Incidental Vascular Findings in Computed Tomography Performed in the Qualification for the TAVI Procedure
Abstract
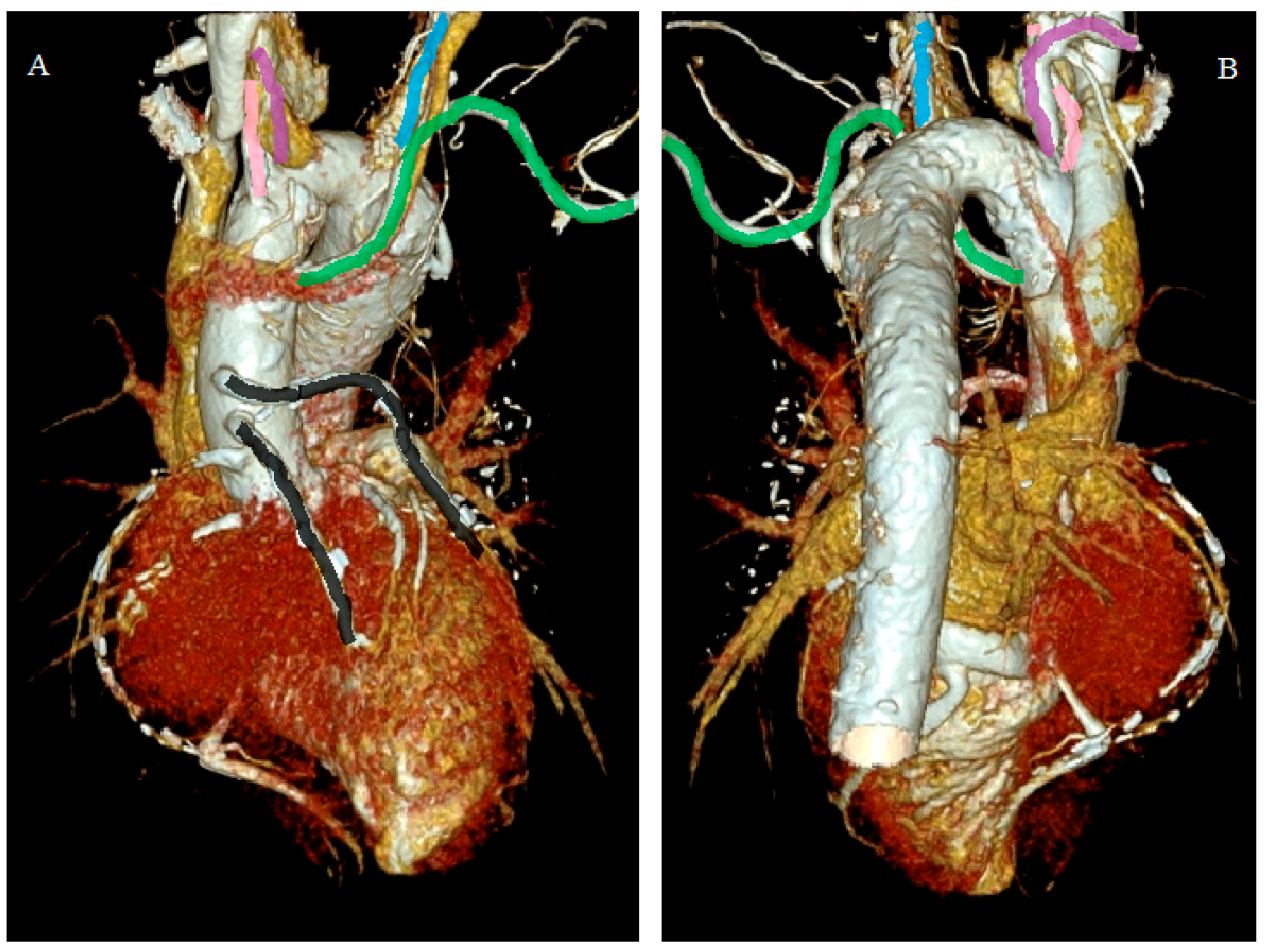
- Incidental vascular finding No. 1: Persistent Left Superior Vena Cava with Absent Right Superior Vena Cava.
- -
- Difficulty inserting central venous cannula, pulmonary artery catheter, and transvenous pacing leads through the left internal jugular/subclavian vein. The dilated coronary sinus (CS), which is thin-walled and stretched, predisposes it to perforation, tamponade, various arrhythmias, and cardiac arrest.
- -
- Persistent left SVC may require separate cannulation during right-sided open-heart surgical procedures, either directly or through CS.
- -
- Difficulty in performing cavo-pulmonary anastomoses.
- -
- Inability to deliver retrograde cardioplegia during open-heart surgery and port-access cardiac surgery because of the large size of CS.
- -
- Rhythm abnormalities such as ectopic atrial rhythm, wandering pacemaker, first-degree atrioventricular block, tachyarrhythmia, and complete heart block because of the stretching or fragmentation of the atrioventricular node and His bundle secondary to the dilatation of CS. Sinoatrial nodal abnormalities have been detected in patients with absent right SVC, predisposing the patient to sick sinus syndrome.
- -
- Rarely, enlarged CS can impinge on the vestibule of the mitral valve that may produce obstruction to left ventricular inflow.
- -
- An orthotopic heart transplant may require the recipient’s heart to be excised along the atrioventricular grove, preserving the persistent left superior vena cava and CS [9].
- Incidental vascular finding No. 2: Right Aortic Arch.
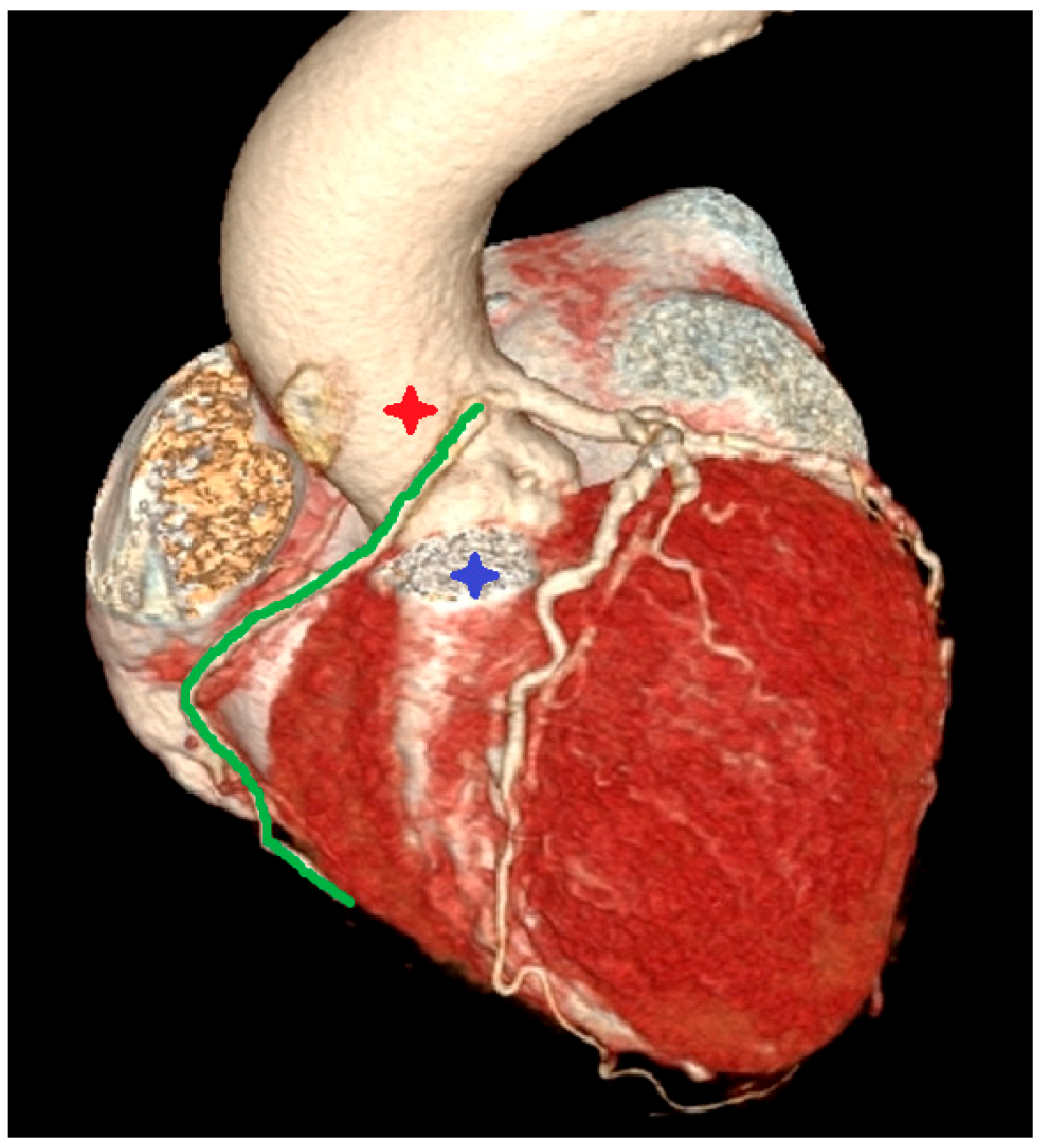
- Incidental vascular finding No. 3: Ectopic Right Coronary Artery Ostium.
- Incidental vascular finding No. 4: Left Superior Pulmonary Vein Draining into Left Brachiocephalic Vein.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Kachel, M.; Milewski, K.; Buszman, P.; Michalak, M.; Domaradzki, W.; Gerber, W.; Śliwka, J.; Nożyński, J.; Sobota, M.; Hirnle, P.; et al. State-of-the-art of transcatheter treatment of aortic valve stenosis and the overview of the InFlow project aiming at developing the first Polish TAVI system. Cardiol. J. 2017, 24, 685–694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Norgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2019, 13, 1–20. [Google Scholar] [CrossRef]
- Liu, H.; Wingert, A.; Wang, J.; Zhang, J.; Wang, X.; Sun, J.; Chen, F.; Khalid, S.G.; Jiang, J.; Zheng, D. Extraction of coronary atherosclerotic plaques from computed tomography imaging: A review of recent methods. Front. Cardiovasc. Med. 2021, 8, 597568. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wingert, A.; Wang, X.; Zhang, J.; Sun, J.; Chen, F.; Khalid, S.G.; Gong, Y.; Xia, L.; Jiang, J.; et al. Consistency in geometry among coronary atherosclerotic plaques extracted from computed tomography angiography. Front. Physiol. 2021, 12, 715265. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; De Maria, G.L.; Joseph, J.; Fan, L.; Cahill, T.J.; Kotronias, R.A.; Burzotta, F.; Newton, J.D.; Kharbanda, R.; Prendergast, B.; et al. Impact of complications during transfemoral transcatheter aortic valve replacement: How can they be avoided and managed? J. Am. Heart Assoc. 2019, 8, e013801. [Google Scholar] [CrossRef] [PubMed]
- Minamino-Muta, E.; Kato, T.; Morimoto, T.; Taniguchi, T.; Nakatsuma, K.; Kimura, Y.; Inoko, M.; Shirai, S.; Kanamori, N.; Murata, K.; et al. Malignant disease as a comorbidity in patients with severe aortic stenosis: Clinical presentation, outcomes, and management. European heart journal. Qual. Care Clin. Outcomes 2018, 4, 180–188. [Google Scholar] [CrossRef]
- Dave, V.; Sesham, K.; Mehra, S.; Roy, T.S.; Ahuja, M.S. Persistent left superior vena cava: An anatomical variation. Med. J. Armed India 2022, 78 (Suppl. 1), S277–S281. [Google Scholar] [CrossRef] [PubMed]
- Mariscal-López, E.; Jiménez, M.P.; Alberca, A.V.; Mateas, F.R. Implantation of a leadless pacemaker in a patient with persistent left superior vena cava and right superior vena cava atresia. Europace 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Arazińska, A.; Polguj, M.; Szymczyk, K.; Kaczmarska, M.; Trębiński, Ł.; Stefańczyk, L. Right aortic arch analysis—Anatomical variant or serious vascular defect? BMC Cardiovasc. Disord. 2017, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Yashima, F.; Hayashida, K.; Munakata, M.; Arai, T.; Shimizu, H.; Fukuda, K. Aortic stenosis with right-sided aortic arch treated with transfemoral aortic valve implantation. Cardiovasc. Interv. Ther. 2019, 34, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gong, Y.; Leng, X.; Xia, L.; Wong, K.; Ou, S.; Leung, W.; Wang, D.; Shi, L. Estimating current and long-term risks of coronary artery in silico by fractional flow reserve, wall shear stress and low-density lipoprotein filtration rate. Biomed. Phys. Eng. Express 2018, 4, 025006. [Google Scholar] [CrossRef]
- Marcucci, M.; Fogante, M.; Schicchi, N.; Agliata, G.; Giovagnoni, A. Rare malignant anomalous right coronary artery incidentally detected by dual source computed tomography angiography in an adult referred for transcatheter aortic valve implantation. Radiol. Case Rep. 2021, 16, 3481–3484. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.P.; Dennie, C.; Pena, E.; Nguyen, E.; LaBounty, T.; Yang, B.; Patel, S. Anomalous coronary arteries that need intervention: Review of pre- and postoperative imaging appearances. Radiographics 2017, 37, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Ku, L.; Song, L.; Ma, X. A rare pulmonary-to-systemic venous connection associated with the partially anomalous pulmonary venous connection. J. Card. Surg. 2022, 37, 3884–3886. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, T.; Sughimoto, K.; Miyaji, K. Partial anomalous pulmonary venous drainage repair concomitant with bilateral semilunar valve replacements and pulmonary artery reconstruction for an adult female 20 years after initial truncus arteriosus repair. Cardiol. Young 2018, 28, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, S.; Almogheer, B.; Mahon, C.; Houshmand, G.; Uygur, B.; Giblin, G.T.; Krupickova, S.; Baksi, A.J.; Alpendurada, F.; Prasad, S.K.; et al. Clinical significance of partial anomalous pulmonary venous connections (isolated and atrial septal defect associated) determined by cardiovascular magnetic resonance. circulation. Cardiovasc. Imaging 2021, 14, e012371. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gać, P.; Grochulska, A.; Poręba, R. Incidental Vascular Findings in Computed Tomography Performed in the Qualification for the TAVI Procedure. Diagnostics 2022, 12, 2773. https://doi.org/10.3390/diagnostics12112773
Gać P, Grochulska A, Poręba R. Incidental Vascular Findings in Computed Tomography Performed in the Qualification for the TAVI Procedure. Diagnostics. 2022; 12(11):2773. https://doi.org/10.3390/diagnostics12112773
Chicago/Turabian StyleGać, Paweł, Aleksandra Grochulska, and Rafał Poręba. 2022. "Incidental Vascular Findings in Computed Tomography Performed in the Qualification for the TAVI Procedure" Diagnostics 12, no. 11: 2773. https://doi.org/10.3390/diagnostics12112773
APA StyleGać, P., Grochulska, A., & Poręba, R. (2022). Incidental Vascular Findings in Computed Tomography Performed in the Qualification for the TAVI Procedure. Diagnostics, 12(11), 2773. https://doi.org/10.3390/diagnostics12112773






