Correlation of Shear-Wave Elastography and Apparent Diffusion Coefficient Values in Breast Cancer and Their Relationship with the Prognostic Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
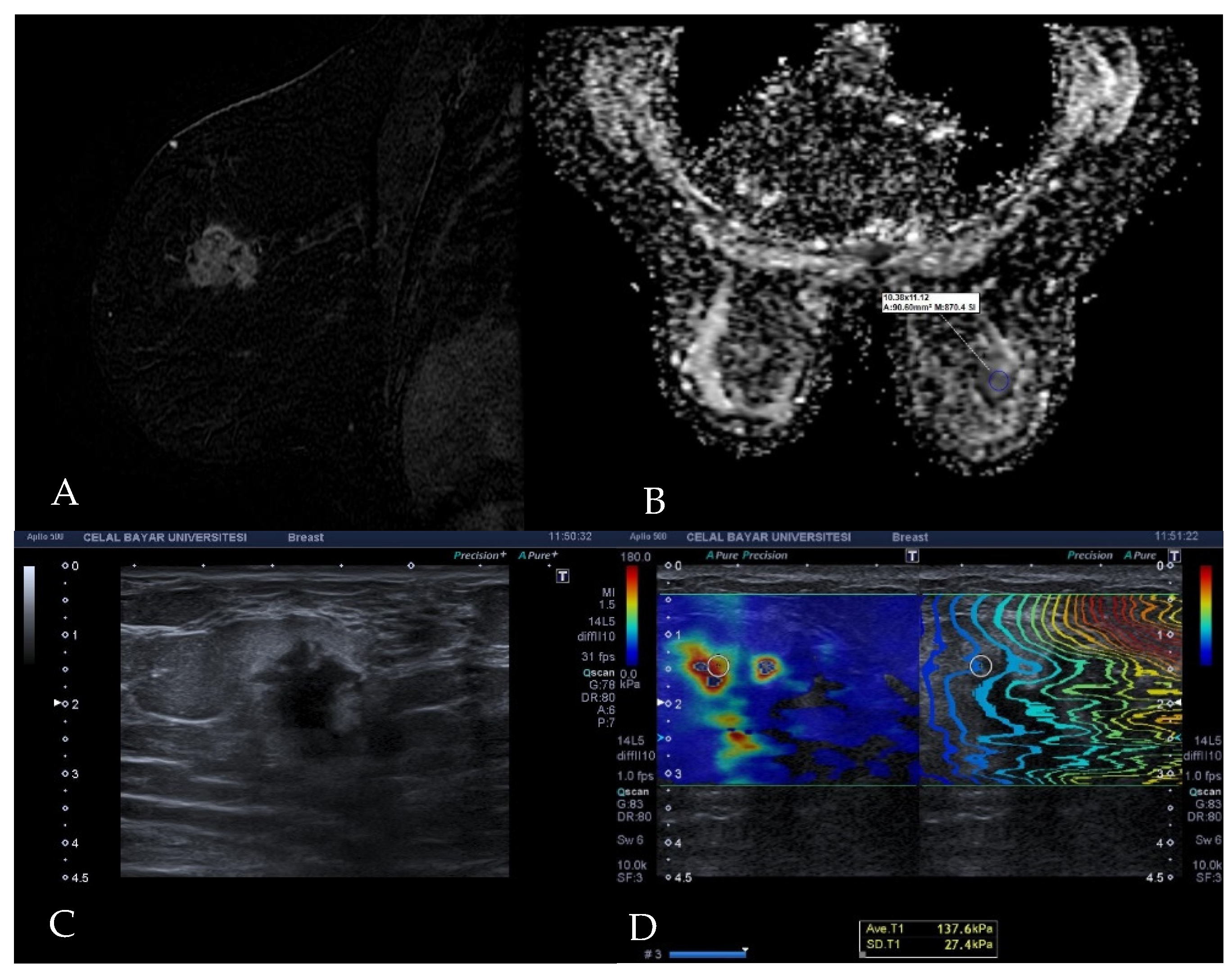
2.2. Elastography Examination Technique
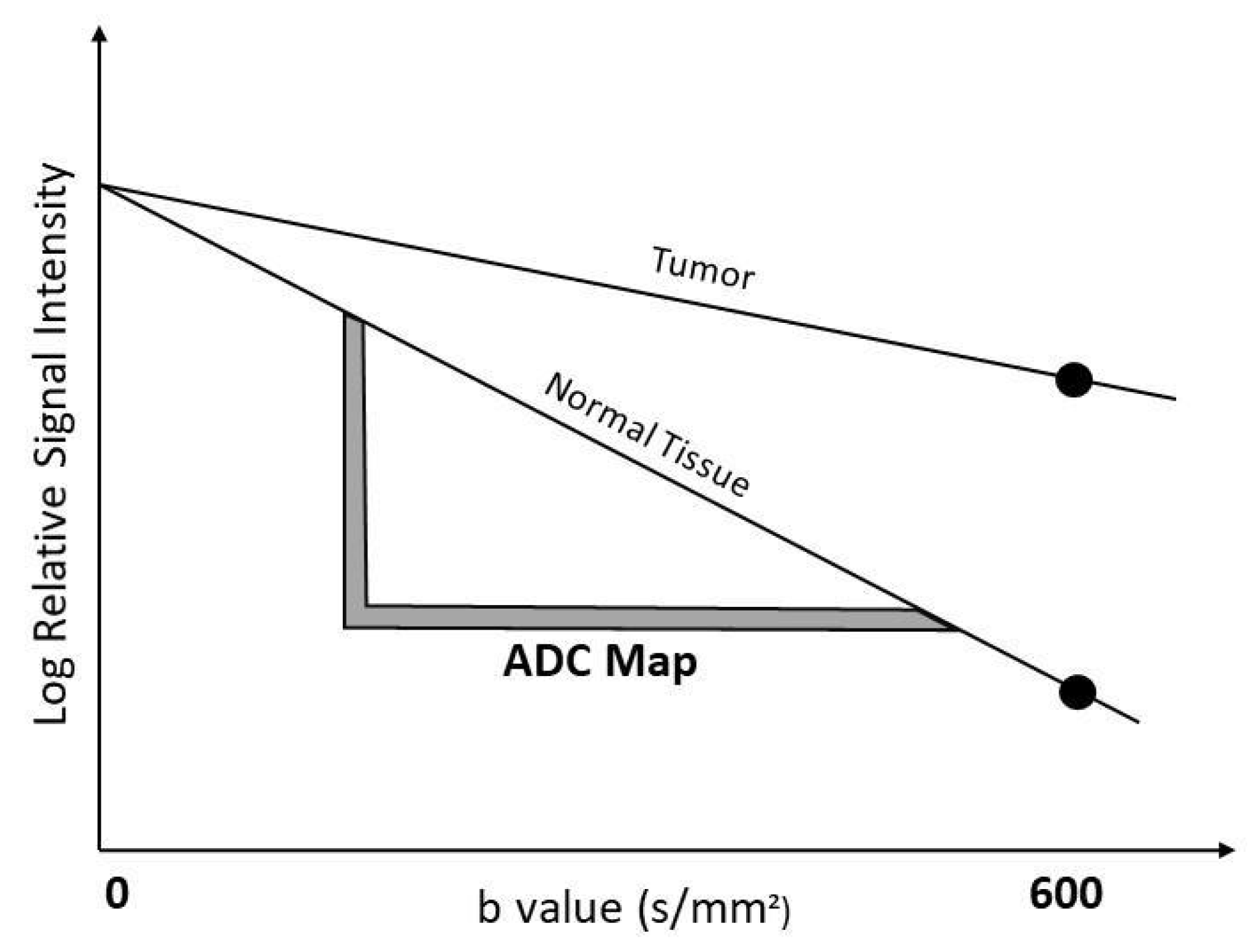
2.3. Diffusion MRI Examination Technique
2.4. Histopathological Analysis
2.5. Statistical Analysis
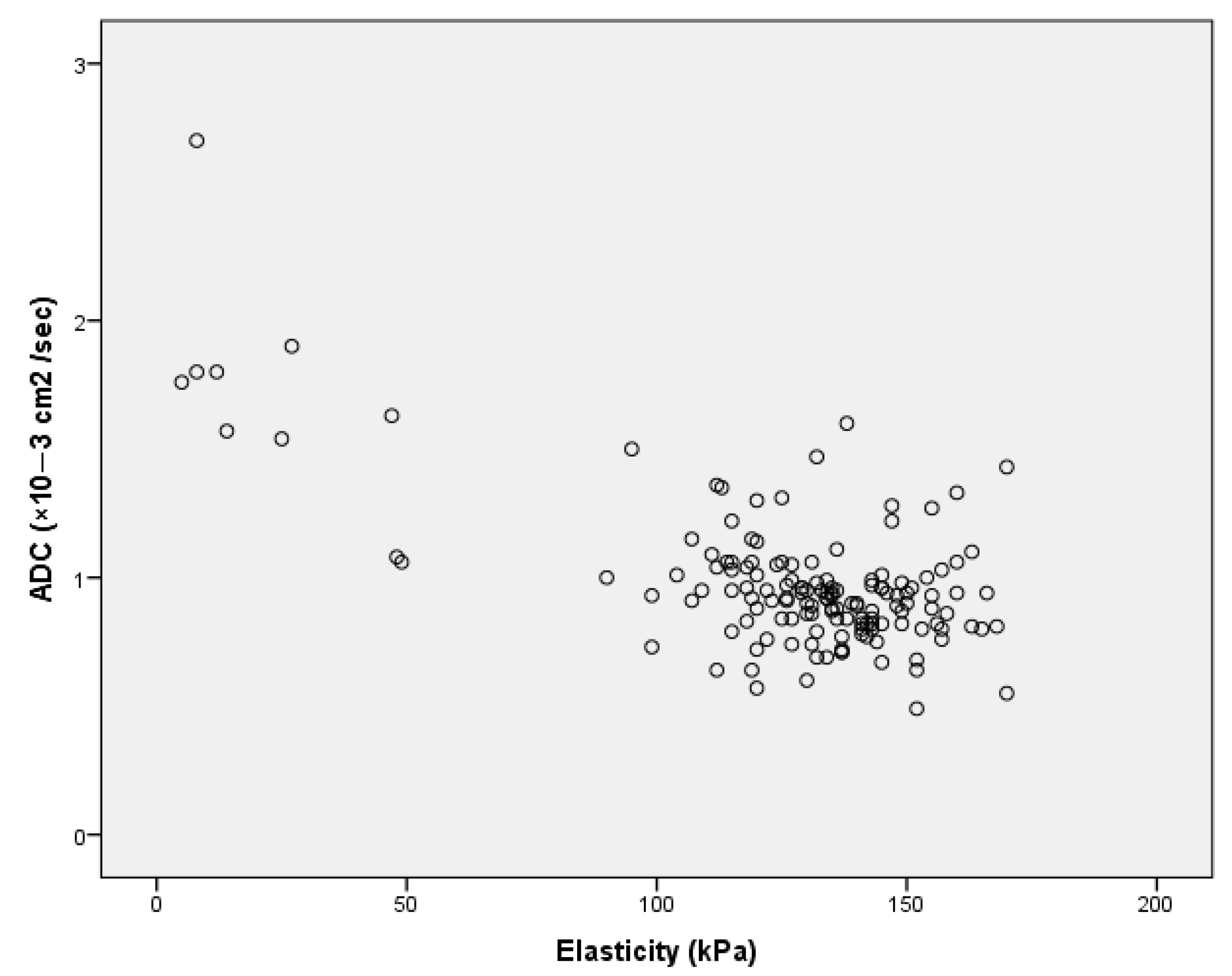
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24, S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Cho, N. Molecular subtypes and imaging phenotypes of breast cancer. Ultrasonography 2016, 35, 281–288. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Q.; Cui, C.; Fei, J.; Su, X.; Li, L.; Ma, J.; Zhang, M. Correlation between imaging features and molecular subtypes of breast cancer in young women (≤30 years old). Jpn. J. Radiol. 2020, 38, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, R.; Wen, C.; Xu, W.; Xu, Z.; Wang, S.; Wu, J.; Pan, D.; Zheng, B.; Qin, G.; et al. Predicting the molecular subtype of breast cancer and identifying interpretable imaging features using machine learning algorithms. Eur. Radiol. 2022, 32, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Drukteinis, J.S.; Mooney, B.P.; Flowers, C.I.; Gatenby, R.A. Beyond mammography: New frontiers in breast cancer screening. Am. J. Med. 2013, 126, 472–479. [Google Scholar] [CrossRef]
- Mann, M.R.; Cho, N.; Moy, L. Breast MRI: State of the art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Hari, S.; Paul, S.; Vidyasagar, R.; Dhamija, E.; Adarsh, A.; Thulkar, S.; Mathur, S.; Sreenivas, V.; Sharma, S.; Srivastava, A.; et al. Breast mass characterization using shear wave elastography and ultrasound. Diagn. Interv. Imaging 2018, 99, 699–707. [Google Scholar] [CrossRef]
- Youk, J.H.; Gweon, H.M.; Son, E.J.; Han, K.H.; Kim, J.A. Diagnostic value of commercially available shear-wave elastography for breast cancers: Integration into BI-RADS classification with subcategories of category 4. Eur. Radiol. 2013, 23, 2695–2704. [Google Scholar] [CrossRef]
- Youk, J.H.; Son, E.J.; Gweon, H.M.; Kim, H.; Park, Y.J.; Kim, J.A. Comparison of strain and shear wave elastography for the differentiation of benign from malignant breast lesions, combined with b-mode ultrasonography: Qualitative and quantitative assessments. Ultrasound Med. Biol. 2014, 40, 2336–2344. [Google Scholar] [CrossRef]
- Cosgrove, D.O.; Berg, W.A.; Doré, C.J.; Skyba, D.M.; Henry, J.-P.; Gay, J.; Cohen-Bacrie, C.; The BE1 Study Group. Shear wave elastography for breast masses is highly reproducible. Eur. Radiol. 2012, 22, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Wallis, M.; Tarvidon, A.; Helbich, T.; Schreer, I. Guidelines from the European Society of Breast Imaging for diagnostic interventional breast procedures. Eur. Radiol. 2007, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, I.D.; Gullo, R.L.; Saccarelli, C.; Thakur, S.B.; Bitencourt, A.; Morris, E.A.; Jochelson, M.S.; Sevilimedu, V.; Martinez, D.F.; Pinker-Domenig, K. Diagnostic value of diffusion-weighted imaging with synthetic b-values in breast tumors: Comparison with dynamic contrast-enhanced and multiparametric MRI. Eur. Radiol. 2021, 31, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Kapetas, P.; Clauser, P.; Milos, R.-I.; Vigano, S.; Bernathova, M.; Helbich, T.H.; Baltzer, P.A. Microstructural breast tissue characterization: A head-to-head comparison of Diffusion Weighted Imaging and Acoustic Radiation Force Impulse elastography with clinical implications. Eur. J. Radiol. 2021, 143, 109926. [Google Scholar] [CrossRef]
- Matsubayashi, R.N.; Imanishi, M.; Nakagawa, S.; Takahashi, R.; Akashi, M.; Momosaki, S.; Muranaka, T. Breast ultrasound elastography and magnetic resonance imaging of fibrotic changes of breast disease: Correlations between elastography findings and pathologic and short Tau inversion recovery imaging results, including the enhancement ratio and apparent. J. Comput. Assist. Tomogr. 2015, 39, 94–101. [Google Scholar] [CrossRef]
- Baltacioglu, N.A.; Tureli, D. Ultrasound shear-wave elasticity and magnetic resonance diffusion coefficient show strong inverse correlation in small fibroadenomas. Marmara Med. J. 2021, 34, 24–28. [Google Scholar] [CrossRef]
- Youk, J.H.; Gweon, H.M.; Son, E.J.; Kim, J.A.; Jeong, J. Shear-wave elastography of invasive breast cancer: Correlation between quantitative mean elasticity value and immunohistochemical profile. Breast Cancer Res. Treat. 2013, 138, 119–126. [Google Scholar] [CrossRef]
- Chang, J.M.; Moon, W.K.; Cho, N.; Yi, A.; Koo, H.R.; Han, W.; Noh, D.-Y.; Moon, H.-G.; Kim, S.J. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res. Treat. 2011, 129, 89–97. [Google Scholar] [CrossRef]
- Choi, W.J.; Kim, H.H.; Cha, J.H.; Shin, H.J.; Kim, H.; Chae, E.Y.; Hong, M.J. Predicting prognostic factors of breast cancer using shear wave elastography. Ultrasound Med. Biol. 2014, 40, 269–274. [Google Scholar] [CrossRef]
- Ganau, S.; Andreu, F.J.; Escribano, F.; Martín, A.; Tortajada, L.; Villajos, M.; Baré, M.; Teixidó, M.; Ribé, J.; Sentís, M. Shear-wave elastography and immunohistochemical profiles in invasive breast cancer: Evaluation of maximum and mean elasticity values. Eur. J. Radiol. 2015, 84, 617–622. [Google Scholar] [CrossRef]
- Makal, G.B.; Güvenç, İ. The Role of Shear Wave Elastography in Differentiating Idiopathic Granulomatous Mastitis From Breast Cancer. Acad. Radiol. 2021, 28, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.K.; Cha, E.S.; Kim, H.S.; Kang, B.J.; Choi, J.J.; Jung, J.H.; Park, Y.G.; Suh, Y.J. Diffusion-weighted imaging of breast cancer: Correlation of the apparent diffusion coefficient value with prognostic factors. J. Magn. Reson. Imaging 2009, 30, 615–620. [Google Scholar] [CrossRef]
- Jeh, S.K.; Kim, S.H.; Kim, H.S.; Kang, B.J.; Jeong, S.H.; Yim, H.W.; Song, B.J. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J. Magn. Reson. Imaging 2011, 33, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Chang, Y.W.; Park, H.J.; Kim, H.J.; Hong, S.S.; Seo, D.Y. Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br. J. Radiol. 2012, 85, e474–e479. [Google Scholar] [CrossRef]
- Moutinho-Guilherme, R.; Oyola, J.H.; Sanz-Rosa, D.; Vassallo, I.T.; García, R.M.; Pisco, J.M.; de Vega, V.M. Correlation between apparent diffusion coefficient values in breast magnetic resonance imaging and prognostic factors of breast invasive ductal carcinoma. Porto. Biomed. J. 2019, 4, e27. [Google Scholar] [CrossRef]
- Tezcan, S.; Uslu, N.; Ozturk, F.U.; Akcay, E.Y.; Tezcaner, T. Diffusion-Weighted Imaging of Breast Cancer: Correlation of the Apparent Diffusion Coefficient Value with Pathologic Prognostic Factors. Eur. J. Breast Health 2019, 15, 262–267. [Google Scholar] [CrossRef]
- Ren, C.; Zou, Y.; Zhang, X.; Li, K. Diagnostic value of diffusion-weighted imaging-derived apparent diffusion coefficient and its association with histological prognostic factors in breast cancer. Oncol. Lett. 2019, 18, 3295–3303. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Can apparent diffusion coefficient (ADC) distinguish breast cancer from benign breast findings? A meta-analysis based on 13 847 lesions. BMC Cancer 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Linh, L.T.; Duc, N.M.; My, T.T.T.; Bang, L.V.; Tien, N.C.; Thong, P.M. Correlations between apparent diffusion coefficient values and histopathologic factors in breast cancer. Clin. Ther. 2021, 72, 218–224. [Google Scholar] [CrossRef]
- Satake, H.; Nishio, A.; Ikeda, M.; Ishigaki, S.; Shimamoto, K.; Hirano, M.; Naganawa, S. Predictive value for malignancy of suspicious breast masses of BI-RADS categories 4 and 5 using ultrasound elastography and MR diffusion-weighted imaging. Am. J. Roentgenol. 2011, 196, 202–209. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, Y.-Q.; Cai, Z.-L.; Gao, Y.-G.; An, N.-Y.; Ma, L.; Mahankali, S.; Gao, J.-H. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J. Magn. Reson. Imaging 2002, 16, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Maçin, S.; Deniz, M.A.; Bükte, Y.; Deniz, Z.T.; Sarıca, Ö.; Oysu, A.S. Effectiveness Of Sonoelastography and Diffusion MRI ADC Value In Discriminating Between Malignant and Benign Lesions of the Breast. Med. J. Bakırkoy 2020, 16, 203–211. [Google Scholar] [CrossRef]
- Azab, E.; Ibrahim, M. Diffusion weighted (DW) MRI role in characterization of breast lesions using absolute and normalized ADC values. Egypt. J. Radiol. Nucl. Med. 2018, 49, 564–570. [Google Scholar] [CrossRef]
- Akın, Y.; Uğurlu, M.Ü.; Kaya, H.; Arıbal, E. Diagnostic Value of Diffusion-weighted Imaging and Apparent Diffusion Coefficient Values in the Differentiation of Breast Lesions, Histpathologic Subgroups and Correlatıon with Prognostıc Factors using 3.0 Tesla MR. J. Breast Health 2016, 12, 123–132. [Google Scholar] [CrossRef]
- Au, F.W.F.; Ghai, S.; Moshonov, H.; Kahn, H.; Brennan, C.; Dua, H.; Crystal, P. Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: Determination of the most discriminatory parameter. Am. J. Roentgenol. 2014, 202, E328–E336. [Google Scholar] [CrossRef]
- Berg, W.A.; Cosgrove, D.O.; Doré, C.J.; Schäfer, F.K.W.; Svensson, W.E.; Hooley, R.J.; Ohlinger, R.; Mendelson, E.B.; Balu-Maestro, C.; Locatelli, M.; et al. Shear-wave elastography improves the specificity of breast US: The BE1 multinational study of 939 masses. Radiology 2012, 262, 435–449. [Google Scholar] [CrossRef]
- Athanasiou, A.; Tardivon, A.; Tanter, M.; Sigal-Zafrani, B.; Bercoff, J.; Deffieux, T.; Gennisson, J.-L.; Fink, M.; Neuenschwander, S. Breast lesions: Quantitative elastography with supersonic shear imaging--preliminary results. Radiology 2010, 256, 297–303. [Google Scholar] [CrossRef]
- Song, E.J.; Sohn, Y.M.; Seo, M. Diagnostic performances of shear-wave elastography and B-mode ultrasound to differentiate benign and malignant breast lesions: The emphasis on the cutoff value of qualitative and quantitative parameters. Clin. Imaging 2018, 50, 302–307. [Google Scholar] [CrossRef]
- Youk, J.H.; Son, E.J.; Gweon, H.M.; Han, K.H.; Kim, J.A. Quantitative lesion-to-fat elasticity ratio measured by shear-wave elastography for breast mass: Which area should be selected as the fat reference? PLoS ONE 2015, 10, e0138074. [Google Scholar] [CrossRef]
- Lee, S.H.; Chang, J.M.; Kim, W.H.; Bae, M.S.; Cho, N.; Yi, A.; Koo, H.R.; Kim, S.J.; Kim, J.Y.; Moon, W.K. Differentiation of benign from malignant solid breast masses: Comparison of two-dimensional and three-dimensional shear-wave elastography. Eur. Radiol. 2013, 23, 1015–1026. [Google Scholar] [CrossRef]
- Marguerite, C.; D’Angelo, A.; Ferrara, F.; Santoro, A.; Belli, P.; Manfredi, R. Radial Scar: A management dilemma. Radiol. Med. 2021, 126, 774–785. [Google Scholar] [CrossRef]
- Taboada, J.L.; Stephens, T.W.; Krishnamurthy, S.; Brandt, K.R.; Whitman, G.J. The Many Faces of Fat Necrosis in the Breast. Am. J. Roentgenol. 2009, 192, 815–825. [Google Scholar] [CrossRef]
- Evans, A.; Whelehan, P.; Thomson, K.; McLean, D.; Brauer, K.; Purdie, C.; Jordan, L.; Baker, L.; Thompson, A. Quantitative shear wave ultrasound elastography: Initial experience in solid breast masses. Breast Cancer Res. 2010, 12, R104. [Google Scholar] [CrossRef]
- Cheng, L.; Bai, Y.; Zhang, J.; Liu, M.; Li, X.; Zhang, A.; Zhang, X.; Ma, L. Optimization of apparent diffusion coefficient measured by diffusion-weighted MRI for diagnosis of breast lesions presenting as mass and non-mass-like enhancement. Tumor Biol. 2013, 34, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.I.; Ohsumi, S.; Sugata, S.; Kataoka, M.; Takashima, S.; Mochizuki, T.; Ikura, H.; Imai, Y. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat. Med. Med. Imaging Radiat. Oncol. 2008, 26, 222–226. [Google Scholar] [CrossRef]
- Squillaci, E.; Manenti, G.; Cova, M.; Di Roma, M.; Miano, R.; Palmieri, G.; Simonetti, G. Correlation of diffusion-weighted MR imaging with cellularity of renal tumours. Anticancer Res. 2004, 24, 4175–4179. [Google Scholar] [PubMed]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Shigematu, Y.; Hirai, T.; Okuda, T.; Liang, L.; Ge, Y.; Komohara, Y.; et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 1999, 9, 53–60. [Google Scholar] [CrossRef]
- Dunnwald, L.K.; Rossing, M.A.; Li, C.I. Hormone receptor status, tumor characteristics, and prognosis: A prospective cohort of breast cancer patients. Breast Cancer Res. 2007, 9, 1–10. [Google Scholar] [CrossRef]
- Ludovini, V.; Sidoni, A.; Pistola, L.; Bellezza, G.; De Angelis, V.; Gori, S.; Mosconi, A.M.; Bisagni, G.; Cherubini, R.; Bian, A.R.; et al. Evaluation of the prognostic role of vascular endothelial growth factor and microvessel density in stages I and II breast cancer patients. Breast Cancer Res. Treat. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Donegan, W.L. Tumor-related prognostic factors for breast cancer. CA Cancer J. Clin. 1997, 47, 28–51. [Google Scholar] [CrossRef]
- Masters, J.R.W.; Hawkins, R.; Sangster, K.; Hawkins, W.; Smith, I.; Shivas, A.; Roberts, M.; Forrest, A. Oestrogen receptors, cellularity, elastosis and menstrual status in human breast cancer. Eur. J. Cancer (1965) 1978, 14, 303–307. [Google Scholar] [CrossRef]
- Mason, R.C.; Steele, R.J.C.; Hawkins, R.A.; Miller, W.R.; Forrest, A.P.M. Cellularity and the quantitation of estrogen receptors. Breast Cancer Res. Treat. 1982, 2, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Hefti, M.M.; Hu, R.; Knoblauch, N.W.; Collins, L.C.; Haibe-Kains, B.; Tamimi, R.M.; Beck, A.H. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 2013, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siersbæk, R.; Kumar, S.; Carroll, J.S. Signaling pathways and steroid receptors modulating estrogen receptor α function in breast cancer. Genes Dev. 2018, 32, 1141–1154. [Google Scholar] [CrossRef]
- Ménard, S.; Fortis, S.; Castiglioni, F.; Agresti, R.; Balsari, A. HER2 as a Prognostic Factor in Breast Cancer. Oncology 2001, 61 (Suppl. S2), 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rubin, I.; Yarden, Y. The basic biology of HER2. Ann. Oncol. 2001, 12, S3–S8. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Y.; Tang, W.; Luo, J.; Wang, M.; Lin, H.; Guo, H.; Ma, Y.; Zhang, J.; Li, Q. Association between the degree of fibrosis in fibrotic focus and the unfavorable clinicopathological prognostic features of breast cancer. PeerJ 2019, 2019, e8067. [Google Scholar] [CrossRef]
- Ju, Y.J.; Hwan, S.P.; Hee, S.M.; Gyu, S.K.; Lee, S.; Kyu, H.O. Association between lysyl oxidase and fibrotic focus in relation with inflammation in breast cancer. Oncol. Lett. 2018, 15, 2431–2440. [Google Scholar] [CrossRef]
- Mujtaba, S.S.; Ni, Y.-B.; Tsang, J.Y.S.; Chan, S.-K.; Yamaguchi, R.; Tanaka, M.; Tan, P.-H.; Tse, G.M. Fibrotic Focus in Breast Carcinomas: Relationship with Prognostic Parameters and Biomarkers. Ann. Surg. Oncol. 2013, 20, 2842–2849. [Google Scholar] [CrossRef]
- Hasebe, T.; Tsuda, H.; Hirohashi, S.; Shimosato, Y.; Iwai, M.; Imoto, S.; Mukai, K. Fibrotic Focus in Invasive Ductal Carcinoma: An Indicator of High Tumor Aggressiveness. Jpn. J. Cancer Res. 1996, 87, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, T.; Mukai, K.; Tsuda, H.; Ochiai, A. New prognostic histological parameter of invasive ductal carcinoma of the breast: Clinicopathological significance of fibrotic focus. Pathol. Int. 2000, 50, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Shin, J.; Kim, D.-H.; Kim, E.-K.; Moon, H.J.; Yoon, J.H.; You, J.K.; Kim, M.J. Correlation between electrical conductivity and apparent diffusion coefficient in breast cancer: Effect of necrosis on magnetic resonance imaging. Eur. Radiol. 2018, 28, 3204–3214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Moon, W.K.; Cho, N.; Chang, J.M.; Moon, H.-G.; Han, W.; Noh, D.Y.; Lee, J.C.; Kim, H.C.; Lee, K.B.; et al. Shear-Wave Elastographic Features of Breast Cancers: Comparison with Mechanical Elasticity and Histopathologic Characteristics. Investig. Radiol. 2014, 49, 147–155. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed16&NEWS=N&AN=52842387 (accessed on 20 February 2021). [CrossRef]
- Surov, A.; Chang, Y.-W.; Li, L.; Martincich, L.; Partridge, S.C.; Kim, J.Y.; Wienke, A. Apparent diffusion coefficient cannot predict molecular subtype and lymph node metastases in invasive breast cancer: A multicenter analysis. BMC Cancer 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Chang, J.M.; Park, I.A.; Lee, S.H.; Kim, W.H.; Bae, M.S.; Koo, H.R.; Yi, A.; Kim, S.J.; Cho, N.; Moon, W.K. Stiffness of tumours measured by shear-wave elastography correlated with subtypes of breast cancer. Eur. Radiol. 2013, 23, 2450–2458. [Google Scholar] [CrossRef]
- Waage, J.E.R.; Rafaelsen, S.R.; Borley, N.R.; Havre, R.F.; Gubberud, E.T.; Leh, S.; Kolbro, T.; Hagen, K.K.; Eide, G.E.; Pfeffer, F. Strain Elastography Evaluation of Rectal Tumors: Inter- and Intraobserver Reproducibility. Ultraschall Med. 2015, 36, 611–617. [Google Scholar] [CrossRef]


| Estrogen Receptor | Progesterone Receptor | HER2 | Ki-67 Index | |
|---|---|---|---|---|
| Luminal A | + | +/− | − | <14% |
| Luminal B | ||||
| Luminal B (HER2 negative) | + | +/− | − | ≥14% |
| Luminal B (HER2 positive) | + | +/− | + | |
| HER2 + Subtype | − | − | + | |
| Triple-negative Subtype | − | − | − | |
| Histopathological Type of Malignant Lesions | Number of Lesions |
|---|---|
| n | |
| Malignant | 134 |
| Invasive ductal carcinoma | 115 |
| Invasive lobular carcinoma | 13 |
| Ductal carcinoma in situ | 3 |
| Malignant epithelial tumor | 1 |
| Mucinous carcinoma | 1 |
| Malignant phyllodes tumor | 1 |
| Benign | 13 |
| Fibrocystic changes | 4 |
| Inflammatory changes | 1 |
| Ductal hyperplasia | 1 |
| Fat necrosis | 1 |
| Apocrine metaplasia | 1 |
| Phyllodes tumor | 1 |
| Radial scar | 1 |
| Fibroadenoma | 1 |
| Sclerosing adenosis | 1 |
| Fibrotic changes | 1 |
| Characteristics | Number of Lesions n (%) | ADC (Median [IQR], ×10−3 cm2/s) | Elasticity (Median [IQR], kPA) | p Value |
|---|---|---|---|---|
| Estrogen | ||||
| Positive | 108 (81.2%) | 0.84 (0.30) | 135.00 (24.00) | ADC → 0.323 Elasticity → 0.530 |
| Negative | 25 (18.8%) | 0.94 (0.09) | 136.00 (24.50) | |
| Progesterone | ||||
| Positive | 82 (61.65%) | 0.88 (0.19) | 135.33 (28.00) | ADC → 0.211 Elasticity → 0.422 |
| Negative | 41 (38.35%) | 0.94 (0.15) | 136.00 (23.00) | |
| HER2 | ||||
| Positive | 35 (26.31%) | 0.95 (0.17) | 134.00 (29.00) | ADC → 0.051 Elasticity → 0.812 |
| Negative | 98 (73.69%) | 0.88 (0.25) | 130.00 (19.00) | |
| Ki-67 Proliferation Index | ||||
| High | 92 (69.17%) | 0.89 (0.18) | 137.00 (22.50) | ADC → 0.638 Elasticity → 0.240 |
| Low | 41 (30.83%) | 0.91 (0.25) | 131.00 (22.50) |
| Characteristics | Number of Lesions n (%) | ADC (Median ± [IQR], ×10−3 cm2/s) | Elasticity (Median ± [IQR], kPA) | Tumor Size (Median ± [IQR], mm) | Lesion Morphology | |
|---|---|---|---|---|---|---|
| Mass n (%) | Non-Mass n (%) | |||||
| Malignant Lesions | 134 (91.15%) | 0.92 (0.18) | 135.00 (24.45) | 25.00 (22.00) | 117 (87.2%) | 17 (12.8%) |
| Luminal A | 37 (27.61%) | 0.90 (0.29) | 131.00 (22.50) | 20.00 (17.00) | 33 (89.2%) | 4 (10.8%) |
| Luminal B | 69 (51.49%) | 0.91 (0.19) | 135.00 (23.00) | 27.00 (24.50) | 59 (85.5%) | 10 (14.5%) |
| HER2 positive | 12 (8.95%) | 0.93 (0.16) | 142.00 (20.00) | 21.50 (19.00) | 10 (83.3%) | 2 (16.7%) |
| Triple-negative | 15 (11.19%) | 0.95 (0.09) | 136.00 (35.00) | 32.00 (17.00) | 14 (93.3%) | 1 (6.7%) |
| Benign Lesions | 13 (8.84%) | 1.60 (0.55) | 27.00 (105.00) | 20.00 (39.50) | 6 (46.1%) | 7 (53.8%) |
| All lesions | 147 (100%) | 0.93 (0.23) | 134.00 (25.75) | 34.50 (22.00) | 123(83.7%) | 24(16.3%) |
| Lesion Morphology | ADC (Median ± [IQR], ×10−3 cm2/s) | Elasticity (Median ± [IQR], kPa) | p Value |
|---|---|---|---|
| Malignant Lesions | |||
| Mass (n = 117) | 0.94 (0.25) | 126.00 (27.50) | ADC → 0.444 Elasticity → 0.718 |
| Non-mass enhancement (n = 17) | 0.93 (0.28) | 135.00 (22.00) | |
| Benign Lesions | |||
| Mass (n = 6) | 1.60 (0.67) | 30.50 (94.75) | ADC → 0.836 Elasticity → 0.628 |
| Non-mass enhancement (n = 7) | 1.57 (0.81) | 64.50 (129.00) |
| ELASTICITY | |||||||
| Year of Publication | Number of Malignant Lesions | ER and PR Positivity Cut-Off Value | Ki-67 Proliferation Index Cut-Off Value | HER2 Positivity Assessment Method | Elastography Type | Findings | |
| Youk et al. [17] | 2013 | 166 | Method of Quick (Allred) Score (QS) | ≥14% | Score 3 or 2 and HER2 amplification | SWE |
|
| Chang et al. [18] | 2013 | 337 | ≥10% | Not mentioned | Score 3 or 2 and HER2 amplification | SWE |
|
| Choi et al. [19] | 2014 | 122 | Not mentioned | Not mentioned | Not mentioned | SWE |
|
| Ganau et al. [20] | 2015 | 216 | ≥10% | ≥14% | Score 3 or 2 and HER2 amplification | SWE |
|
| Makal et al. [21] | 2021 | 112 lesions (full malign) | ≥1% | ≥14% | Score 3 or 2 and HER2 amplification | SWE |
|
| Our study | 133 | ≥1% | ≥14% | Score 3 or 2 and HER2 amplification | SWE |
| |
| ADC | |||||||
| Year of Publication | Number of Malignant Lesions | ER and PR positivity cut-off value | Ki-67 proliferation index cut-off value | HER2 positivity assessment method | b Value | Findings | |
| Sung et al. [22] | 2009 | 62 | ≥10% | Not mentioned | Score 2 and 3 | 0 and 1000 |
|
| Jeh et al. [23] | 2011 | 107 | ≥10% | ≥15% | Score 2 and 3 | 0, 750 and 1000 |
|
| Choi et al. [24] | 2012 | 335 | ≥10% | ≥20% | Score 2 and 3 | 0 and 1000 |
|
| Moutinho-Guilherme et al. [25] | 2018 | 100 | Method of Quick (Allred) Score (QS) | ≥20% | Score 3 or 2 and HER2 amplification | 0 and 700 |
|
| Tezcan et al. [26] | 2019 | 83 | ≥10% | Not mentioned | Score 3 or 2 and HER2 amplification | 0 and 500 |
|
| Ren et al. [27] | 2019 | 307 | ≥10% | ≥14% | Score 3 or 2 and HER2 amplification | 0 and 1000 |
|
| Surov et al. [65] | 2019 | 661 | Not mentioned | ≥14% | Not mentioned | Different b values |
|
| Linh et al. [29] | 2021 | 49 | ≥1% | ≥14% | Score 3 or 2 and HER2 amplification | 0 and 1000 |
|
| Our study | 133 | ≥1% | ≥14% | Score 3 or 2 and HER2 amplification | 0 and 600 |
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orguc, S.; Açar, Ç.R. Correlation of Shear-Wave Elastography and Apparent Diffusion Coefficient Values in Breast Cancer and Their Relationship with the Prognostic Factors. Diagnostics 2022, 12, 3021. https://doi.org/10.3390/diagnostics12123021
Orguc S, Açar ÇR. Correlation of Shear-Wave Elastography and Apparent Diffusion Coefficient Values in Breast Cancer and Their Relationship with the Prognostic Factors. Diagnostics. 2022; 12(12):3021. https://doi.org/10.3390/diagnostics12123021
Chicago/Turabian StyleOrguc, Sebnem, and Çağdaş Rıza Açar. 2022. "Correlation of Shear-Wave Elastography and Apparent Diffusion Coefficient Values in Breast Cancer and Their Relationship with the Prognostic Factors" Diagnostics 12, no. 12: 3021. https://doi.org/10.3390/diagnostics12123021
APA StyleOrguc, S., & Açar, Ç. R. (2022). Correlation of Shear-Wave Elastography and Apparent Diffusion Coefficient Values in Breast Cancer and Their Relationship with the Prognostic Factors. Diagnostics, 12(12), 3021. https://doi.org/10.3390/diagnostics12123021








