Abstract
There is evidence of an association between hypertension and retinal arteriolar narrowing. Manual measurement of retinal vessels comes with additional variability, which can be eliminated using automated software. This scoping review aims to summarize research on automated retinal vessel analysis systems. Searches were performed on Medline, Scopus, and Cochrane to find studies examining automated systems for the diagnosis of retinal vascular alterations caused by hypertension using the following keywords: diagnosis; diagnostic screening programs; image processing, computer-assisted; artificial intelligence; electronic data processing; hypertensive retinopathy; hypertension; retinal vessels; arteriovenous ratio and retinal image analysis. The searches generated 433 articles. Of these, 25 articles published from 2010 to 2022 were included in the review. The retinographies analyzed were extracted from international databases and real scenarios. Automated systems to detect alterations in the retinal vasculature are being introduced into clinical practice for diagnosis in ophthalmology and other medical specialties due to the association of such changes with various diseases. These systems make the classification of hypertensive retinopathy and cardiovascular risk more reliable. They also make it possible for diagnosis to be performed in primary care, thus optimizing ophthalmological visits.
1. Introduction
Fundus photography, also known as retinography, is a popular imaging technique used to visualize changes in the retinal vessels through the pupil. It can capture changes in vascular caliber and the global geometric patterns of the retina [1]. It is also able to detect signs of retinopathy—such as microaneurysms, hemorrhages, cotton wool spots and hard exudates, and symptoms of the retinal arteriolar wall (e.g., generalized and focal arteriolar narrowing and arteriovenous nicking)—all of which are often observed in patients with systemic diseases, such as diabetes and hypertension.
Regarding diabetes, the 1989 Saint Vincent Declaration set the goal of reducing diabetes-related blindness by one-third over the next five years. This was restated in the 2005 Liverpool Declaration’s objective of establishing systematic screening programs to reach at least 80% of the population with diabetes by 2021 [2]. The increased demand for diabetic retinopathy (DR) screening resulting from systematic programs could be met using automated retinal image analysis systems. Such systems can be used in different DR screening scenarios and offer relatively high sensitivity and a substantial reduction in the workload of the health system. Moreover, they are now mature enough to be safely used in DR screening [3,4]. Automated tools improve the quality of DR screening and accessibility to medical care while reducing the cost of the disease by promoting early detection and treatment, which is essential to stop progression [5].
Regarding hypertension, there is evidence that it is associated with retinal arteriolar narrowing. Retinal vessel diameter is expressed as arteriovenous ratio (AVR). According to the Keith–Wagener–Barker classification, AVR values of less than 0.66 reflect hypertensive retinopathy [6].
Arteriolar narrowing is associated with more severe coronary heart disease, stroke, and mortality [6,7,8,9]. There has also been a recent increase in evidence showing that retinal arteriolar narrowing, retinal venular widening, and a suboptimal retinal vascular network are associated with poorer cognitive performance [10,11,12]. Retinal imaging techniques provide unique information about the state of the microvasculature and neuronal structure, different from current neuroimaging markers, such as brain magnetic resonance imaging (MRI), and systemic markers, such as blood pressure. While retinal imaging cannot fully replace PET scans or MRIs in the diagnosis of disease, it does offer a complementary approach to these brain imaging techniques and has considerable potential in clinical and research settings [13].
For all these reasons, retinal imaging can be used as a risk stratification tool because studies suggest that the addition of retinal measures improves the prediction of stroke (an improvement on established risk factors of approximately 10%) [14,15]. Although just a modest improvement in prediction, these findings suggest that adding a combination of various retinal features and/or retinal functional parameters (i.e., a “multimarker approach”) may further improve the prediction of dementia and stroke. It might also enable the identification of a more specific subgroup of patients who could benefit from more intensive and expensive examinations, such as brain MRI.
Retinal vascular imaging has also been used to examine the effects of antihypertensive therapy, showing that lowering blood pressure leads to the regression of retinal vascular signs [16,17]. While there have been no significant intervention studies using changes in retinal images as alternative outcome measurements in dementia and stroke, this approach has substantial potential. In addition to its clinical value, retinal imaging may also be a worthwhile research tool in major brain and neurological diseases, such as multiple sclerosis [18,19,20], depression [21,22], and schizophrenia [23,24].
Evaluating retinographies manually implies additional variability in retinal vessel measurements, even when following a standardized protocol. This variability is eliminated if fully automated software is used to measure retinal vascular caliber and other anomalies, although there may be other additional sources of variation in the measurements, such as retinal pigmentation, pupil dilation, the presence of cataracts and other media opacities, photographic technique, type of camera (mydriatic/non-mydriatic or desktop/portable), and image quality (brightness, focus, and contrast) [25]. Manually segmenting vessels, labeling arteries and veins, and localizing the optic disc is a time-consuming task that decreases process efficiency. However, over the past two decades, multiple software systems have been developed to measure and semi-automatically assess the retinal vessel caliber from fundus photographs using artificial intelligence (AI) algorithms [26].
Research question: What is the current stage of implementation of automated retinal vessel analysis systems retinographies?
Aim: This scoping review aims to summarize the research available on automated retinal vessel measurement systems so they may be considered in future research and introduced into clinical practice.
2. Materials and Methods
This review followed the PRISMA extension checklist for scoping reviews [27].
2.1. Search Strategy, Data Sources, and Selection
Searches were performed in the Medline, Scopus, and Cochrane electronic databases to locate studies published between 1 january 2004 and 1 september 2022 examining automated systems for the diagnosis of retinal vessel alterations caused by hypertension. The following keywords were used: diagnosis; diagnostic screening programs; image processing, computer-assisted; artificial intelligence; electronic data processing; hypertensive retinopathy; hypertension; retinal vessels; arteriovenous ratio (no MeSH); retinal image analysis (no MeSH).
2.2. Selection Criteria
Articles were included in the review if they met the following inclusion criteria:
- (1)
- Automated systems were used to partially or totally analyze photographic images of the retina.
- (2)
- Changes in the retinal vascular network and/or retinal vascular measurements were analyzed.
- (3)
- The publication was peer-reviewed.
- (4)
- The study was observational, descriptive (population, cross-sectional), analytical (case studies and controls, cohorts), experimental (clinical trials), or a validation of experiments/new image analysis methods.
Only papers written in English were selected. Studies using automated systems for diabetes screening were excluded. Qualitative studies and gray literature were excluded.
2.3. Selection of Studies
Abstracts and articles were independently reviewed by two members of the research team based on predetermined inclusion and exclusion criteria. When it was unclear whether an article should be included or some discrepancy appeared, the coordinating researcher of the study also reviewed it.
2.4. Data Extraction
Initial data extraction elements included: author, country, research aim, study design, study setting, interpretation system, degree of software automation (semi-automatic, automatic), lesions that the system was able to detect, focus of the photograph and area analyzed, sensitivity, specificity, diagnostic precision, economic evaluation, time savings, local management or the possibility of electronically sending the image to a repository, possibility of comparison for patient follow-up.
Data from each article were independently extracted by two of the authors and then verified by two others.
3. Results
3.1. Search Process
The PRISMA flow diagram (Figure 1) describes the steps taken to select the articles [28]. The search strategies generated 433 articles, of which 58 full-length articles were evaluated for eligibility. Of these, 25 articles were included in the scoping review.
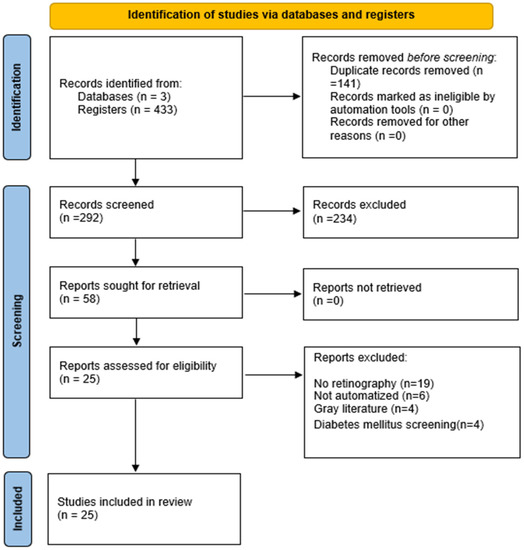
Figure 1.
PRISMA 2020 flow diagram.
3.2. Characteristics of the Articles
The articles included in the review were published between 2010 and 2022, and the number of retinographies analyzed ranged from 20 to 95,716. These retinographies were provided by international databases, as well as real scenarios. Table 1 presents a summary of the characteristics of the studies, including first author, year of publication, the country where the study was conducted, study aim, sample, number of retinographies, name of the software used, degree of automation, and, lastly, the scenario in which it was tested.

Table 1.
Characteristics of the studies.
The median number of retinographies analyzed in the included studies was 180, with a maximum of 54,714 and a minimum of 40. In relation to the degree of automation, in 10 articles they used automatic software and in 15 semi-automated systems.
3.3. Interpretation Procedures
The analysis systems included in these articles detect various alterations of the vessels, including tortuosity, arteriolar and venular caliber, and even AVR calculation. Table 2 shows a description of the retinal lesions detected in each study, the focus of the photograph, and the area of the retina that was analyzed. The focus of the image was mainly on the macula and optic disc, and the analyzed area ranged between 2 to 3 radii from the optic disc.

Table 2.
Lesions detected.
Lastly, the sensitivity, specificity, and diagnostic accuracy were recorded, although this information was only reported in seven articles.
The researchers were initially interested in studying additional data, such as economic evaluation, time savings, local management or the possibility of electronically sending the image to a repository, and the possibility of comparison for patient follow-up. However, the articles selected did not provide this information; therefore, such data were not collected.
3.4. Summary of the Results
Most of the articles reviewed fall into two main categories. The first includes publications dealing with automated or semi-automated systems that measure retinal vessels as a diagnostic method for other pathological processes. Retinal vessel measurement can be useful for diagnosing pathologies related to cardiovascular risk [26,34,41] and hypertension [26,33,34,36], dementia and stroke [35], glaucoma [37], chronic kidney disease [11], glycemic control in children [40], myopia [39], and severity of diabetic macular edema [45].
The second category comprises articles aimed at developing automated retinal vessel measurement systems. These articles cover several levels of development: those that use systems limited to vessel segmentation [46,49,51]; those that include vessel labeling and creation of the vascular tree [31,42,45,47,52]; and those that calculate AVR in order to grade hypertensive retinopathy [43,44,49,53].
4. Discussion
This scoping review aimed to summarize the research available on automated retinal vessel analysis systems in order to determine where automated AVR calculation systems are currently at in terms of implementation. The results indicate that interest in developing technology that facilitates the analysis of the retinal microvascular network has increased over the past eight years. Publications from 2014 and earlier refer to experiments to test the algorithms developed. As of 2015, the software developed from these algorithms has been introduced into clinical practice, demonstrating advantages in real-life scenarios, even though it is not yet widely nor systematically employed. The results of this review confirm that automated AVR calculation systems have not just been introduced as a diagnostic tool for retinal vascular disorders in the field of ophthalmology, but they have been extensively applied in other medical specialties as an accessible and efficient diagnostic tool for other pathologies. Numerous findings indicate that retinal vascular caliber is associated with various systemic diseases, such as hypertension, obesity, diabetes, chronic kidney disease, and stroke. Moreover, since AVR is associated with the development of cardiovascular disease, examining it in this way offers a non-invasive view into the systemic microvasculature.
Hypertensive retinopathy is an indicator of damage to other target organs. However, it is difficult for ophthalmologists to study hypertensive retinopathy in the early stages. Therefore, further research should be conducted on computer-assisted diagnoses that use AVR calculation to automatically detect hypertensive retinopathy and grade it in its early stages [54].
The automation of processes is a prerequisite to improving the affordability, efficiency, and accessibility of these procedures [55] and reducing the high subjectivity of manually assessing AVR [56]. Deep learning methods arise to compute AVR. Convolutional Neural Networks (CNN, Atlanta, GA, USA) obtain a good approximation of AVR value by applying a sequence of spatial filters, subsampling, and non-linear operations.
The clinical applications of artificial intelligence in automated AVR reading could cover a wide range of tasks, including automating hypertensive retinopathy screening, supporting treatment decision-making, assessing systemic vascular status and cardiovascular mortality [57], prescribing medications and diagnostic tests, and creating prognostic models of different diseases [58] to provide more efficient, precise, and sensitive methods in the interpretation of clinical data.
Nonetheless, using artificial intelligence to analyze retinal microvasculature does present some limitations. Firstly, the images used to validate and train the AI may not have enough ethnic variability to provide high external validity because there must be enough, but not too many, images for the processes to be efficient. Secondly, the data should be restricted to those criteria of greatest prognostic relevance, maintaining maximum diagnostic accuracy and minimum algorithm complexity. Consequently, if these processes were automated, they could be implemented in primary care for use by trained health professionals and in rural settings, thus facilitating the classification of cardiovascular risk and reducing the need to refer cases for evaluation by an ophthalmologist. Overall, this would result in the optimization of available health resources.
5. Conclusions
In recent years, there has been increased interest in developing technology that facilitates the analysis of the retinal microvascular network. Software has been developed and is being introduced into clinical practice not just as a diagnostic tool in the field of ophthalmology, but also in other medical specialties, because there is an established association between various diseases and retinal vessel alterations.
If automated processes for retinal vessel measurement were implemented in primary care for use by trained health professionals, fewer cases would need evaluation by an ophthalmologist, thus optimizing the available health resources.
Moreover, such processes improve the reliability of vasculature measurements, which, in turn, leads to better classification of hypertensive retinopathy by eliminating observer subjectivity and taking cardiovascular risk into account. Also, the more reliable the measurements, the better the early diagnosis of other pathologies, such as dementia and stroke. Further research on the evaluation and implementation of these technologies is needed to recommend their use.
Author Contributions
Conceptualization, P.T.-M. and V.M.L.-L.; methodology, R.G.-S.; formal analysis, R.G.-S.; A.H.; E.I.G.; and V.M.L.-L.; investigation, D.V.L.; M.T.A.; R.F.; P.M.-A.; I.B.; and J.U.A.; writing—original draft preparation, R.G.-S. and V.M.L.-L.; writing—review and editing, R.G.-S. and A.H.; supervision, P.T.-M.; project administration, R.G.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheung, C.Y.; Ikram, M.K.; Sabanayagam, C.; Wong, T.Y. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 2012, 60, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Screening Programmes by Geography—Retina International’s Diabetic Eye Disease Toolkit. Available online: http://ded.retinaint.org/screening-innovation-and-clinical-trials/screening-programs-by-geography/ (accessed on 19 May 2021).
- Nørgaard, M.F.; Grauslund, J. Automated screening for diabetic retinopathy—A systematic review. Ophthalmic. Res. 2018, 60, 9–17. [Google Scholar] [CrossRef]
- Nielsen, K.B.; Lautrup, M.L.; Andersen, J.K.H.; Savarimuthu, T.R.; Grauslund, J. Deep Learning—Based Algorithms in Screening of Diabetic Retinopathy: A Systematic Review of Diagnostic Performance. Ophthalmol. Retin. 2019, 3, 294–304. [Google Scholar] [CrossRef]
- Islam, M.M.; Yang, H.C.; Poly, T.N.; Jian, W.S.; Li, Y.C. Deep learning algorithms for detection of diabetic retinopathy in retinal fundus photographs: A systematic review and meta-analysis. Comput. Methods Programs Biomed. 2020, 191, 105320. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Mittal, D. Measurement of Arterio-Venous Ratio for Detection of Hypertensive Retinopathy through Digital Color Fundus Images. J. Biomed. Eng. Med. Imaging 2015, 2, 35. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Tay, W.T.; Mitchell, P.; Wang, J.J.; Hsu, W.; Lee, M.L.; Lau, Q.P.; Zhu, A.L.; Klein, R.; Saw, S.M.; et al. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J. Hypertens. 2011, 29, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Bravo, P.E.; Gupta, A.; Farhad, H.; Klein, B.E.; Klein, R.; Di Carli, M.; Solomon, S.D. Retinal Vessel Calibers in Predicting Long-Term Cardiovascular Outcomes: The Atherosclerosis Risk in Communities Study. Circulation 2016, 134, 1328–1338. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Ong, Y.T.; Hilal, S.; Cheung, C.Y.; Xu, X.; Chen, C.; Venketasubramanian, N.; Wong, T.Y.; Ikram, M.K. Retinal Vascular Fractals and Cognitive Impairment. Dement. Geriatr. Cogn. Dis. Extra 2014, 4, 305–313. [Google Scholar] [CrossRef]
- Yip, W.; Ong, P.G.; Teo, B.W.; Cheung, C.Y.-L.; Tai, E.S.; Cheng, C.-Y.; Lamoureux, E.; Wong, T.Y.; Sabanayagam, C. Retinal vascular imaging markers and incident chronic kidney disease: A prospective cohort study. Sci. Rep. 2017, 7, 9374. [Google Scholar] [CrossRef]
- Taylor, A.M.; MacGillivray, T.J.; Henderson, R.D.; Ilzina, L.; Dhillon, B.; Starr, J.M.; Deary, I.J. Retinal vascular fractal dimension, childhood IQ, and cognitive ability in old age: The lothian birth cohort study 1936. PLoS ONE 2015, 10, e0121119. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Ikram, M.K.; Chen, C.; Wong, T.Y. Imaging retina to study dementia and stroke. Prog. Retin. Eye Res. 2017, 57, 89–107. [Google Scholar] [CrossRef] [PubMed]
- McGeechan, K.; Liew, G.; MacAskill, P.; Irwig, L.; Klein, R.; Klein, B.E.; Wang, J.J.; Mitchell, P.; Vingerling, J.R.; de Jong, P.T.; et al. Prediction of incident stroke events based on retinal vessel caliber: A systematic review and individual-participant meta-analysis. Am. J. Epidemiol. 2009, 170, 1323–1332. [Google Scholar] [CrossRef]
- Forés, R.; Manresa, J.M.; López-Lifante, V.M.; Heras, A.; Delgado, P.; Vázquez, X.; Ruiz, S.; Alzamora, M.T. Relationship between retinal microvasculature, cardiovascular risk and silent brain infarction in hypertensive patients. Diagnostics 2021, 11, 937. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.; Stettler, C.; Stanton, A.; Witt, N.; Tapp, R.; Chaturvedi, N.; Allemann, S.; Mayet, J.; Sever, P.; Poulter, N.; et al. Differential effects of antihypertensive treatment on the retinal microcirculation: An anglo-scandinavian cardiac outcomes trial substudy. Hypertension 2009, 54, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.M.; Storey, E.; Wong, T.Y.; Woods, R.; Tonkin, A.; Wang, J.J.; Kam, A.; Janke, A.; Essex, R.; Abhayaratna, W.; et al. Aspirin for the prevention of cognitive decline in the elderly: Rationale and design of a neuro-vascular imaging study (ENVIS-ion). BMC Neurol. 2012, 12, 3. [Google Scholar] [CrossRef]
- Bhaduri, B.; Nolan, R.M.; Shelton, R.L.; Pilutti, L.A.; Motl, R.W.; Moss, H.E.; Pula, J.H.; Boppart, S.A. Detection of retinal blood vessel changes in multiple sclerosis with optical coherence tomography. Biomed. Opt. Express 2016, 7, 2321. [Google Scholar] [CrossRef]
- Petzold, A.; de Boer, J.F.; Schippling, S.; Vermersch, P.; Kardon, R.; Green, A.; Calabresi, P.A.; Polman, C. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 921–932. [Google Scholar] [CrossRef]
- Saidha, S.; Sotirchos, E.S.; Ibrahim, M.A.; Crainiceanu, C.M.; Gelfand, J.M.; Sepah, Y.J.; Ratchford, J.N.; Oh, J.; Seigo, M.A.; Newsome, S.D.; et al. Microcystic macular oedema, Thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: A retrospective study. Lancet Neurol. 2012, 11, 963–972. [Google Scholar] [CrossRef]
- Meier, M.H.; Gillespie, N.A.; Hansell, N.K.; Hewitt, A.W.; Hickie, I.B.; Lu, Y.; MacGregor, S.; Medland, S.E.; Sun, C.; Wong, T.Y.; et al. Associations between depression and anxiety symptoms and retinal vessel caliber in adolescents and young adults. Psychosom. Med. 2014, 76, 732–738. [Google Scholar] [CrossRef]
- Yildiz, M.; Alim, S.; Batmaz, S.; Demir, S.; Songur, E.; Ortak, H.; Demirci, K. Duration of the depressive episode is correlated with ganglion cell inner plexifrom layer and nasal retinal fiber layer thicknesses: Optical coherence tomography findings in major depression. Psychiatry Res.-Neuroimaging 2016, 251, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Kalenderoglu, A.; Sevgi Karadag, A.; Bekir Egilmez, O.; Han-Almis, B.; Şimşek, A. Decreases in ganglion cell layer and inner plexiform layer volumes correlate better with disease severity in schizophrenia patients than retinal nerve fiber layer thickness: Findings from spectral optic coherence tomography. Eur. Psychiatry 2016, 32, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.H.; Shalev, I.; Moffitt, T.E.; Kapur, S.; Keefe, R.S.; Wong, T.Y.; Belsky, D.W.; Harrington, H.; Hogan, S.; Houts, R.; et al. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am. J. Psychiatry 2013, 170, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, A.; Liew, G.; Burlutsky, G.; Rochtchina, E.; Zhang, Y.P.; Hsu, W.; Lee, J.M.; Wong, T.Y.; Mitchell, P.; Wang, J.J. Effect of image quality, color, and format on the measurement of retinal vascular fractal dimension. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5525–5529. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo-Fernandez, J.A.; Garcia-Garcia, A.; Chamoso, P.; Recio-Rodríguez, J.I.; Rodríguez-González, S.; Patino-Alonso, M.C.; Rodriguez-Sanchez, E.; Corchado-Rodríguez, J.M.; Gómez-Marcos, M.A.; Garcia-Ortiz, L. Automatic image analyser to assess retinal vessel calibre (ALTAIR). A new tool to evaluate the thickness, area and length of the vessels of the retina. Int. J. Med. Inform. 2020, 136, 104090. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 89. [Google Scholar] [CrossRef]
- Badawi, S.A.; Fraz, M.M.; Shehzad, M.; Mahmood, I.; Javed, S.; Mosalam, E.; Nileshwar, A.K. Detection and Grading of Hypertensive Retinopathy Using Vessels Tortuosity and Arteriovenous Ratio. J. Digit. Imaging 2022, 35, 281–301. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, H.; Zhang, S.; Zhong, X.; Lin, Y.; Xiong, Z.; Liu, M.; Yimamu, A.; Christopher, O.; Zhou, Z.; et al. Mid- to Late-Life Time-Averaged Cumulative Blood Pressure and Late-Life Retinal Microvasculature: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e25226. [Google Scholar] [CrossRef]
- Irshad, S.; Yin, X.; Zhang, Y. A new approach for retinal vessel differentiation using binary particle swarm optimization. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2021, 9, 510–522. [Google Scholar] [CrossRef]
- Dai, G.; He, W.; Xu, L.; Pazo, E.E.; Lin, T.; Liu, S.; Zhang, C. Exploring the effect of hypertension on retinal microvasculature using deep learning on East Asian population. PLoS ONE 2020, 15, e0230111. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.; Fleming, A.; Williams, M.C.; Trucco, E.; Quinn, N.; Hogg, R.; McKay, G.J.; Kee, F.; Young, I.; Pellegrini, E.; et al. Association between hypertension and retinal vascular features in ultra-widefield fundus imaging. Open Hear. 2020, 7, e001124. [Google Scholar] [CrossRef] [PubMed]
- Tapp, R.J.; Owen, C.G.; Barman, S.A.; Welikala, R.A.; Foster, P.J.; Whincup, P.H.; Strachan, D.P.; Rudnicka, A.R.; UK Biobank Eye and Vision Consortium. Associations of retinal microvascular diameters and tortuosity with blood pressure and arterial stiffness United Kingdom biobank. Hypertension 2019, 74, 1383–1390. [Google Scholar] [CrossRef]
- Lau, A.Y.; Mok, V.; Lee, J.; Fan, Y.; Zeng, J.; Lam, B.; Wong, A.; Kwok, C.; Lai, M.; Zee, B. Retinal image analytics detects white matter hyperintensities in healthy adults. Ann. Clin. Transl. Neurol. 2019, 6, 98–105. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, S.M.; Kang, M.T.; Liu, L.R.; Li, H.; Wei, S.F.; Ran, A.R.; Wang, N. Anyang Childhood Eye Study Group. Association between blood pressure and retinal arteriolar and venular diameters in Chinese early adolescent children, and whether the association has gender difference: A cross-sectional study. BMC Ophthalmol. 2018, 18, 133. [Google Scholar] [CrossRef]
- Adiarti, R.; Ekantini, R.; Agni, A.N.; Wong, T.Y.; Sasongko, M.B. Retinal Arteriolar Narrowing in Young Adults with Glaucomatous Optic Disc. J. Glaucoma 2018, 27, 699–702. [Google Scholar] [CrossRef]
- Akbar, S.; Akram, M.U.; Sharif, M.; Tariq, A.; Yasin, U. Arteriovenous ratio and papilledema based hybrid decision support system for detection and grading of hypertensive retinopathy. Comput. Methods Programs Biomed. 2018, 154, 123–141. [Google Scholar] [CrossRef]
- Iwase, A.; Sekine, A.; Suehiro, J.; Tanaka, K.; Kawasaki, Y.; Kawasaki, R.; Sinai, M.J.; Araie, M. A new method of magnification correction for accurately measuring retinal vessel calibers from fundus photographs. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1858–1864. [Google Scholar] [CrossRef]
- Li, L.J.; Lamoureux, E.; Wong, T.Y.; Lek, N. Short-term poor glycemic control and retinal microvascular changes in pediatric Type 1 Diabetes patients in Singapore: A pilot study. BMC Ophthalmol. 2017, 17, 60. [Google Scholar] [CrossRef]
- Vázquez Dorrego, X.M.; Manresa Domínguez, J.M.; Heras Tebar, A.; Forés, R.; Girona Marcé, A.; Alzamora Sas, M.T.; Delgado Martínez, P.; Riba-Llena, I.; Ugarte Anduaga, J.; Beristain Iraolai, A.; et al. Semi-automatic measuring of arteriovenous relation as a possible silent brain infarction risk index in hypertensive patients. Arch. Soc. Española Oftalmol. 2016, 91, 513–519. [Google Scholar] [CrossRef]
- Cavallari, M.; Stamil, C.; Umeton, R.; Calimeri, F.; Orzi, F. Novel Method for Automated Analysis of Retinal Images: Results in Subjects with Hypertensive Retinopathy and CADASIL. BioMed Res. Int. 2015, 2015, 752957. [Google Scholar] [CrossRef] [PubMed]
- Fraz, M.M.; Welikala, R.A.; Rudnicka, A.R.; Owen, C.G.; Strachan, D.P.; Barman, S.A. QUARTZ: Quantitative analysis of retinal vessel topology and size - An automated system for quantification of retinal vessels morphology. Expert Syst. Appl. 2015, 42, 7221–7234. [Google Scholar] [CrossRef]
- Estrada, R.; Allingham, M.J.; Mettu, P.S.; Cousins, S.W.; Tomasi, C.; Farsiu, S. Retinal Artery-Vein Classification via Topology Estimation. IEEE Trans. Med. Imaging 2015, 34, 2518–2534. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Sepah, Y.J.; Ibrahim, M.A.; Sophie, R.; Moazez, C.; Bittencourt, M.G.; Annam, R.E.; Hanout, M.; Liu, H.; Ferraz, D.; et al. Association of retinal vessel calibre and visual outcome in eyes with diabetic macular oedema treated with ranibizumab. Eye 2014, 28, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.W.; Rajan, S.E. Retinal vessel segmentation employing ANN technique by Gabor and moment invariants-based features. Appl. Soft Comput. J. 2014, 22, 94–100. [Google Scholar] [CrossRef]
- Dashtbozorg, B.; Mendonca, A.M.; Campilho, A. An automatic graph-based approach for artery/Vein classification in retinal images. IEEE Trans. Image Process. 2014, 23, 1073–1083. [Google Scholar] [CrossRef]
- Vázquez, S.G.; Barreira, N.; Penedo, M.G.; Rodríguez-Blanco, M. Reliable monitoring system for arteriovenous ratio computation. Comput. Med. Imaging Graph. 2013, 37, 337–345. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Huang, Y. An automated computational framework for retinal vascular network labeling and branching order analysis. Microvasc. Res. 2012, 84, 169–177. [Google Scholar] [CrossRef]
- Ortega, M.; Barreira, N.; Novo, J.; Penedo, M.G.; Pose-Reino, A.; Gómez-Ulla, F. Sirius: A web-based system for retinal image analysis. Int. J. Med. Inform. 2010, 79, 722–732. [Google Scholar] [CrossRef]
- Villalobos-Castaldi, F.M.; Felipe-Riverón, E.M.; Sánchez-Fernández, L.P. A fast, efficient and automated method to extract vessels from fundus images. J. Vis. 2010, 13, 263–270. [Google Scholar] [CrossRef]
- Vázquez, S.G.; Cancela, B.; Barreira, N.; Penedo, M.G.; Rodríguez-Blanco, M.; Seijo, M.P.; de Tuero, G.C.; Barceló, M.A.; Saez, M. Improving retinal artery and vein classification by means of a minimal path approach. Mach. Vis. Appl. 2013, 24, 919–930. [Google Scholar] [CrossRef]
- Akbar, S.; Akram, M.U.; Sharif, M.; Tariq, A.; Khan, S.A. Decision support system for detection of hypertensive retinopathy using arteriovenous ratio. Artif. Intell. Med. 2018, 90, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Lou, Y.; Erginay, A.; Clarida, W.; Amelon, R.; Folk, J.C.; Niemeijer, M. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5200–5206. [Google Scholar] [CrossRef]
- Manresa, J.M.; Forés, R.; Vázquez, X.; Alzamora, M.T.; Heras, A.; Delgado, P.; Torán-Montserrat, P. Reliability of retinography for the detection of hypertensive retinopathy in Primary Care. Aten. Primaria 2020, 52, 410–417. [Google Scholar] [CrossRef]
- Arnould, L.; Binquet, C.; Guenancia, C.; Alassane, S.; Kawasaki, R.; Daien, V.; Tzourio, C.; Kawasaki, Y.; Bourredjem, A.; Bron, A.; et al. Association between the retinal vascular network with Singapore ‘i’ Vessel Assessment (SIVA) software, cardiovascular history and risk factors in the elderly: The Montrachet study, population-based study. PLoS ONE 2018, 13, e0194694. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunović, H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).