Radiation Exposure and Lifetime Attributable Risk of Cancer Incidence and Mortality from Low- and Standard-Dose CT Chest: Implications for COVID-19 Pneumonia Subjects
Abstract
1. Introduction
2. Health Effects of Radiation Exposure
3. Radiation Dose Quantities in CT
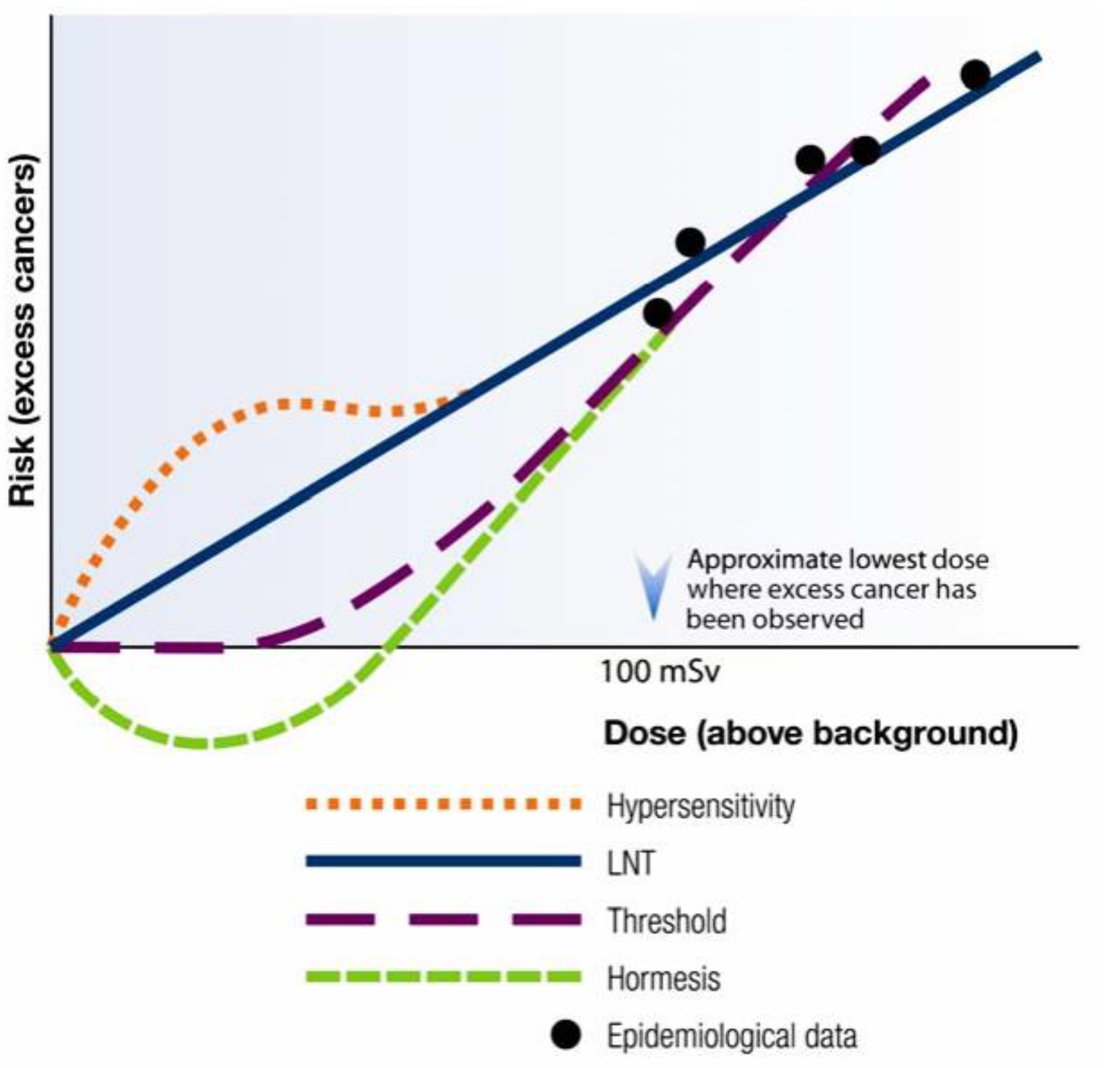
4. Cancer Risk at Low-Dose Radiation in Human Subjects
5. Low- and Standard-Dose Chest CT in COVID-19: Radiation Exposure
6. Low- and Standard-Dose Chest CT in COVID-19: Radiation Risk
7. Projected Number of Future Cancers That Could Be Related to Chest CT Scans Performed during COVID-19 Pandemic Worldwide
8. Radiation Risk from Chest CT ‘in Perspective’
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulz, R.A.; Stein, J.A.; Pelc, N.J. How CT happened: The early development of medical computed tomography. J. Med. Imaging 2021, 8, 052110–26. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.H.; Tsai, K.; Kim, S.; Wu, Y.J.; Demissie, K. Exposure to tomographic scans and cancer risks. JNCI Cancer Spectr. 2020, 4, pkz072. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.; Ciet, P.; Tiddens, H.A.; Zhang, W.; Guillerman, R.P.; Van Straten, M. Monitoring cystic fibrosis lung disease by computed tomography. Radiation risk in perspective. Am. J. Respir. Crit. Care Med. 2014, 189, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.S.; Chu, P.; Szczykutowicz, T.P.; Smith-Bindman, R. Comparison of Strategies to Conserve Iodinated Intravascular Contrast Media for Computed Tomography During a Shortage. JAMA 2022, 328, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Miglioretti, D.L.; Johnson, E.; Williams, A. Pediatric computed tomography and associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013, 167, 700–707. [Google Scholar] [CrossRef]
- Brenner, D.; Hall, E. Computed tomography: An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef]
- Coursey, C.; Frush, D.P.; Yoshizumi, T.; Toncheva, G.; Nguyen, G.; Greenberg, S.B. Pediatric chest MDCT using tube current modulation: Effect on radiation dose with breast shielding. Am. J. Roentgenol. 2008, 190, 54–61. [Google Scholar] [CrossRef]
- World Health Organization. Communicating Radiation Risks in Paediatric Imaging: Information to Support Health Care Discussions about Benefit and Risk, 1st ed.; World Health Organization: Geneva, Switzerland, 2016; pp. 14–27. [Google Scholar]
- Devic, C.; Bodgi, L.; Sonzogni, L.; Pilleul, F.; Ribot, H.; Charry, C.D.; Le Moigne, F.; Paul, D.; Carbillet, F.; Munier, M.; et al. Influence of cellular models and individual factor in the biological response to chest CT scan exams. Eur. Radiol. Exp. 2022, 6, 14. [Google Scholar] [CrossRef]
- Grant, E.J.; Brenner, A.; Sugiyama, H.; Sakata, R.; Sadakane, A.; Utada, M.; Cahoon, E.K.; Milder, C.M.; Soda, M.; Cullings, H.M.; et al. Solid cancer incidence among the life span study of atomic bomb survivors: 1958–2009. Radiat. Res. 2017, 187, 513–537. [Google Scholar] [CrossRef]
- De Gonzalez, A.B.; Salotti, J.A.; McHugh, K.; Little, M.P.; Harbron, R.W.; Lee, C.; Ntowe, E.; Braganza, M.Z.; Parker, L.; Rajaraman, P.; et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: Assessment of the impact of underlying conditions. Br. J. Cancer 2016, 114, 388–394. [Google Scholar] [CrossRef]
- Huang, W.; Muo, C.; Lin, C.; Jen, Y.; Yang, M.; Lin, J.; Sung, F.C.; Kao, C.H. Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: A nation-wide population-based cohort study. Br. J. Cancer 2014, 110, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, Q.; Wang, J.; Yu, N. Risk of developing cancers due to low-dose radiation exposure among medical X-ray workers in China—Results of a prospective study. Int. J. Clin. Exp. Pathol. 2016, 9, 11897–11903. [Google Scholar]
- Preston, D.; Kitahara, C.; Freedman, D.; Sigurdson, A.; Simon, S.; Little, M.; Cahoon, E.K.; Rajaraman, P.; Miller, J.S.; Alexander, B.H.; et al. Breast cancer risk and protracted low-to-moderate dose occupational radiation exposure in the US Radiologic Technologists Cohort, 1983–2008. Br. J. Cancer 2016, 115, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalez, A.B.; Darby, S. Risk of cancer from diagnostic X-rays: Estimates for the UK and 14 other countries. Lancet 2004, 363, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, Y.; Wen, Y.; Dai, X.; Liu, W.; Gong, Q.; Chaoqiong, H.; Fajin, L.; Jiahui, W. Radiation dose levels in chest computed tomography scans of coronavirus disease 2019 pneumonia: A survey of 2119 patients in Chongqing, southwest China. Medicine 2021, 100, e26692. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Prabhakar, N.; Muthu, V.; Farookh, S.; Kaur, H.; Suri, V.; Ritesh, A. CT findings of COVID-19–associated pulmonary mucormycosis: A case series and literature review. Radiology 2022, 302, 214–217. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 October 2022).
- COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 12 October 2022).
- Yurdaisik, I.; Nurili, F.; Aksoy, S.H.; Agirman, A.G.; Aktan, A. Ionizing radiation exposure in patients with COVID-19: More than needed. Radiat. Prot. Dosim. 2021, 194, 135–143. [Google Scholar] [CrossRef]
- Radmard, A.R.; Gholamrezanezhad, A.; Montazeri, S.A.; Kasaeian, A.; Nematollahy, N.; Langrudi, R.M.; Reza Javad, R.; Dehghan, A.; Hekmatnia, A.; Shakourirad, A.; et al. A multicenter survey on the trend of chest CT scan utilization: Tracing the first footsteps of COVID-19 in Iran. Arch. Iran. Med. 2020, 23, 787–793. [Google Scholar] [CrossRef]
- Bahrami-Motlagh, H.; Abbasi, S.; Haghighimorad, M.; Salevatipour, B.; Alavi Darazam, I.; Sanei Taheri, M.; Esmaeili Tarki, F.; Naghibi Irvani, S.S. Performance of low-dose chest CT scan for initial triage of COVID-19. Iran. J. Radiol. 2020, 17, 104950. [Google Scholar] [CrossRef]
- Kang, Z.; Li, X.; Zhou, S. Recommendation of low-dose CT in the detection and management of COVID-2019. Eur. Radiol. 2020, 30, 4356–4357. [Google Scholar] [CrossRef] [PubMed]
- Homayounieh, F.; Holmberg, O.; Umairi, R.A.; Aly, S.; Basevičius, A.; Costa, P.R.; Darweesh, A.; Gershan, V.; Ilves, P.; Kostova-Lefterova, D.; et al. Variations in CT utilization, protocols, and radiation doses in COVID-19 pneumonia: Results from 28 countries in the IAEA study. Radiology 2021, 298, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Dauda, A.M.; Ozoh, J.O.; Towobola, O.A. Medical doctors’ awareness of radiation exposure in diagnostic radiology investigations in a South African academic institution. S. Afr. J. Radiol. 2019, 23, a1707. [Google Scholar] [CrossRef] [PubMed]
- ICRP. The 2007 recommendations of the International Commission on Radiological Protection: ICRP publication 103. Ann. ICRP 2007, 37, 1–322. [Google Scholar]
- Dowd, S.B.; Tilson, E.R. Practical Radiation Protection and Applied Radiobiology; WB Saunders: Philadelphia, PA, USA, 1999. [Google Scholar]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Linet, M.S.; Slovis, T.L.; Miller, D.L.; Kleinerman, R.; Lee, C.; Rajaraman, P.; de Gonzalez, A.B. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J. Clin. 2012, 62, 75–100. [Google Scholar] [CrossRef]
- Garg, M.; Prabhakar, N.; Bhalla, A.S. Cancer risk of CT scan in COVID-19: Resolving the dilemma. Indian J. Med. Res. 2021, 153, 568–571. [Google Scholar] [CrossRef]
- American Cancer Society. Lifetime Risk of Developing or Dying from Cancer. Available online: http://bit.ly/2hyGDR (accessed on 12 October 2022).
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; National Academies Press: Washington, DC, USA, 2006.
- Andersson, M.; Eckerman, K.; Mattsson, S. Lifetime attributable risk as an alternative to effective dose to describe the risk of cancer for patients in diagnostic and therapeutic nuclear medicine. Phys. Med. Biol. 2017, 62, 9177–9188. [Google Scholar] [CrossRef]
- Azadbakht, J.; Khoramian, D.; Lajevardi, Z.S.; Elikaii, F.; Aflatoonian, A.H.; Farhood, B.; Najafi, M.; Bagheri, H. A review on chest CT scanning parameters implemented in COVID-19 patients: Bringing low-dose CT protocols into play. Egypt. J. Radiol. Nucl. 2021, 52, 13. [Google Scholar] [CrossRef]
- Nuclear Regulatory Commission. Linear no-threshold model and standards for protection against radiation. Fed. Reg. 2021, 86, 45923–45936. [Google Scholar]
- Calabrese, E.J. Linear non-threshold (LNT) fails numerous toxicological stress tests: Implications for continued policy use. Chem.-Biol. Interact. 2022, 365, 110064. [Google Scholar] [CrossRef]
- Health Physics Society. Position statement of the health physics society PS010-4: Radiation risk in perspective. Health Phys. 2020, 118, 79–80. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Sources, Effects and Risks of Ionizing Radiation: UNSCEAR 2013. United Nations Scientific Committee on the Effects of Atomic Radiation. Available online: https://www.unscear.org/docs/publications/2013/UNSCEAR_2013_Report_Vol.I.pdf (accessed on 12 October 2022).
- United States Nuclear Regulatory Commission. Radiation Exposure and Cancer. Available online: https://www.nrc.gov/about-nrc/radiation/health-effects/rad-exposure-cancer.html (accessed on 12 October 2022).
- American Nuclear Society. Health Effects of Low-Level Radiation: Position Statement 41. Available online: https://ans.org/pi/ps/docs/ps41.pdf (accessed on 12 October 2022).
- Calabrese, E.J.; Shamoun, D.Y.; Agathokleous, E. Dose response and risk assessment: Evolutionary foundations. Environ. Pollut. 2022, 309, 119787. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Tharmalingam, S. The LNT model for cancer induction is not supported by radiobiological data. Chem. Biol. Interact. 2019, 301, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Pennington, C.W.; Siegel, J.A. The linear no-threshold model of low-dose radiogenic cancer: A failed fiction. Dose-Response 2019, 17, 824200. [Google Scholar] [CrossRef] [PubMed]
- Canadian Nuclear Safety Commission. Linear-Non-Threshold Model. Available online: https://nuclearsafety.gc.ca/eng/resources/health/linear-non-threshold-model/index.cfm (accessed on 12 October 2022).
- Vaiserman, A.; Koliada, A.; Socol, Y. Hormesis through Low-Dose Radiation. Sci. Hormesis Health Longev. 2019, 22, 129–138. [Google Scholar] [CrossRef]
- Shore, R.E.; Beck, H.L.; Boice, J.D.; Caffrey, E.A.; Davis, S.; Grogan, H.; Mettler, F.A.; Preston, R.J.; Till, J.E.; Wakeford, R.; et al. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J. Radiol. Prot. 2018, 38, 1217–1233. [Google Scholar] [CrossRef] [PubMed]
- Puskin, J.S. Perspective on the use of LNT for radiation protection and risk assessment by the US Environmental Protection Agency. Dose-Response 2009, 7, 284–291. [Google Scholar] [CrossRef]
- Leblanc, J.E.; Burtt, J.J. Radiation biology and its role in the Canadian radiation protection framework. Health Phys. 2019, 117, 319–329. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.-P.; Mahesh, M.; Gould, R.; de González, A.B.; Miglioretti, D.L. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef]
- Pochin, E. Problems involved in detecting increased malignancy rates in areas of high natural radiation background. Health Phys. 1976, 31, 148–151. [Google Scholar] [PubMed]
- International Atomic Energy Agency. Methods for Estimating the Probability of Cancer from Occupational Radiation Exposure. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/te_870_web.pdf (accessed on 12 October 2022).
- Williams, D. Radiation carcinogenesis: Lessons from Chernobyl. Oncogene 2008, 27, 9–18. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Primak, A.N.; Braun, N.; Kofler, J.; Yu, L.; Christner, J. Strategies for reducing radiation dose in CT. Radiol. Clin. 2009, 47, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Huda, W.; Mettler, F.A. Volume CT dose index and dose-length product displayed during CT: What good are they? Radiology 2011, 258, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Allisy-Roberts, P.J.; Williams, J. Farr’s Physics for Medical Imaging; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Khoramian, D.; Sistani, S. Estimation and comparison of the radiation effective dose during coronary computed tomography angiography examinations on single-source 64-MDCT and dual-source 128-MDCT. J. Radiol. Prot. 2017, 37, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Huda, W.; Ogden, K.M.; Khorasani, M.R. Converting dose-length product to effective dose at CT. Radiology 2008, 248, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Deak, P.D.; Small, Y.; Kalender, W.A. Multisection CT protocols: Sex- and age-specific conversion factors used to determine effective dose from doselength product. Radiology 2010, 25, 158–166. [Google Scholar] [CrossRef]
- Lin, E.C. Radiation risk from medical imaging. Mayo Clin. Proc. 2010, 85, 1142–1146. [Google Scholar] [CrossRef]
- Pierce, D.A.; Preston, D.L. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat. Res. 2000, 154, 178–186. [Google Scholar] [CrossRef]
- Preston, D.L.; Shimizu, Y.; Pierce, D.A.; Suyama, A.; Mabuchi, K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat. Res. 2003, 160, 381–407. [Google Scholar] [CrossRef]
- Ashmore, J.; Krewski, D.; Zielinski, J.; Jiang, H.; Semenciw, R.; Band, P. First analysis of mortality and occupational radiation exposure based on the National Dose Registry of Canada. Am. J. Epidemiol. 1998, 148, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Sont, W.; Zielinski, J.; Ashmore, J.; Jiang, H.; Krewski, D.; Fair, M.; Band, P.R.; Létourneau, E.G. First analysis of cancer incidence and occupational radiation exposure based on the National Dose Registry of Canada. Am. J. Epidemiol. 2001, 153, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Doody, M.M.; Lonstein, J.E.; Stovall, M.; Hacker, D.G.; Luckyanov, N.; Land, C.E. Breast cancer mortality after diagnostic radiography: Findings from the US Scoliosis Cohort Study. Spine 2000, 25, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.; Modan, B.; Preston, D.; Alfandary, E.; Stovall, M.; Boice, J.D., Jr. Thyroid neoplasia following low-dose radiation in childhood. Radiat. Res. 1989, 120, 516–531. [Google Scholar] [CrossRef]
- Cardis, E.; Gilbert, E.; Carpenter, L.; Howe, G.; Kato, I.; Armstrong, B.; Beral, V.; Cowper, G.; Douglas, A.; Fix, J.; et al. Effects of low doses and low dose rates of external ionizing radiation: Cancer mortality among nuclear industry workers in three countries. Radiat. Res. 1995, 142, 117–132. [Google Scholar] [CrossRef]
- Brenner, D. Does fractionation decrease the risk of breast cancer induced by low-LET radiation? Radiat. Res. 1999, 151, 225–229. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.S.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Krille, L.; Dreger, S.; Schindel, R.; Albrecht, T.; Asmussen, M.; Barkhausen, J.; Berthold, J.D.; Chavan, A.; Claussen, C.; Forsting, M.; et al. Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: Results from a German cohort study. Radiat. Environ. Biophys. 2015, 54, 1–12. [Google Scholar] [CrossRef]
- Journy, N.; Rehel, J.; Ducou Le Pointe, H.; Lee, C.; Brisse, H.; Chateil, J.; Caer-Lorho, S.; Laurier, D.; Bernier, M.O. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br. J. Cancer 2015, 112, 185–193. [Google Scholar] [CrossRef]
- White, I.K.; Shaikh, K.A.; Moore, R.J.; Bullis, C.L.; Sami, M.T.; Gianaris, T.J.; Fulkerson, D.H. Risk of radiation-induced malignancies from CT scanning in children who underwent shunt treatment before 6 years of age: A retrospective cohort study with a minimum 10-year follow-up. J. Neurosurg. Pediatr. 2014, 13, 514–519. [Google Scholar] [CrossRef]
- Hauptmann, M.; Daniels, R.D.; Cardis, E.; Cullings, H.M.; Kendall, G.; Laurier, D.; Linet, M.S.; Little, M.P.; Lubin, J.H.; Preston, D.; et al. Epidemiological studies of low-dose ionizing radiation and cancer: Summary bias assessment and meta-analysis. JNCI Monographs. 2020, 2020, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Abalo, K.D.; Rage, E.; Leuraud, K.; Richardson, D.B.; Le Pointe, H.D.; Laurier, D.; Bernier, M.O. Early life ionizing radiation exposure and cancer risks: Systematic review and meta-analysis. Pediatr. Radiol. 2021, 51, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-Y.; Han, K.; Jung, J.-H.; Kim, J.S. Association of exposure to diagnostic low-dose ionizing radiation with risk of cancer among youths in South Korea. JAMA Netw. Open 2019, 2, e1910584. [Google Scholar] [CrossRef] [PubMed]
- De González, A.B.; Mahesh, M.; Kim, K.-P.; Bhargavan, M.; Lewis, R.; Mettler, F.; Land, C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch. Intern. Med. 2009, 169, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Managing Patient Dose in Computed Tomography. ICRP Publication 87. Ann. ICRP 2000, 30, 7–45. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.H.; Fairley, R.; Murphy, L.S.-L.; Doss, M. The risk of cancer from CT scans and other sources of low-dose radiation: A critical appraisal of methodologic quality. Prehosp. Disaster Med. 2020, 35, 3–16. [Google Scholar] [CrossRef]
- Rehani, M.M.; Yang, K.; Melick, E.R.; Heil, J.; Šalát, D.; Sensakovic, W.F.; Bob, L. Patients undergoing recurrent CT scans: Assessing the magnitude. Eur. Radiol. 2020, 30, 1828–1836. [Google Scholar] [CrossRef]
- Brambilla, M.; Vassileva, J.; Kuchcinska, A.; Rehani, M.M. Multinational data on cumulative radiation exposure of patients from recurrent radiological procedures: Call for action. Eur. Radiol. 2020, 30, 2493–2501. [Google Scholar] [CrossRef]
- Agostini, A.; Floridi, C.; Borgheresi, A.; Badaloni, M.; Esposto Pirani, P.; Terilli, F.; Ottaviani, L.; Giovagnoni, A. Proposal of a low-dose, long-pitch, dual-source chest CT protocol on third-generation dual-source CT using a tin filter for spectral shaping at 100 kVp for CoronaVirus Disease 2019 (COVID-19) patients: A feasibility study. La Radiol. Med. 2020, 125, 365–373. [Google Scholar] [CrossRef]
- Greffier, J.; Hoballah, A.; Sadate, A.; De Oliveira, F.; Claret, P.-G.; De Forges, H.; Loubet, P.; Mauboussin, J.M.; Hamard, A.; Beregi, J.P.; et al. Ultra-low-dose chest CT performance for the detection of viral pneumonia patterns during the COVID-19 outbreak period: A monocentric experience. Quant. Imaging Med. Surg. 2021, 11, 3190–3199. [Google Scholar] [CrossRef]
- Tabatabaei, S.M.H.; Talari, H.; Gholamrezanezhad, A.; Farhood, B.; Rahimi, H.; Razzaghi, R.; Narges, M.; Rajebi, H. A low-dose chest CT protocol for the diagnosis of COVID-19 pneumonia: A prospective study. Emerg. Radiol. 2020, 27, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Dangis, A.; Gieraerts, C.; De Bruecker, Y.; Janssen, L.; Valgaeren, H.; Obbels, D.; Gillis, M.; Van Ranst, M.; Frans, J.; Demeyere, A. Accuracy and reproducibility of low-dose submillisievert chest CT for the diagnosis of COVID-19. Radiol. Cardiothorac. Imaging 2020, 2, 196. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; El-Husseiny, R.M.; Sweed, R.A.; Abd El, N.A.E.-M.; Masoud, M. Ultra-low-dose chest CT protocol during the second wave of COVID-19 pandemic: A double-observer prospective study on 250 patients to evaluate its detection accuracy. Egypt. J. Radiol. Nucl. Med. 2021, 52, 136. [Google Scholar] [CrossRef]
- Aslan, S.; Bekci, T.; Çakır, İ.M.; Ekiz, M.; Yavuz, I.; Şahin, A.M. Diagnostic performance of low-dose chest CT to detect COVID-19: A Turkish population study. Diagn. Interv. Radiol. 2021, 27, 181–187. [Google Scholar] [CrossRef]
- Atlı, E.; Uyanık, S.A.; Öğüşlü, U.; Cenkeri, H.Ç.; Yılmaz, B.; Gümüş, B. The Feasibility of Low-dose Chest CT Acquisition Protocol for the Imaging of COVID-19 Pneumonia. Curr. Med. Imaging Rev. 2022, 18, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Karami, V.; Albosof, M.; Najarian, M.; Gholami, M. Assessment of commercially available in-plane bismuth breast shields for clinical use in patients undergoing thoracic computed tomography. Hong Kong J. Radiol. 2021, 24, 108–115. [Google Scholar] [CrossRef]
- Bernier, M.; Rehel, J.; Brisse, H.; Wu-Zhou, X.; Caer-Lorho, S.; Jacob, S.; Chateil, J.F.; Aubert, B.; Laurier, D. Radiation exposure from CT in early childhood: A French large-scale multicentre study. Br. J. Radiol. Suppl. 2012, 85, 53–60. [Google Scholar] [CrossRef]
- Niemann, T.; Zbinden, I.; Roser, H.; Bremerich, J.; Remy-Jardin, M.; Bongartz, G. Computed tomography for pulmonary embolism: Assessment of a 1-year cohort and estimated cancer risk associated with diagnostic irradiation. Acta Radiol. 2013, 54, 778–784. [Google Scholar] [CrossRef]
- Lahham, A.; AL Masri, H.; Kameel, S. Estimation of female radiation doses and breast cancer risk from chest CT examinations. Radiat. Prot. Dosim. 2018, 179, 303–309. [Google Scholar] [CrossRef]
- Ghetti, C.; Ortenzia, O.; Maddalo, M.; Altabella, L.; Sverzellati, N. Dosimetric and radiation cancer risk evaluation of high resolution thorax CT during COVID-19 outbreak. Phys. Med. 2020, 80, 119–124. [Google Scholar] [CrossRef]
- Matkevich, E.I.; Ivanov, I.V. Radiation Doses and Risk Assessment during Computed Tomography of the Chest in COVID-19 Patients. In Computed-Tomography (CT) Scan; IntechOpen: London, UK, 2021; pp. 1–13. [Google Scholar] [CrossRef]
- Razavi, E.; Zare, M.H.; Zamani, H.; Masjedi, H.; Dalvand, S.; Razavi-Ratki, S.K.; Omidi, R.; Hazbavi, M. Estimation of Effective Doses and Lifetime Risk of Exposure-Induced Cancer Death in Pediatric CT Scans. Int. J. Pediatr. 2022, 10, 15755–15771. [Google Scholar] [CrossRef]
- American Association of Physicists in Medicine (AAPM) 2019. Lung Cancer Screening CT Protocols Version 5.1. Available online: https://www.aapm.org/pubs/CTProtocols/documents/LungCancerScreeningCT.pdf (accessed on 12 October 2022).
- Tahmasebzadeh, A.; Paydar, R.; Soltani-Kermanshahi, M.; Maziar, A.; Reiazi, R. Lifetime attributable cancer risk related to prevalent CT scan procedures in pediatric medical imaging centers. Int. J. Radiat. Biol. 2021, 97, 1282–1288. [Google Scholar] [CrossRef]
- De Basea, M.B.; Moriña, D.; Figuerola, J.; Barber, I.; Muchart, J.; Lee, C.; Elisabeth, C. Subtle excess in lifetime cancer risk related to CT scanning in Spanish young people. Environ. Int. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Brenner, D.J. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 2004, 231, 440–445. [Google Scholar] [CrossRef]
- Berrington de González, A.; Kim, K.P.; Berg, C.D. Low-dose lung computed tomography screening before age 55: Estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J. Med. Screen. 2008, 15, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Karami, V.; Zabihzadeh, M. Prevalence of radiosensitive organ shielding in patients undergoing computed tomography examinations: An observational service audit in Ahvaz, Iran. Asian Biomed. 2015, 9, 771–775. [Google Scholar] [CrossRef]
- Jansen-van der Weide, M.C.; Greuter, M.J.; Jansen, L.; Oosterwijk, J.C.; Pijnappel, R.M.; de Bock, G.H. Exposure to low-dose radiation and the risk of breast cancer among women with a familial or genetic predisposition: A meta-analysis. Eur. Radiol. 2010, 20, 2547–2556. [Google Scholar] [CrossRef]
- Ron, E.; Lubin, J.H.; Shore, R.E.; Mabuchi, K.; Modan, B.; Pottern, L.M.; Schneider, A.B.; Tucker, M.A.; Boice Jr, J.D. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat. Res. 1995, 141, 259–277. [Google Scholar] [CrossRef]
- Toossi, M.T.B.; Malekzadeh, M. Radiation dose to newborns in neonatal intensive care units. Iran. J. Radiol. 2012, 9, 144–149. [Google Scholar] [CrossRef]
- Bourguignon, M.; Gisone, P.; Perez, M.; Michelin, S.; Dubner, D.; Giorgio, M.; Carosella, E.D. Genetic and epigenetic features in radiation sensitivity. Part II: Implications for clinical practice and radiation protection. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 351–368. [Google Scholar] [CrossRef]
- Moser, J.; Sheard, S.; Edyvean, S.; Vlahos, I. Radiation dose-reduction strategies in thoracic CT. Clin. Radiol. 2017, 72, 407–420. [Google Scholar] [CrossRef] [PubMed]
- The Associated Press. Report Links Increased Cancer Risk to CT Scans. The New York Times. 2007. Available online: https://www.nytimes.com/2007/11/29/us/29scan.html (accessed on 12 October 2022).
- UNSCEAR. UNSCEAR 2008 Report. Sources and Effects of Ionizing Radiation. Volume I: Sources: Report to the General Assembly, Sscientific Annexes A and B; UNSCEAR 2008 Report. United Nations Scientific Committee on the Effects of Atomic Radiation; United Nations: New York, NY, USA, 2010.
- Hendry, J.H.; Simon, S.L.; Wojcik, A.; Sohrabi, M.; Burkart, W.; Cardis, E.; Laurier, D.; Tirmarche, M.; Hayata, I. Human exposure to high natural background radiation: What can it teach us about radiation risks? J. Radiol. Prot. 2009, 29, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, L.; Fornalski, K.W.; Feinendegen, L.E. Cancer mortality among people living in areas with various levels of natural background radiation. Dose-Response 2015, 13, 92391. [Google Scholar] [CrossRef] [PubMed]
- Preventable Deaths. Available online: https://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/ (accessed on 12 October 2022).
- Lindsay, R.; Paterson, A.; Edgar, D. Preparing for severe contrast media reactions in children–results of a national survey, a literature review and a suggested protocol. Clin. Radiol. 2011, 66, 340–348. [Google Scholar] [CrossRef]
| Risk Qualification | LAR of Cancer Incidence per 100,000 People | LBR a (%) | % LBR + % LAR b |
|---|---|---|---|
| Negligible | <0.2 | 42 | 42.00 |
| Minimal | 0.2–2 | 42 | 42.00 |
| Very low | 2–20 | 42 | 42.02 |
| Low | 20–200 | 42 | 42.25 |
| Moderate | 200–400 | 42 | 42.50 |
| Risk Qualification | LAR of Fatal Cancer per 100,000 People | LBR a (%) | % LBR + % LAR b |
|---|---|---|---|
| Negligible | <0.1 | 20 | 20.00 |
| Minimal | 0.1–1 | 20 | 20.00 |
| Very low | 1–10 | 20 | 20.01 |
| Low | 10–100 | 20 | 20.10 |
| Moderate | 100–200 | 20 | 20.20 |
| All Solid Cancers | Leukemia | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Excess cancer cases | 800 (400–1600) | 1300 (690–2500) | 100 (30–300) | 70 (20–250) |
| Excess deaths | 410 (200–830) | 610 (300–1200) | 70 (20–220) | 50 (10–190) |
| Ref | LAR of Cancer per 100,000 Persons * | ED (mSv) | DLP (mGy.cm) | CTDIvol (mGy) | Pitch | mA/mAs | kVp | Sample Size | Mean Age [Range] (Year) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Incidence | |||||||||
| [84] | 5.5 | 7.5 c | 1.80 | 112 | 3.50 | 1 | 30 a | 120 | 20 | 64 [≥50] |
| [85] | 2.3 | 3.7 | 0.56 | 40 | 1.27 | 1.2 | 21.5 a | 100, 120 | 192 | 61.8 |
| [23] | 3.6 | 6.1 | 0.91 | 64.7 | 1.77 | 1.5 | 20, 30 b | 110, 120 | 163 | 65 [21–97] |
| [86] | 3.7 | 6.7 | 0.85 | 61 | 1.6 | 1.4 | 45 a | 120 | 250 | 50 [16–84] |
| [86] | 2.6 | 4.7 | 0.59 | 42 | 1.1 | 1.4 | 22 a | 120 | 250 | 50 [16–84] |
| [87] | 1.1 | 1.9 | 0.28 | 20.4 | - | 1.5 | 35–50 b | 80 | 250 | 60 [18–97] |
| [83] | 1 | 1.5 | 0.20 | 14.2 | 0.39 | 1.7 | 10 b | 100 | 380 | 66.3 [>18] |
| [88] | 2.5 | 4.3 | 0.56 | 40.3 | 1 | 1.37 | 50 b | 100 | 141 | 37 |
| Ref | LAR of Cancer per 100,000 Persons * | ED (mSv) | DLP (mGy.cm) | CTDIvol (mGy) | Pitch | mAs | kVp | Sample Size | Mean Age [Range] (Year) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Incidence | |||||||||
| [84] | 20.3 | 27.1 a | 6.6 | 413 | 13 | 1 | 150 | 120 | 20 | 64 [≥50] |
| [89] | 28.6 | 50 | 6.6 | 415 | 9.50 | - | 100 | 120 | 180 | 41.5 [18–74] |
| [77] | - | 195 a | - | - | - | - | - | - | - | 15 |
| [8] | - | 150 a | - | - | - | - | - | - | - | [≤15] |
| [25] | 19 | 28.2 | 5.3 b | 329 | 8 | - | - | 100–130 | 782 | 59 |
| [90] | - | 15.18 a | 2.2 | - | - | - | - | 5746 | [≤5] | |
| [91] | 17.25 | 29.4 | 4.3 | 318 | 9 | 1.3 | 100 | 120 | 691 | 66 [20–≥80] |
| [92] | 31 | 55.6 | 7 | 650 | 8.8 | 0.9–1 | 168–350 | 120 | 200 | [15–80] |
| [93] | 17.4 | 21 a | 4.4 | 239 | 6.8 | 1.2 | 132 | 110, 120 | 3224 | 67 [17–105] |
| [94] | 14 | 16.1 a | 3.1 | - | - | 1.2, 1.4 | 130 | 1003 | [>12] | |
| [17] | 20.7 | 33.2 | 5 | 355 | 10.5 | 0.7–1.5 | - | 80–120 | 550 | 47 |
| [50] | 34.5 | 87 a | 8.7 | - | - | - | 120 | [≥18] | ||
| [95] | 40 | 51.3 a | 3.8 | - | - | 1.42 | 40 | 120 | 765 | [≤15] |
| [88] | 20.4 | 35.4 | 4.6 | 330 | 8 | 1.37 | 90–400 | 120 | 92 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, M.; Karami, V.; Moazen, J.; Kwee, T.; Bhalla, A.S.; Shahbazi-Gahrouei, D.; Shao, Y.-H.J. Radiation Exposure and Lifetime Attributable Risk of Cancer Incidence and Mortality from Low- and Standard-Dose CT Chest: Implications for COVID-19 Pneumonia Subjects. Diagnostics 2022, 12, 3043. https://doi.org/10.3390/diagnostics12123043
Garg M, Karami V, Moazen J, Kwee T, Bhalla AS, Shahbazi-Gahrouei D, Shao Y-HJ. Radiation Exposure and Lifetime Attributable Risk of Cancer Incidence and Mortality from Low- and Standard-Dose CT Chest: Implications for COVID-19 Pneumonia Subjects. Diagnostics. 2022; 12(12):3043. https://doi.org/10.3390/diagnostics12123043
Chicago/Turabian StyleGarg, Mandeep, Vahid Karami, Javad Moazen, Thomas Kwee, Ashu Seith Bhalla, Daryoush Shahbazi-Gahrouei, and Yu-Hsuan Joni Shao. 2022. "Radiation Exposure and Lifetime Attributable Risk of Cancer Incidence and Mortality from Low- and Standard-Dose CT Chest: Implications for COVID-19 Pneumonia Subjects" Diagnostics 12, no. 12: 3043. https://doi.org/10.3390/diagnostics12123043
APA StyleGarg, M., Karami, V., Moazen, J., Kwee, T., Bhalla, A. S., Shahbazi-Gahrouei, D., & Shao, Y.-H. J. (2022). Radiation Exposure and Lifetime Attributable Risk of Cancer Incidence and Mortality from Low- and Standard-Dose CT Chest: Implications for COVID-19 Pneumonia Subjects. Diagnostics, 12(12), 3043. https://doi.org/10.3390/diagnostics12123043









