Duration of Diabetes as a Risk Factor for Retinal Microvasculature Alterations Detected with Optical Coherence Tomography Angiography in Patients without Clinical Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. OCTA Image Acquisition and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic Retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Classification of Diabetic Retinopathy from Fluorescein Angiograms: ETDRS Report Number 11. Ophthalmology 1991, 98, 807–822. [Google Scholar] [CrossRef]
- Kwiterovich, K.A.; Maguire, M.G.; Murphy, R.P.; Schachat, A.P.; Bressler, N.M.; Bressler, S.B.; Fine, S.L. Frequency of Adverse Systemic Reactions after Fluorescein Angiography. Results of a Prospective Study. Ophthalmology 1991, 98, 1139–1142. [Google Scholar] [CrossRef]
- Khadamy, J.; Aghdam, K.; Falavarjani, K. An Update on Optical Coherence Tomography Angiography in Diabetic Retinopathy. J. Ophthalmic Vis. Res. 2018, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Gao, S.S.; Liu, L.; Lauer, A.K.; Bailey, S.T.; Flaxel, C.J.; Wilson, D.J.; Huang, D.; Jia, Y. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2016, 134, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Wiley, H.E.; Ferris, F.L. Retina, 5th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012; pp. 940–968. ISBN 1-4557-0737-6. [Google Scholar]
- Thompson, I.A.; Durrani, A.K.; Patel, S. Optical Coherence Tomography Angiography Characteristics in Diabetic Patients without Clinical Diabetic Retinopathy. Eye 2019, 33, 648–652. [Google Scholar] [CrossRef] [PubMed]
- de Carlo, T.E.; Chin, A.T.; Bonini Filho, M.A.; Adhi, M.; Branchini, L.; Salz, D.A.; Baumal, C.R.; Crawford, C.; Reichel, E.; Witkin, A.J.; et al. Detection of Microvascular Changes in Eyes of Patients with Diabetes but not Clinical Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Retina 2015, 35, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Takase, N.; Nozaki, M.; Kato, A.; Ozeki, H. Enlargement of Foveal Avascular Zone in Diabetic Eyes Evaluated by En Face Optical Coherence Tomography Angiography. Retina 2015, 35, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Yasin Alibhai, A.; Moult, E.M.; Shahzad, R.; Rebhun, C.B.; Moreira-Neto, C.; McGowan, M.; Lee, D.; Lee, B.; Baumal, C.R.; Witkin, A.J.; et al. Quantifying Microvascular Changes Using OCT Angiography in Diabetic Eyes without Clinical Evidence of Retinopathy. Ophthalmol. Retina 2018, 2, 418–427. [Google Scholar] [CrossRef]
- Dimitrova, G.; Chihara, E.; Takahashi, H.; Amano, H.; Okazaki, K. Quantitative Retinal Optical Coherence Tomography Angiography in Patients With Diabetes Without Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 190–196. [Google Scholar] [CrossRef]
- Cao, D.; Yang, D.; Huang, Z.; Zeng, Y.; Wang, J.; Hu, Y.; Zhang, L. Optical Coherence Tomography Angiography Discerns Preclinical Diabetic Retinopathy in Eyes of Patients with Type 2 Diabetes without Clinical Diabetic Retinopathy. Acta Diabetol. 2018, 55, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and Risk of Diabetic Retinopathy When Age at Diagnosis Is Less than 30 Years. Arch. Ophthalmol. 1984, 102, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. III. Prevalence and Risk of Diabetic Retinopathy When Age at Diagnosis Is 30 or More Years. Arch. Ophthalmol. 1984, 102, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Keenan, H.A.; Cavallerano, J.D.; Asztalos, B.F.; Schaefer, E.J.; Sell, D.R.; Strauch, C.M.; Monnier, V.M.; Doria, A.; Aiello, L.P.; et al. Protection from Retinopathy and Other Complications in Patients with Type 1 Diabetes of Extreme Duration: The Joslin 50-Year Medalist Study. Diabetes Care 2011, 34, 968–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, G.; Banaee, T.; Conti, F.F.; Singh, R.P. Optical Coherence Tomography Angiography in Eyes with Retinal Vein Occlusion. J. Ophthalmic Vis. Res. 2018, 13, 315–332. [Google Scholar] [CrossRef]
- Rao, H.L.; Pradhan, Z.S.; Suh, M.H.; Moghimi, S.; Mansouri, K.; Weinreb, R.N. Optical Coherence Tomography Angiography in Glaucoma. J. Glaucoma 2020, 29, 312–321. [Google Scholar] [CrossRef]
- Katulanda, P.; Ranasinghe, P.; Jayawardena, R. Prevalence of Retinopathy among Adults with Self-Reported Diabetes Mellitus: The Sri Lanka Diabetes and Cardiovascular Study. BMC Ophthalmol. 2014, 14, 100. [Google Scholar] [CrossRef] [Green Version]
- Seferovic, J.P.; Bentley-Lewis, R.; Claggett, B.; Diaz, R.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Lewis, E.F.; Maggioni, A.P.; McMurray, J.J.V.; et al. Retinopathy, Neuropathy, and Subsequent Cardiovascular Events in Patients with Type 2 Diabetes and Acute Coronary Syndrome in the ELIXA: The Importance of Disease Duration. J. Diabetes Res. 2018, 2018, 1631263. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.L.C.; Pankow, J.S.; Heiss, G.; Selvin, E. Validity and Reliability of Self-Reported Diabetes in the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2012, 176, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, P.J.; Durbin, M.K.; Roisman, L.; Zheng, F.; Miller, A.; Robbins, G.; Schaal, K.B.; Gregori, G. ZEISS AngioplexTM Spectral Domain Optical Coherence Tomography Angiography: Technical Aspects. OCT Angiogr. Retin. Macular Dis. 2016, 56, 18–29. [Google Scholar] [CrossRef]
- Laotaweerungsawat, S.; Psaras, C.; Liu, X.; Stewart, J.M. OCT Angiography Assessment of Retinal Microvascular Changes in Diabetic Eyes in an Urban Safety-Net Hospital. Ophthalmol. Retina 2020, 4, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Waheed, N.K.; Moult, E.M.; Adhi, M.; Lee, B.; De Carlo, T.; Jayaraman, V.; Baumal, C.R.; Duker, J.S.; Fujimoto, J.G. Ultrahigh Speed Swept Source Optical Coherence Tomography Angiography of Retinal and Choriocapillaris Alterations in Diabetic Patients With and Without Retinopathy. Retina 2017, 37, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Jiang, C.; Wang, X.; Zhu, L.; Gu, R.; Xu, H.; Jia, Y.; Huang, D.; Sun, X. Macular Perfusion in Healthy Chinese: An Optical Coherence Tomography Angiogram Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3212–3217. [Google Scholar] [CrossRef]
- Iafe, N.A.; Phasukkijwatana, N.; Chen, X.; Sarraf, D. Retinal Capillary Density and Foveal Avascular Zone Area Are Age-Dependent: Quantitative Analysis Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5780–5787. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, A.P.; Mankad, R.N.; Marek, L.; Das, R.; Rangasamy, S.; Monickaraj, F.; Das, A. Genotypes and Phenotypes: A Search for Influential Genes in Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 2712. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, A.P.; Monickaraj, F.; Rangasamy, S.; Hobbs, S.; McGuire, P.; Das, A. Do Genomic Factors Play a Role in Diabetic Retinopathy? J. Clin. Med. 2020, 9, 216. [Google Scholar] [CrossRef] [Green Version]
- Coscas, F.; Sellam, A.; Glacet-Bernard, A.; Jung, C.; Goudot, M.; Miere, A.; Souied, E.H. Normative Data for Vascular Density in Superficial and Deep Capillary Plexuses of Healthy Adults Assessed by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT211–OCT223. [Google Scholar] [CrossRef]

| DM Duration | ||||
|---|---|---|---|---|
| Short Cohort: 0–5 Years (n = 571) | Medium Cohort: 6–10 Years (n = 306) | Long Cohort: Over 10 Years (n = 241) | p Values | |
| Age, year | p < 0.001 (SC vs. MC and SC vs. LC) | |||
| Mean (SD) [range] | 54.6 (11.7) [15–84] | 59.3 (10.2) [31–84] | 61.7 (10.3) [22–85] | p = 0.012 (MC vs. LC) |
| Gender, No./total No. (%) | p = 0.072 (SC vs. MC) | |||
| Female | 279/571 (48.9) | 169/306 (55.2) | 138/241 (57.3) | p = 0.029 (SC vs. LC) |
| Male | 292/571 (51.1) | 137/306 (44.8) | 103/241 (42.7) | p = 0.634 (MC vs. LC) |
| Body mass index (BMI) | ||||
| Mean (SD) | 31.32 (7.27) | 30.37 (6.61) | 30.62 (7.56) | p = 0.067 |
| HbA1c, % | p = 0.012 (SC vs. MC) | |||
| Mean (SD) [range] | 7.64 (2.05) [4.1–20.4] | 7.76 (1.74) [4.4–15.1] | 7.97 (2.27) [5.4–27.1] | p = 0.001 (SC vs. LC) |
| p = 0.387 (MC vs. LC) | ||||
| Hypertension | p < 0.001 (SC vs. MC and SC vs. LC) | |||
| No./total No. (%) | 303/571 (53.1) | 209/306 (68.3) | 177/241 (73.4) | p = 0.190 (MC vs. LC) |
| Hyperlipidemia | p = 0.002 (SC vs. MC) | |||
| No./total No. (%) | 299/571 (52.4) | 194/306 (63.4) | 156/241 (64.7) | p = 0.001 (SC vs. LC) |
| p = 0.747 (MC vs. LC) | ||||
| Diabetes treatment, No./total No. (%) | ||||
| Diet control | 117/571 (20.5) | 26/306 (8.5) | 15/241 (6.2) | p < 0.001 (SC vs. MC and SC vs. LC) |
| Oral medicine | 385/571 (67.4) | 215/306 (70.3) | 159/241 (66.0) | p = 0.158 (MC vs. LC) |
| Insulin use | 69/571 (12.1) | 65/306 (21.2) | 67/241 (27.8) |
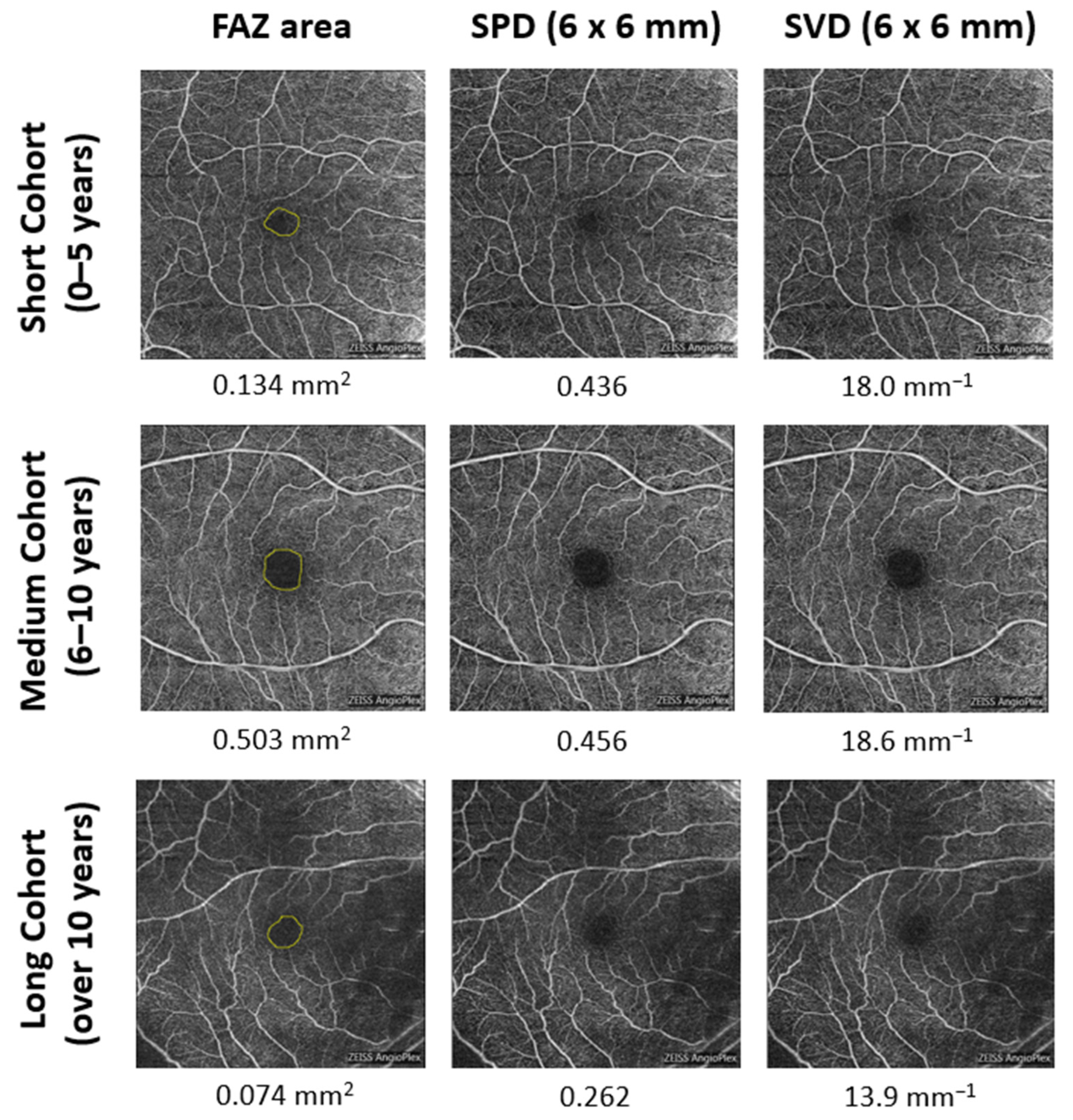
| Perfusion Density | Vessel Density, mm−1 | FAZ Area, mm2 | FAZ Perimeter, mm | FAZ Circularity Index | |||||
|---|---|---|---|---|---|---|---|---|---|
| Statistic | 6 mm Patch | 1 mm Patch | Inner Ring | 6 mm Patch | 1 mm Patch | Inner Ring | |||
| Short cohort (SC): | |||||||||
| Mean (SD) | 0.360 (0.040) | 0.168 (0.054) | 0.381 (0.036) | 19.3 (1.9) | 9.4 (3.0) | 20.5 (2.0) | 0.287 (0.112) | 2.35 (0.51) | 0.643 (0.110) |
| Medium cohort (MC): | |||||||||
| Mean (SD) | 0.357 (0.038) | 0.157 (0.053) | 0.377 (0.034) | 19.0 (1.9) | 8.7 (2.9) | 20.3 (2.0) | 0.300 (0.118) | 2.43 (0.55) | 0.622 (0.112) |
| Long cohort (LC): | |||||||||
| Mean (SD) | 0.348 (0.041) | 0.160 (0.060) | 0.369 (0.037) | 18.6 (2.0) | 8.9 (3.3) | 19.7 (2.2) | 0.272 (0.119) | 2.35 (0.58) | 0.609 (0.114) |
| p value | p = 0.119 (SC vs. MC) | p = 0.003 (SC vs. MC) | p = 0.172 (SC vs. MC) | p = 0.028 (SC vs. MC) | p = 0.001 (SC vs. MC) | p = 0.127 (SC vs. MC) | p = 0.105 (SC vs. MC) | p = 0.053 | p = 0.002 (SC vs. MC) |
| p = 0.027 (MC vs. LC) | p = 0.441 (MC vs. LC) | p = 0.008 (MC vs. LC) | p = 0.011 (MC vs. LC) | p = 0.458 (MC vs. LC) | p = 0.003 (MC vs. LC) | p = 0.004 (MC vs. LC) | (*) | p = 0.268 (MC vs. LC) | |
| p < 0.001 (SC vs. LC) | p = 0.062 (SC vs. LC) | p < 0.001 (SC vs. LC) | p < 0.001 (SC vs. LC) | p = 0.021 (SC vs. LC) | p < 0.001 (SC vs. LC) | p = 0.087 (SC vs. LC) | (*) | p < 0.001 (SC vs. LC) | |
| Perfusion Density | Vessel Density, mm−1 | FAZ Area, mm2 | FAZ Perimeter, mm | FAZ Circularity Index | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 6 mm Patch | 1 mm Patch | Inner Ring | 6 mm Patch | 1 mm Patch | Inner Ring | |||
| Duration of diabetes | |||||||||
| 0–5 years | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 6–10 years | −0.025 | −0.068 | −0.014 | −0.006 | −0.077 | 0.000 | 0.061 | 0.070 | −0.049 |
| p value | 0.447 | 0.038 | 0.673 | 0.860 | 0.018 | 0.989 | 0.069 | 0.037 | 0.145 |
| 10+ years | −0.065 | −0.007 | −0.071 | −0.059 | −0.014 | −0.060 | −0.045 | −0.021 | −0.061 |
| p value | 0.061 | 0.839 | 0.036 | 0.075 | 0.672 | 0.075 | 0.193 | 0.550 | 0.076 |
| Age | −0.212 | −0.153 | −0.261 | −0.349 | −0.164 | −0.336 | −0.091 | −0.006 | −0.201 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.008 | 0.866 | <0.001 |
| Gender | 0.012 | 0.200 | 0.007 | 0.034 | 0.213 | 0.006 | −0.209 | −0.185 | −0.024 |
| p value | 0.681 | <0.001 | 0.819 | 0.235 | <0.001 | 0.843 | <0.001 | <0.001 | 0.419 |
| BMI | 0.005 | 0.085 | 0.000 | 0.056 | 0.111 | 0.051 | −0.057 | −0.092 | 0.075 |
| p value | 0.865 | 0.005 | 0.995 | 0.057 | <0.001 | 0.087 | 0.065 | 0.004 | 0.016 |
| HbA1c | −0.014 | −0.029 | −0.030 | −0.037 | −0.029 | −0.027 | −0.002 | 0.003 | −0.013 |
| p value | 0.668 | 0.356 | 0.339 | 0.229 | 0.349 | 0.375 | 0.957 | 0.915 | 0.685 |
| Hypertension | −0.082 | −0.035 | −0.062 | −0.004 | −0.021 | 0.020 | −0.007 | 0.021 | −0.012 |
| p value | 0.010 | 0.264 | 0.050 | 0.897 | 0.501 | 0.511 | 0.832 | 0.526 | 0.705 |
| Hyperlipidemia | 0.059 | 0.021 | 0.050 | 0.014 | 0.005 | 0.005 | 0.021 | 0.012 | 0.012 |
| p value | 0.051 | 0.491 | 0.096 | 0.638 | 0.855 | 0.875 | 0.495 | 0.687 | 0.692 |
| Treatment of diabetes | |||||||||
| Oral medicine | 0.020 | 0.002 | 0.016 | −0.004 | −0.002 | −0.006 | −0.021 | −0.007 | −0.002 |
| p value | 0.632 | 0.952 | 0.703 | 0.915 | 0.954 | 0.874 | 0.619 | 0.870 | 0.971 |
| Insulin use | −0.004 | −0.004 | −0.001 | −0.030 | −0.010 | −0.025 | −0.016 | 0.006 | −0.023 |
| p value | 0.937 | 0.931 | 0.979 | 0.482 | 0.824 | 0.552 | 0.727 | 0.891 | 0.602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, J.; Haq, Z.; Yang, D.; Jin, J.Q.; Stewart, J.M. Duration of Diabetes as a Risk Factor for Retinal Microvasculature Alterations Detected with Optical Coherence Tomography Angiography in Patients without Clinical Retinopathy. Diagnostics 2022, 12, 3020. https://doi.org/10.3390/diagnostics12123020
Qian J, Haq Z, Yang D, Jin JQ, Stewart JM. Duration of Diabetes as a Risk Factor for Retinal Microvasculature Alterations Detected with Optical Coherence Tomography Angiography in Patients without Clinical Retinopathy. Diagnostics. 2022; 12(12):3020. https://doi.org/10.3390/diagnostics12123020
Chicago/Turabian StyleQian, Jing, Zeeshan Haq, Daphne Yang, Joy Q. Jin, and Jay M. Stewart. 2022. "Duration of Diabetes as a Risk Factor for Retinal Microvasculature Alterations Detected with Optical Coherence Tomography Angiography in Patients without Clinical Retinopathy" Diagnostics 12, no. 12: 3020. https://doi.org/10.3390/diagnostics12123020
APA StyleQian, J., Haq, Z., Yang, D., Jin, J. Q., & Stewart, J. M. (2022). Duration of Diabetes as a Risk Factor for Retinal Microvasculature Alterations Detected with Optical Coherence Tomography Angiography in Patients without Clinical Retinopathy. Diagnostics, 12(12), 3020. https://doi.org/10.3390/diagnostics12123020







