Contrast-Enhanced Ultrasonography with Arrival Time Parametric Imaging as a Non-Invasive Diagnostic Tool for Liver Cirrhosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethical Consideration
2.3. Contrast-Enhanced Ultrasonography with Arrival Time Parametric Imaging
2.4. Vibration-Controlled Transient Elastography (VTCE) and Controlled Attenuation Parameter (CAP) Measurements
2.5. Surrogate Serum Fibrosis Markers
2.6. Clinical and Biological Assessment
2.7. Statistical Analysis
2.7.1. Sample Size Calculation
2.7.2. Analysis of Data
2.7.3. Bootstrap Resampling, Sensitivity Analysis
3. Results
3.1. Clinical and Biochemical Characteristics
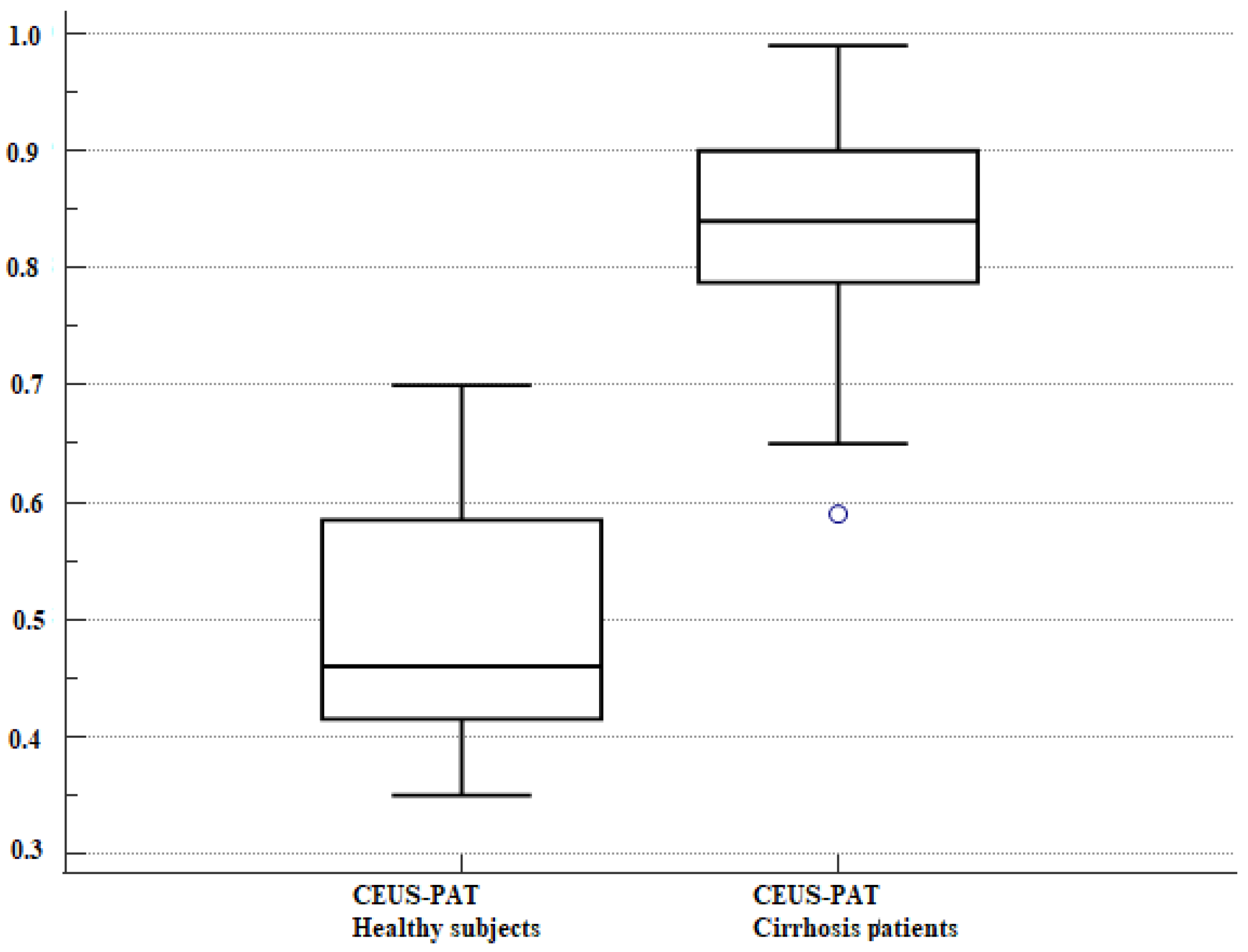
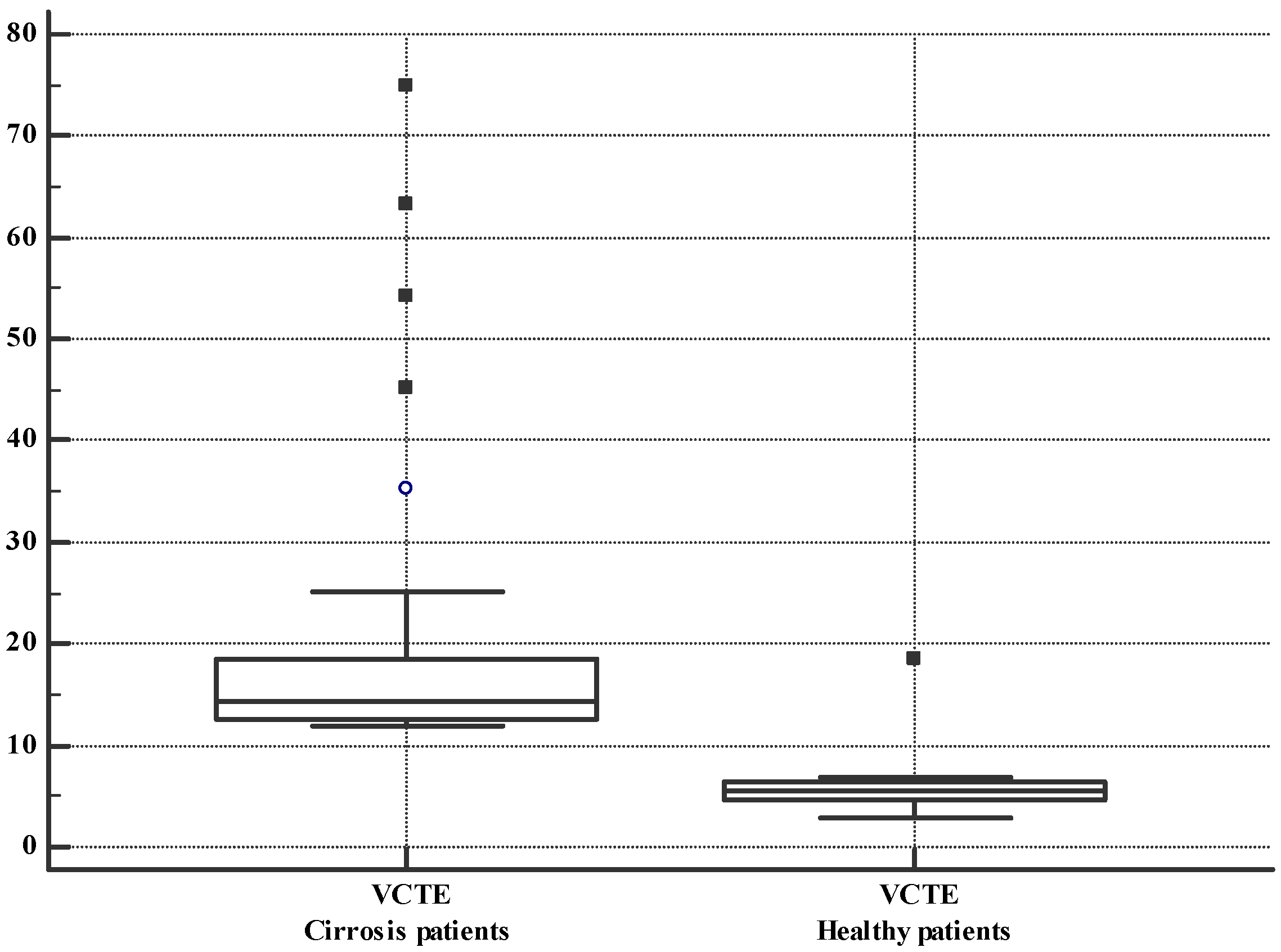
3.2. VCTE and CEUS-PAT Evaluation
3.3. Performance of CEUS-PAT in Detecting Liver Cirrhosis
3.4. Comparison between VCTE, CEUS-PAT, FIB-4 Score, and APRI Score in Diagnosing Liver Cirrhosis
3.5. Univariate and Multivariate Analysis of CEUS-PAT
3.6. Bootstrapping for Sensitivity Analysis
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khalifa, A.; Rockey, D.C. The utility of liver biopsy in 2020. Curr. Opin. Gastroenterol. 2020, 36, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T. Faut-il encore faire des biopsies du foie? [Is liver biopsy still useful?]. Rev. Med. Interne 2007, 28, 67–70. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall. Med. 2017, 38, e16–e47, Erratum in Ultraschall Med. 2017, 38, e48. [Google Scholar]
- Giuffrè, M.; Fouraki, S.; Comar, M.; Masutti, F.; Crocè, L.S. The Importance of Transaminases Flare in Liver Elastography: Characterization of the Probability of Liver Fibrosis Overestimation by Hepatitis C Virus-Induced Cytolysis. Microorganisms 2020, 8, 348. [Google Scholar] [CrossRef]
- Giuffrè, M.; Giuricin, M.; Bonazza, D.; Rosso, N.; Giraudi, P.J.; Masutti, F.; Palmucci, S.; Basile, A.; Zanconati, F.; de Manzini, N.; et al. Optimization of Point-Shear Wave Elastography by Skin-to-Liver Distance to Assess Liver Fibrosis in Patients Undergoing Bariatric Surgery. Diagnostics 2020, 10, 795. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.H.; Xia, G.C.; Lei, C.G. Quantitative diagnosis of early-stage liver cirrhosis with contrast-enhanced ultrasound--a clinical study. Adv. Clin. Exp. Med. 2012, 21, 385–390. [Google Scholar] [PubMed]
- Knottnerus, J.A.; Muris, J.W. Assessment of the accuracy of diagnostic tests: The cross-sectional study. J. Clin. Epidemiol. 2003, 56, 1118–1128. [Google Scholar] [CrossRef]
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Med Ultrason 2020; 22, 13-19 19 Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new non-invasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Foucher, J.; Bernard, P.H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Gurusamy, K.S.; Ntaoula, S.; Cholongitas, E.; Davidson, B.R.; Burroughs, A.K. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 2011, 54, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Strobel, D.; Krodel, U.; Martus, P.; Hahn, E.G.; Becker, D. Clinical evaluation of contrast-enhanced color Doppler sonography in the differential diagnosis of liver tumors. J. Clin. Ultrasound 2000, 28, 1–13. [Google Scholar] [CrossRef]
- Kim, G.; Shim, K.Y.; Baik, S.K. Diagnostic Accuracy of Hepatic Vein Arrival Time Performed with Contrast-Enhanced Ultrasonography for Cirrhosis: A Systematic Review and Meta-Analysis. Gut Liver 2017, 11, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Flahault, A.; Cadilhac, M.; Thomas, G. Sample size calculation should be performed for design accuracy in diagnostic test studies. J. Clin. Epidemiol. 2005, 58, 859–862. [Google Scholar] [CrossRef]
- Fieberg, J.R.; Vitense, K.; Johnson, D.H. Resampling-based methods for biologists. PeerJ 2020, 8, e9089. [Google Scholar] [CrossRef]
- Rutter, C.M. Bootstrap estimation of diagnostic accuracy with patient-clustered data. Acad. Radiol. 2000, 7, 413–419. [Google Scholar] [CrossRef]
- Obuchowski, N.A.; Lieber, M.L. Confidence intervals for the receiver operating characteristic area in studies with small samples. Acad. Radiol. 1998, 5, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Cortese, G.; Baumgartner, R. A comparison of confidence/credible interval methods for the area under the ROC curve for continuous diagnostic tests with small sample size. Stat. Methods Med. Res. 2017, 26, 2603–2621. [Google Scholar] [CrossRef] [PubMed]
- Moga, T.V.; David, C.; Popescu, A.; Lupusoru, R.; Heredea, D.; Ghiuchici, A.M.; Foncea, C.; Burdan, A.; Sirli, R.; Danilă, M.; et al. Multiparametric Ultrasound Approach Using a Tree-Based Decision Classifier for Inconclusive Focal Liver Lesions Evaluated by Contrast Enhanced Ultrasound. J. Pers. Med. 2021, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Albrecht, T.; Blomley, M.J.; Cosgrove, D.O.; Taylor-Robinson, S.D.; Jayaram, V.; Eckersley, R.; Urbank, A.; Butler-Barnes, J.; Patel, N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet 1999, 353, 1579–1583. [Google Scholar] [CrossRef]
- Ridolfi, F.; Abbattista, T.; Marini, F.; Vedovelli, A.; Quagliarini, P.; Busilacchi, P.; Brunelli, E. Contrast-enhanced ultrasound to evaluate the severity of chronic hepatitis C. Dig. Liver Dis. 2007, 39, 929–935. [Google Scholar] [CrossRef]
- Fujita, Y.; Watanabe, M.; Sasao, K.; Wakui, N.; Shinohara, M.; Ishii, K.; Sumino, Y. Investigation of liver parenchymal flow using contrast-enhanced ultrasound in patients with alcoholic liver disease. Alcohol. Clin. Exp. Res. 2004, 28 (Suppl. S8), 169S–173S. [Google Scholar] [CrossRef]
- Wakui, N.; Takayama, R.; Kanekawa, T.; Ichimori, M.; Otsuka, T.; Shinohara, M.; Ishii, K.; Kamiyama, N.; Sumino, Y. Usefulness of arrival time parametric imaging in evaluating the degree of liver disease progression in chronic hepatitis C infection. J. Ultrasound Med. 2012, 31, 373–382. [Google Scholar] [CrossRef]
- Cocciolillo, S.; Parruti, G.; Marzio, L. CEUS and Fibroscan in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. World J. Hepatol. 2014, 6, 496–503. [Google Scholar] [CrossRef]
- Nasr, P.; Hilliges, A.; Thorelius, L.; Kechagias, S.; Ekstedt, M. Contrast-enhanced ultrasonography could be a non-invasive method for differentiating none or mild from severe fibrosis in patients with biopsy proven non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2016, 51, 1126–1132. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, X.; Liu, D.; Qian, L.; Hu, X. Contrast-Enhanced Ultrasonography Diagnostic Evaluation of Esophageal Varices in Patients With Cirrhosis. Ultrasound Q. 2016, 32, 136–143. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.C.; Peng, X.Y.; Wu, X.W.; Du, T.T.; Wang, J.J.; Tian, S.X.; Lu, G.L. Usefulness of Contrast-Enhanced Ultrasonography for Predicting Esophageal Varices in Patients with Hepatitis B Virus (HBV)-Related Cirrhosis. Med. Sci. Monit. 2017, 23, 2241–2249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, T.; Wang, H.; Song, J.; Ling, W.; Shi, Y.; Guo, G.; Luo, Y. Assessment of liver fibrosis by ultrasound elastography and contrast-enhanced ultrasound: A randomised prospective animal study. Exp. Anim. 2018, 67, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Leung, G.; Lau, T.Y.; Tipoe, G.L.; Lee, E.S.; Yuen, Q.W.; Huang, Y.P.; Zheng, Y.P. Evaluation of liver fibrosis by investigation of hepatic parenchymal perfusion using contrast-enhanced ultrasound: An animal study. J. Clin. Ultrasound 2012, 40, 462–470. [Google Scholar] [CrossRef]
- Kayali, S.; Pasta, A.; Pellicano, R.; Fagoonee, S.; Giuliana, E.; Facchini, C.; Pili, S.; Buccilli, S.; Labanca, S.; Borro, P. Effect of contrast-enhanced ultrasound (CEUS) on liver stiffness measurements obtained by transient and shear-wave elastography. Panminerva Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yoshimine, N.; Wakui, N.; Nagai, H.; Igarashi, Y. Arrival-Time Parametric Imaging in Contrast-Enhanced Ultrasound for Diagnosing Fibrosis in Primary Biliary Cholangitis. Ultrasound Q. 2022, 38, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bâldea, V.; Sporea, I.; Lupușoru, R.; Bende, F.; Mare, R.; Popescu, A.; Șirli, R. Comparative Study Between the Diagnostic Performance of Point and 2-D Shear-Wave Elastography for the Non-invasive Assessment of Liver Fibrosis in Patients with Chronic Hepatitis C Using Transient Elastography as Reference. Ultrasound Med. Biol. 2020, 46, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Șirli, R.; Popescu, A.; Bâldea, V.; Lupușoru, R.; Bende, F.; Cotrău, R.; Sporea, I. Ultrasound-Based Quantification of Fibrosis and Steatosis with a New Software Considering Transient Elastography as Reference in Patients with Chronic Liver Diseases. Ultrasound Med. Biol. 2021, 47, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Bende, F.; Sporea, I.; Şirli, R.; Nistorescu, S.; Fofiu, R.; Bâldea, V.; Popescu, A. The Performance of a 2-Dimensional Shear-Wave Elastography Technique for Predicting Different Stages of Liver Fibrosis Using Transient Elastography as the Control Method. Ultrasound Q. 2020, 37, 97–104. [Google Scholar] [CrossRef]
- Sporea, I.; Badea, R.; Brisc, C.; Ioanițescu, S.; Moga, T.; Popescu, A.; Săftoiu, A.; Săndulescu, L.; Spârchez, Z.; Șirli, R. Romanian National Guidelines on Contrast Enhanced Ultrasound in clinical practice. Med. Ultrason. 2017, 19, 401–415. [Google Scholar] [CrossRef]
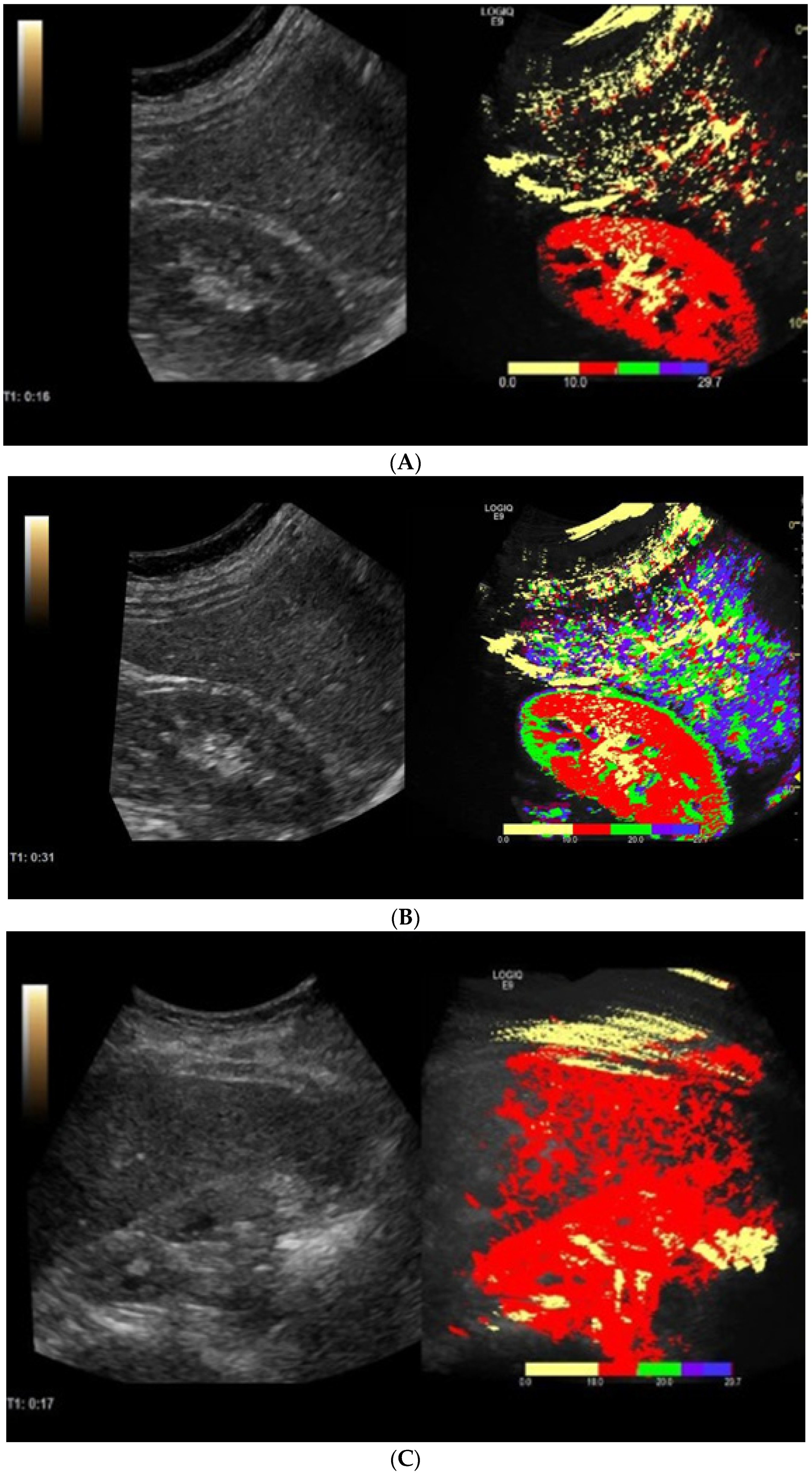


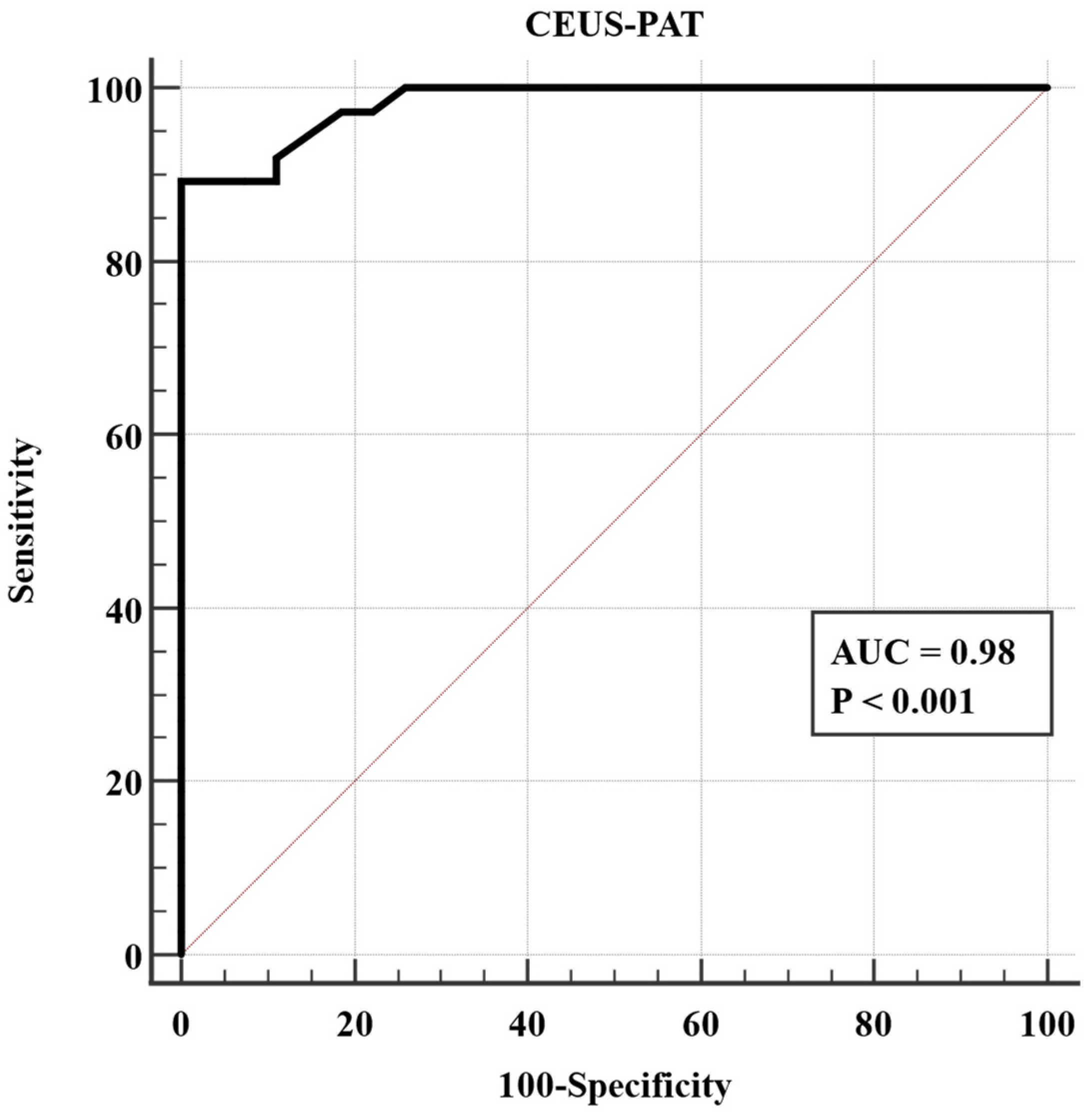
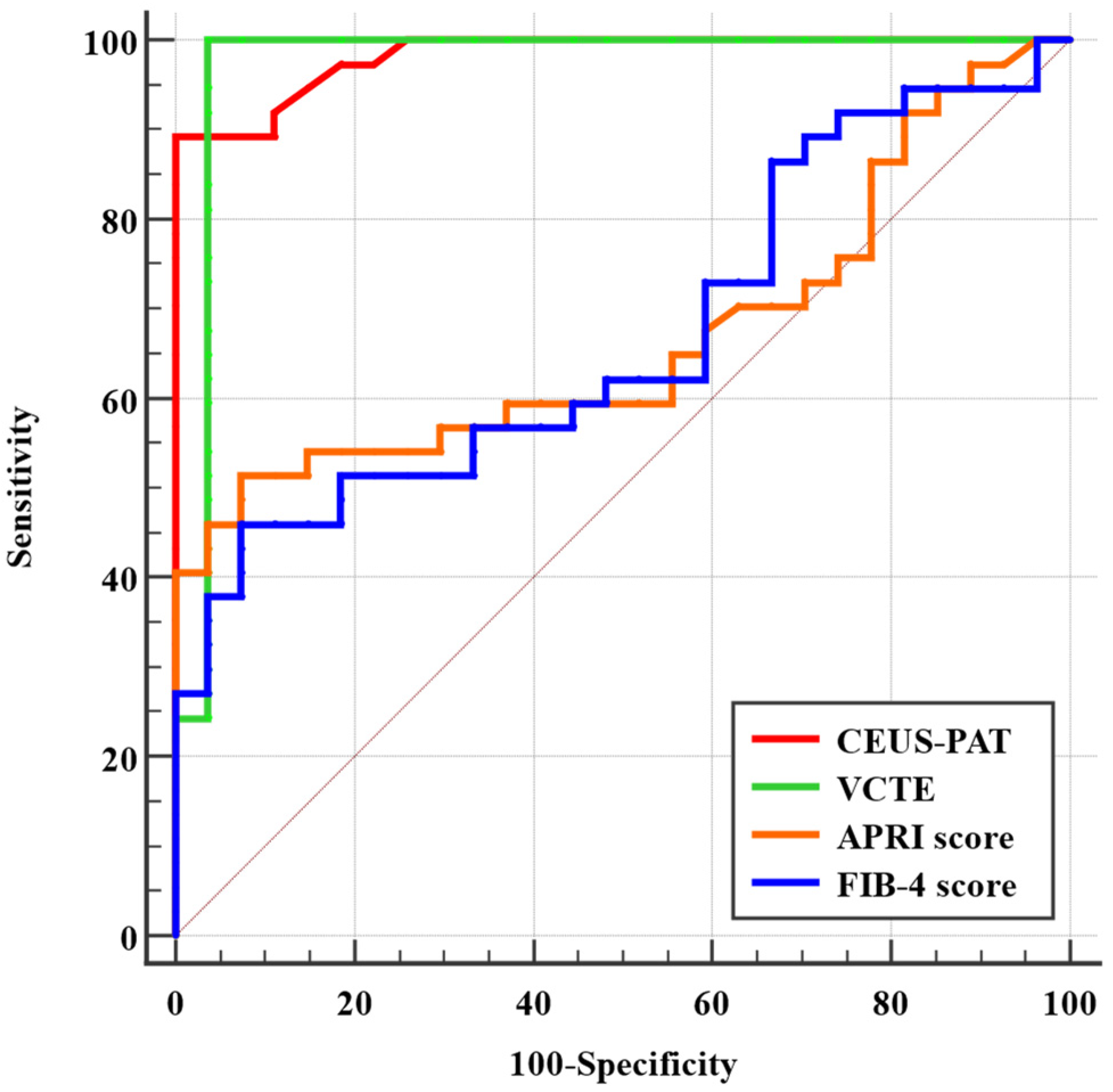
| Parameter | Healthy Volunteers (n = 27) | Liver Cirrhosis Patients (n = 37) | p-Value |
|---|---|---|---|
| Age (years) | 58.00 ± 8.94 | 59.70 ± 8.93 | 0.45 |
| Gender Male Female | 17 (62.9%) 10 (37.1%) | 20 (54.0%) 17 (46.0%) | 0.68 |
| BMI (kg/m2) | 25.47 ± 2.82 | 26.38 ± 5.29 | 0.37 |
| Haemoglobin (g/dL) | 13.46 ± 1.76 | 12.41 ± 1.94 | 0.02 |
| Haematocrit (%) | 37.39 ± 7.55 | 37.42 ± 6.94 | 0.98 |
| Leucocytes (*109/L) | 7.96 ± 1.50 | 7.91 ± 2.06 | 0.90 |
| Thrombocytes (103/L) | 208.77 ± 55.72 | 177.75 ± 97.92 | 0.11 |
| Albumin (mg/dL) | 4.05 ± 0.60 | 2.91 ± 0.91 | <0.0001 |
| Natrium (mmol/L) | 138.40 ± 3.80 | 135.24 ± 5.16 | 0.006 |
| Potassium (mmol/L) | 4.40 ± 0.53 | 4.52 ± 0.57 | 0.38 |
| Urea (mg/dL) | 29.33 ± 7.08 | 37.43 ± 18.22 | 0.01 |
| Creatinine (mg/dL) | 0.97 ± 0.19 | 0.99 ± 0.28 | 0.80 |
| INR | 1.05 (0.8–1.2) | 1.10 (0.8–2.3) | 0.12 |
| AST (U/L) | 29 (13–65) | 43 (17–161) | 0.03 |
| ALT (U/L) | 35 (18–98) | 45 (18–150) | 0.02 |
| AP (U/L) | 72 (42–121) | 78 (37–286) | 0.23 |
| GGT (U/L) | 70 (16–101) | 64 (14–162) | 0.61 |
| CRP mg/L | 2.9 (1.5–123) | 6.4 (1.8–128) | 0.0007 |
| Cholesterol (mg/dL) | 150 (97–294) | 157 (84–272) | 0.44 |
| Triglycerides (mg/dL) | 126 (67–298) | 113 (43–211) | 0.12 |
| Variable | Liver Cirrhosis Patients | Healthy Volunteers | ||||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | Difference | p-Value | |
| CAP (dB/m) | 37 | 269.86 ± 63.46 | 27 | 223.74 ± 41.25 | −46.12 (−74.07; −18.17) | 0.0016 |
| CEUS-PAT | 37 | 0.83 ± 0.09 | 27 | 0.49 ± 0.11 | −0.33 (−0.38; 0.28) | <0.0001 |
| VCTE (kPa) | 37 | 20.21 ± 14.97 | 27 | 5.87 ± 2.87 | −14.34 (−20.19; 8.50) | <0.0001 |
| CEUS-PAT | VCTE | APRI Score | FIB-4 Score | ||
|---|---|---|---|---|---|
| CEUS-PAT | correlation coefficient | 1.00 | 0.81 | 0.41 | 0.33 |
| p-value | 0.0001 | 0.001 | 0.007 | ||
| VCTE | correlation coefficient | 0.81 | 1.0 | 0.38 | 0.21 |
| p-value | 0.0001 | 0.001 | 0.08 | ||
| APRI score | correlation coefficient | 0.41 | 0.38 | 1.0 | 0.53 |
| p-value | 0.001 | 0.001 | 0.0001 | ||
| FIB-4 score | correlation coefficient | 0.33 | 0.21 | 0.53 | 1.0 |
| p-value | 0.007 | 0.08 | 0.0001 | ||
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Coefficient | Std. err | p-Value | Coefficient | Std. err | p-Value | |
| Age (years) | 0.004 | 0.002 | 0.13 | |||
| Gender (male) | 0.0005 | 0.04 | 0.99 | |||
| BMI (kg/m2) | 0.001 | 0.005 | 0.72 | |||
| Haemoglobin (g/dL) | −0.02 | 0.01 | 0.04 | −0.02 | 0.04 | 0.67 |
| Haematocrit (%) | 0.001 | 0.003 | 0.68 | |||
| Leucocytes (*109/L) | 0.000007 | 0.01 | 0.99 | |||
| Thrombocytes (*103/L) | −0.0003 | 0.0002 | 0.19 | |||
| Albumin (mg/dL) | −0.11 | 0.02 | <0.0001 | −0.06 | 0.02 | 0.02 |
| Sodium (mmol/L) | −0.1 | 0.004 | 0.003 | −0.009 | 0.005 | 0.12 |
| Potassium (mmol/L) | 0.08 | 0.4 | 0.06 | |||
| Urea (mg/dL) | 0.002 | 0.001 | 0.18 | |||
| Creatinine (mg/dL) | −0.03 | 0.09 | 0.72 | |||
| INR | −0.01 | 0.02 | 0.53 | |||
| AST (U/L) | 0.001 | 0.006 | 0.01 | 0.0003 | 0.001 | 0.82 |
| ALT (U/L) | 0.001 | 0.006 | 0.02 | 0.0003 | 0.001 | 0.75 |
| AP (U/L) | 0.0009 | 0.0004 | 0.06 | |||
| GGT (U/L) | 0.0005 | 0.0007 | 0.50 | |||
| CRP (mg/L) | 0.0009 | 0.001 | 0.36 | |||
| Cholesterol (mg/dL) | −0.0002 | 0.0006 | 0.65 | |||
| Triglycerides (mg/dL) | −0.0005 | 0.0002 | 0.06 | |||
| Severe steatosis at CAP | 0.007 | 0.001 | 0.03 | 0.002 | 0.001 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupușoru, R.; Sporea, I.; Rațiu, I.; Lungeanu, D.; Popescu, A.; Dănilă, M.; Mare, R.; Marc, L.; Lascău, A.; Moga, T.V.; et al. Contrast-Enhanced Ultrasonography with Arrival Time Parametric Imaging as a Non-Invasive Diagnostic Tool for Liver Cirrhosis. Diagnostics 2022, 12, 3013. https://doi.org/10.3390/diagnostics12123013
Lupușoru R, Sporea I, Rațiu I, Lungeanu D, Popescu A, Dănilă M, Mare R, Marc L, Lascău A, Moga TV, et al. Contrast-Enhanced Ultrasonography with Arrival Time Parametric Imaging as a Non-Invasive Diagnostic Tool for Liver Cirrhosis. Diagnostics. 2022; 12(12):3013. https://doi.org/10.3390/diagnostics12123013
Chicago/Turabian StyleLupușoru, Raluca, Ioan Sporea, Iulia Rațiu, Diana Lungeanu, Alina Popescu, Mirela Dănilă, Ruxandra Mare, Luciana Marc, Andrada Lascău, Tudor Voicu Moga, and et al. 2022. "Contrast-Enhanced Ultrasonography with Arrival Time Parametric Imaging as a Non-Invasive Diagnostic Tool for Liver Cirrhosis" Diagnostics 12, no. 12: 3013. https://doi.org/10.3390/diagnostics12123013
APA StyleLupușoru, R., Sporea, I., Rațiu, I., Lungeanu, D., Popescu, A., Dănilă, M., Mare, R., Marc, L., Lascău, A., Moga, T. V., Bende, F., Ghiuchici, A.-M., & Șirli, R. (2022). Contrast-Enhanced Ultrasonography with Arrival Time Parametric Imaging as a Non-Invasive Diagnostic Tool for Liver Cirrhosis. Diagnostics, 12(12), 3013. https://doi.org/10.3390/diagnostics12123013










