Optics Images. Am. J. Ophthalmol. 2015, 159, 118–123.e1. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Melles, R.B. Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology 2014, 121, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Wynne, N.; Heitkotter, H.; Woertz, E.N.; Cooper, R.F.; Carroll, J. Comparison of cone mosaic metrics from images acquired with the spectralis high magnification module and adaptive optics scanning light ophthalmoscopy. Transl. Vis. Sci. Technol. 2022, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.; Robertson, M.; Kam, S.; Penwarden, A.; Riga, P.; Davies, N. Prevalence of hydroxychloroquine retinopathy using 2018 Royal College of Ophthalmologists diagnostic criteria. Eye 2021, 35, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, I.H.; Foot, B.; Lotery, A.J. The Royal College of Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): Executive summary. Eye 2021, 35, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Results |
|---|---|
| Total n (eyes) | 123 (246) |
| Mean Age ± SD, years | 55.2 ± 13.8 |
| Female n (%) | 111 (90.2) |
| Primary Diagnosis n (%) | |
| RA | 28 (22.8) |
| SLE | 50 (40.7) |
| pSS | 19 (15.4) |
| Other | 24 (19.5) |
| Mean Time of HCQ Use ± SD, months | 84.0 ± 72.3 |
| Mean Weekly HCQ Dose ± SD, mg | 2327 ± 650 |
| Result of the Routine HCQ Screening | ||||
|---|---|---|---|---|
| NIR Imaging | Positive, n (%) | Negative, n (%) | Unsure/Ungradable, n (%) | Total, n (%) |
| Positive, n (%) | 2 (0.8) | 27 (11.0) | 4 (1.6) | 33 (13.4) |
| Negative, n (%) | 11 (4.5) | 154 (62.6) | 10 (4.1) | 175 (71.1) |
| Unsure/ungradable, n (%) | 1 (0.4) | 35 (14.2) | 2 (0.8) | 38 (15.4) |
| Total, n (%) | 14 (5.7) | 216 (87.8) | 16 (6.5) | 246 (100) |
| NIR | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| HCQ toxicity | 14.3 | 71.3 | 6.1 | 88.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.; Leal, I.; Morais Sarmento, T.; Mano, S.S.; José, P.; Vaz-Pereira, S. Bull’s Eye Maculopathy in Near-Infrared Reflectance as An Early Sign of Hydroxychloroquine Toxicity. Diagnostics 2023, 13, 445. https://doi.org/10.3390/diagnostics13030445
Santos M, Leal I, Morais Sarmento T, Mano SS, José P, Vaz-Pereira S. Bull’s Eye Maculopathy in Near-Infrared Reflectance as An Early Sign of Hydroxychloroquine Toxicity. Diagnostics. 2023; 13(3):445. https://doi.org/10.3390/diagnostics13030445
Chicago/Turabian StyleSantos, Miguel, Inês Leal, Tiago Morais Sarmento, Sofia Sousa Mano, Patrícia José, and Sara Vaz-Pereira. 2023. "Bull’s Eye Maculopathy in Near-Infrared Reflectance as An Early Sign of Hydroxychloroquine Toxicity" Diagnostics 13, no. 3: 445. https://doi.org/10.3390/diagnostics13030445
APA StyleSantos, M., Leal, I., Morais Sarmento, T., Mano, S. S., José, P., & Vaz-Pereira, S. (2023). Bull’s Eye Maculopathy in Near-Infrared Reflectance as An Early Sign of Hydroxychloroquine Toxicity. Diagnostics, 13(3), 445. https://doi.org/10.3390/diagnostics13030445







