Abstract
Assessment of midpalatal suture maturation is crucial before deciding which type of maxillary expansion technique will be performed to treat transverse discrepancies. In 2013, Angelieri et al. proposed a new method to evaluate midpalatal maturation using cone-beam computed tomography. The aim of this study was to systematically identify, evaluate, and provide a synthesis of the existing literature about this new method and to rigorously assess the methodological quality of these articles. A bibliographic search was carried out using PubMed, Cochrane Library, SciELO, LILACS, Web of Science, and Scopus using the terms midpalatal suture, cranial sutures, palate, maturation, interdigitation, ossification, maxillary expansion, evaluation, assessment, and assess. Quality assessment was performed using the Observational Cohort and Cross-Sectional Studies tool developed by the National Heart, Lung, and Blood Institute. Hence, 56 articles were obtained, of which only 10 met the selection criteria. We could not include any of the data into an analysis because of the large variation of the data collected and high methodological heterogeneity found among studies. Of all the studies included, 10% had poor quality, 70% fair, and 20% good quality, respectively. Even though age and sex play a role in midpalatal suture obliteration, there is a poor correlation between these variables. Thus, every patient should be assessed individually before choosing the best protocol for maxillary expansion. The midpalatal suture maturation method has the potential to be used for diagnostic purposes, but clinicians should be cautious of routinely using it because an extensive training and calibration program should be performed prior.
1. Introduction
The maxillary deficiency in the transverse plane is called maxillary constriction. The main etiologic factors of this deficiency are mouth breathing, harmful habits, like thumb sucking and/or pacifiers, and atypical phonation and swallowing. The poor positioning of the tongue, the imbalance of perioral muscles, the lack of lip seal, together with the labial hypotonicity, contribute to maxillary constriction [1,2,3,4].
Transverse maxillary deficiency is a relatively frequently encountered orthodontic problem, with a prevalence of approximately 10% in adults, and is often characterized by a unilateral or bilateral posterior crossbite [5,6].
The orthodontic procedure used to achieve the correction of maxillary transverse deficiency is called maxillary expansion. The main goal of this treatment is to widen the maxilla by accomplishing the separation of the midpalatal suture (MPS), maximizing skeletal expansion, and minimizing dentoalveolar expansion [7]. This occurs by the stretching of collagenous fibers as well as the local formation of a new bone, correcting the transverse maxillary constriction with a real increase in the transversal width [8].
Currently, there are four expansion treatment modalities used by clinicians: slow maxillary expansion (SME), rapid maxillary expansion (RME), miniscrew assisted rapid palatal expansion (MARPE) and surgically assisted rapid palatal expansion (SARPE). These methods may vary depending on the force used, the appliance, the activation protocol of the screw positioned on the expander and the duration of treatment [9,10].
In children and young adolescents, transverse maxillary deficiency is effectively treated with slow or rapid maxillary expansion (RME).
SME typically utilizes continuous low-force systems applied over a longer period of time than RME, and it is achieved through a Quad-helix appliance, removable plates, an spring appliances (e.g., Minne expander) [11,12]. With slow maxillary expansion, tooth movements through the alveolar ridge tend to be greater than the orthopedic effects [13,14].
RME is associated with systems of heavy and intermittent forces applied in a short time frame and is achieved through appliances anchored to teeth or tissues (e.g., Hyrax or Haas) in growing patients [11,15,16].
In post pubertal patients (young adults and adults), with the closure of the craniofacial sutures and an increase in density of the MPS, RME cannot be performed using the conventional method [17] and surgically assisted techniques (SARPE) are required to provide skeletal expansion [18,19]. In addition to being invasive and expensive, surgically assisted techniques are associated with surgical risks [20,21,22].
In this context, as a result of the need to develop a non-surgical treatment for maxillary transverse deficiency in patients who would normally apply for a SARPE, Lee et al. in South Korea and Moon et al. in the USA [23,24] developed a new method named miniscrew-assisted rapid palatal expansion (MARPE).
MARPE is either a tooth-bone borne or a solely bone-borne RPE device with a rigid element that connects to miniscrews inserted into the palate, delivering the expansion force directly to the basal bone of the maxilla [24,25].
The time point to shift from a non-surgical to a surgical approach is still not clear enough [26,27]. Existing studies mention that RME should be performed before puberty [28,29] and others have shown successful expansion by RME in adult patients [30,31,32].
Authors have reported histologic studies of patients aged 27, 32, 54, and even 71 [33,34,35,36] years not having signs of MPS fusion. These findings support the existence of great variability in developmental stages in palatal suture fusion and that they are not directly related to chronological age, even more in young adults [33,35,36,37,38].
The skeletal maturation assessment is routinely used in the clinical practice of physical and rehabilitation medicine, orthopedics, pediatrics, and orthodontics to plan an adequate treatment in growing subjects [39,40].
The gold standard for assessing skeletal maturation is the hand wrist maturation (HWM), a method that needs an extra hand and wrist X-ray [41].
In this context, Lamparski et al. [42] introduced a method for assessing cervical vertebral maturation on the cephalometric radiographs. As a result, additional patient radiation was eliminated. Currently, this type of radiograph is routinely applied in orthodontic treatment [43].
Cone-beam computed tomography (CBCT) provides 3-dimensional visualization of the MPS in vivo, without any overlapping of anatomic structures, at relatively low cost [17,44]. This could allow the development of a qualitative or quantitative assessment of MPS maturation to assist the decision about whether conventional or surgically assisted maxillary expansion is more appropriate [7].
In 2013, Angelieri et al. [27] proposed a new method that allows the individual assessment of MPS using CBCT. They divided MPS maturation into five different stages (A, B, C, D, and E), being able to find the suture open in stages A, B, and C, partially closed in D, and totally closed in E.
Since the creation of this method, some systematic revisions have been performed [45,46,47], but this is the first article to systematically assess and standardize the calibration, training, blinding processes, and randomization of the images that each one of the articles used. This is of vital importance to ensure the internal validity of each study.
Therefore, the present systematic review aimed to critically appraise the available literature on the assessment of maturation of the midpalatal suture before maxillary expansion, using the method proposed by Angelieri et al. [27], with a two-fold focus: (1) to summarize and (2) to assess the methodological quality of evidence.
2. Materials and Methods
2.1. Protocol and Registration
The systematic review was conducted and written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [48,49]. The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42022307742).
2.2. Eligibility Criteria
A PICOS (population, intervention, comparator, outcomes, and study design) question was established as an inclusion criteria:
Population (P): human subjects of any gender without restriction of ethnicity or age.
Intervention (I): midpalatal suture maturation method proposed by Angelieri et al. [27] (Table 1).

Table 1.
Skeletal maturation stages of the MPS proposed by Angelieri et al. [27].
Condition (C): not having used another method to assess midpalatal suture maturation.
Outcome (O): degree of ossification-maturation-interdigitation of midpalatal suture before maxillary expansion treatment.
Study design (S): observational studies (cohort studies either prospective or retrospective and cross-sectional studies).
Articles including subjects who had undergone any type of orthodontic or orthopedic treatment, nonhuman studies, syndromic conditions, case reports, cleft lip, and palate, and review articles were excluded.
2.3. Information Sources and Search Strategy
Electronics searches in MEDLINE (via PubMed), Web of Science, Cochrane Library, Scopus, LILACS and SciELO were conducted up to July 2022. Google Scholar was investigated to partially access the gray literature.
Finally, manual searches in the reference list of included articles were also carried out. There was no restriction of language, year, or status of publication for inclusion.
Detailed search strategies were developed for each database based on the strategy developed for MEDLINE, and subsequently adapted for the other databases (Table 2).

Table 2.
Electronic literature search strategy.
2.4. Selection of Sources of Evidence
Study selection was performed in three phases. First, the main researcher (A.S) excluded the duplicate articles using the Reference Manager EndNote X9 (Clarivate Analytics, Philadelphia, Pa). Secondly, two reviewers (A.S and P.S.V) blindly assessed the titles and abstracts of identified records. Then, the same reviewers separately applied eligibility criteria to the full-text studies using the systematic review web application Rayyan [50] (rayyan.qcri.org). Information was cross-checked in a consensus meeting in which disagreements were solved between them. If there was no consensus, a third reviewer was consulted to make a final decision (I.G.C).
2.5. Data Charting Process and Data Items
The data was extracted independently by two reviewers (A.S and P.S.V) using a data extraction sheet designed in Microsoft Excel (Redmond, Wash), and any differences were resolved by discussion and consensus with a third reviewer (I.G.C). The following data were extracted from each included study: first author, publication year, study design, sample size, sex distribution, objectives, inclusion criteria, equipment used, number of examiners, calibration, training, and blinding process, inter and intra-evaluator agreement, statistical analysis used, and the author’s conclusion.
2.6. Quality Assessment of Included Studies Synthesis of Results
As suggested by Ma et al. [51], the Observational Cohort and Cross-Sectional Studies tool developed by the National Heart, Lung, and Blood Institute [52] was used to assess the quality of the articles that met the inclusion criteria.
Two reviewers independently assessed the articles and subsequently discussed the quality of each study (A.S and P.S.V.). In case of discrepancy, a third author was consulted for further evaluation (I.G.C.).
3. Results
A total of 56 studies were identified by electronic searches, and 36 studies remained after removing duplicates. After initial screening, a total of 31 studies met the predetermined inclusion criteria. After the full text review, nine studies were included for this review. In addition, 1 eligible study was identified via hand searches. As a result, 10 studies were included in this systematic review (Figure 1). A summary of the characteristics of each included study is presented in Table 3.
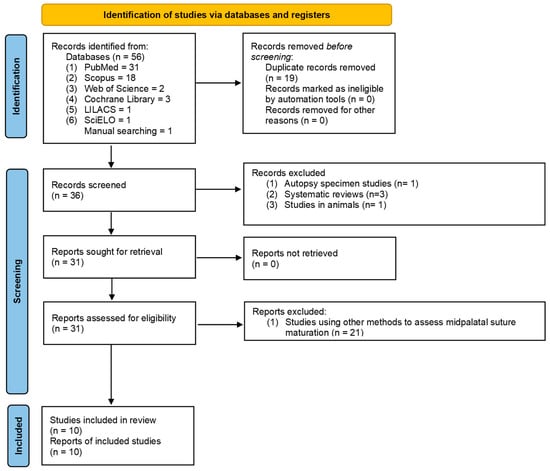
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only. Page, MJ.; McKenzie, JE.; Bossuyt, PM.; Boutron, I.; Hoffmann, TC.; Mulrow, CD.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: n71. doi: 10.1136/bmj.n71 [48].

Table 3.
Summary of characteristics of included studies.
3.1. Results of Individual Sources of Evidence and Synthesis of Results
3.1.1. Angelieri et al. 2013
Angelieri et al. [27] assessed midpalatal suture maturation in 140 subjects between the ages of 5–58. Stages A and B were mainly observed up to 13 years of age (55 subjects), whereas stage C was noted primarily from 11 to 17 years of age but occasionally in younger and older age groups (two subjects under 11 years and four over 18 years old). Fusion of the palatine (stage D) and maxillary (stage E) regions of the midpalatal suture was completed after 11 years only in girls (six subjects). From ages 14 to 17 (no years here), three of 13 (23%) boys showed fusion only in the palatine bone (stage D).
3.1.2. Tonello et al. 2017
Tonello et al. [53] evaluated midpalatal suture maturation in 84 children 11–15 years old. Stage A was only found in one 11-year-old girl. In the age group 11–13 years, it was observed that the unfused stages (A, B, and C) were seen in 90.3% of the subjects.
Stage D was present in six girls and five boys (13.1% of the sample). Stage E was found in 10.7% of the sample. Almost all subjects (eight of nine) were 14 or 15 years of age, except for a 12-year-old girl.
3.1.3. Angelieri et al. 2017
Angelieri et al. [54] assessed midpalatal maturation in 78 adults 18–66 years old. Hence, 19 of the adults presented a fused midpalatal suture in the palatine (Stage D) and/or maxillary bones (50, 42 female and eight male). However, the midpalatal suture was not fused in nine of the subjects (12%)
3.1.4. Ladewig et al. 2018
Ladewig et al. [56] evaluated midpalatal suture maturation in 112 patients 16–20 years old. Stage A was found in none of the subjects and Stage B in 16 of them. Stage C was present in 23 males (52.3%) and 27 females (39.7%). Stage D and E were present in 26 and 27 subjects respectively.
3.1.5. Jiménez et al. 2019
Jimenez et al. [57] assessed midpalatal suture maturation in 200 subjects 10–25 years old. They mention that the possibility to find open midpalatal suture (Stages A, B, and C) in individuals 10–15 years old was 70.8% (35 out of 48), in subjects aged 16–20 and 21–25 years old was 21.2% (11 out of 52) and 17% (17 out of 100), respectively. Stage D was present in 58 subjects (nine of them were less than 15 years old) and Stage E was present in 79 subjects (5% of them were less than 15 years old).
3.1.6. Vahdat et al. 2020
Vahdat et al. [58] evaluated MPS maturation in 178 subjects 10–70 years old. Stage A was found in 0 subjects, B in 25 (14 female and 11 male), C in 72 (35 female and 37 male), D in 47 (23 female and 24 male), and E in 34 (17 female and 17 male).
3.1.7. Katti et al. 2020
Katti et al. [59] assessed MPS maturation in 200 subjects between the ages of 11 to 50. The authors didn’t subdivide their results by gender. Stage A was found in 15 subjects (group under 20 years old). Stage B was found in 40 subjects (15 of them older than 20 years old), C in 70 subjects (60 of them older than 20 years old), D in 25, and E in 40 (all of them over 20 years old).
3.1.8. Gatti reis et al. 2020
Gatti Reis et al. [60] evaluated MPS maturation in 487 subjects 15–40 years old. Stage A was found in 0 subjects, B in five (all of them female), C in 166 (93 female and 73 male), D in 81 (38 female and 43 male), and E in 235 (153 female and 82 male).
3.1.9. Villarroel et al. 2021
Villarroel et al. [61] assessed MPS maturation in 150 subjects between the age of 15 and 30 years old. Stage A was found in 0 subjects, B in two (one female and one male), C in 65 (29 female and 36 male), D in 33 (17 female and 16 male), and E in 50 (30 female and 20 male).
3.2. Quality Assessment of Included Studies
The obtained grade of quality assessment for each study is included in Table 4. Grades for the selected studies ranged from 58.3% to 83.3%. One study [59] had poor quality, seven studies [27,53,54,55,56,58,60] had fair quality, and two studies [57,61] had good quality.

Table 4.
Blinding, Calibration process of included studies.
4. Discussion
4.1. Summary of Evidence
One of the most important factors when making the clinical decision regarding how to deal with a transverse maxillary constriction is defining whether the midpalatal suture is open or closed, thus influencing enormously the treatment that will be given to the pa-tient. This can be especially challenging in late-stage adolescent and young adult patients because there is no consensus in the literature regarding the minimum age for reliable palatal expansion [57].
Even though RME is a more conservative treatment, if it is indicated in patients with totally or partially closed midpalatal sutures, it can lead to consequences such as significant pain, gingival recession, palatal mucosa ulceration or necrosis, buccal tipping of the posterior teeth, reduction of buccal bone thickness [30,62,63,64,65,66], alveolar bone bending [67], buccal root resorption [68], fenestration of the buccal cortex [69], and instability of the expansion [70,71]. On the other hand, it is important to mention that even though a surgical expansion with SARPE is possible at any time throughout life, it implies increasing morbidity, cost, risk, and more days required for patient recovery [54]. It has also been reported to be the most unpredictable procedure among all orthognathic surgery modal-ities. This unpredictability of the surgical expansion has to do with its relapse potential [72,73].
A third option mentioned in the scientific literature is the use of micro implants (MARPE) in cases in which the midpalatal suture is in process of closure [74,75,76].
Despite the unquestionable success of the RME protocol in clinical practice, there is still no consensus regarding the age limit for palatal expansion. This is mainly due to the great physiologic variability, among patients with an obliterated palatal suture earlier or at a more advanced age, without a precise diagnostic [77]. This has been confirmed by histologic studies that have shown the same variability in the maturation of the midpalatal suture [35,36,37,38].
Furthermore, some clinicians have recommended surgical intervention in patients older than 14 years [78], 16 years [79], 20 years [80], or 25 years [81]. To add to the confusion, many case reports have shown that RME is possible in older adult patients [30,32,62].
As mentioned above, a lot of uncertainty and doubt exists in scientific literature because of contradictory information in relation to which is the best clinical approach when performing maxillary expansion.
Within this frame of reference, a diagnostic method in which it is possible to evaluate the maturation of the palatal suture with safety and reliability before maxillary expansion becomes important [53].
The individual evaluation of midpalatal suture maturation on CBCT scans has been proposed by Angelieri et al. [27], to identify the morphology of the midpalatal suture prior to maxillary expansion, trying to guide clinicians in choosing the best clinical procedure to accomplish a successful treatment.
Two important factors mentioned in literature are age and sex. They play an essential role in finding midpalatal suture opening but are not crucial in the decision making because they are not reliable parameters to determine if the MPS is merged or not [35]. Angelieri et al. [27] mention that chronologic age is unreliable for determining the developmental status of the suture during growth, even though it has been suggested that gradual obliteration of MPS occurs as patients get older.
In addition, it has been mentioned that skeletal maturation of the MPS occurs earlier in women than in men [82,83,84,85], especially in the puberal ages [86,87]. This is also possible to observe in all the studies included in this revision [27,53,54,56,57,58,59,60,61].
Related to age, an interesting event that occurs is related to studies that included adults. In them, it is possible to appreciate subjects that, despite having passed their growth phase, still have an open or partially obliterated midpalatal suture [27,53,54,56,57,58,59,60,61] (Table 5).

Table 5.
Quality assessment of the included studies using the Observational Cohort and Cross-Sectional Studies (NHBLI) tool.
Besides age and sex, it is important to consider the existence of other biological factors responsible for the resistance to maxillary transverse expansion, other than the stage of MPS maturity, such as bone density [7,33,88,89], fusion of the zygomaticotemporal, zygomaticofrontal, zygomaticomaxillary, and pterygopalatine sutures [33,36,69,90,91,92,93].
Even though the advent of CBCT has brought many benefits to the field of orthodontics, allowing the clinician to three-dimensionally visualize the maxillary anatomy [94] and evaluate the MPS maturation without the overlap of the surrounding structures [38], we have to remember that radiological assessment is not a risk-free procedure, especially when children are involved, and there is a growing concern of radiation dose [95,96].
The existing guidelines about the use of CBCT in orthodontics have emphasized the need of a stronger justification when prescribing CBCT examinations. Children or young adults should undergo a CBCT examination only when the benefits of the diagnosis or treatment plan outweigh the potential risks of radiation exposure [97]. Jimenez et al. [57] mention that the need for a tomographic examination should be reduced to avoid the load of ionizing radiation in the patient. In this regard, it is an essential clinical procedure to follow the guidelines of imaging proposed by the American Academy of Oral and Maxillofacial Radiology appropriately [97], according to the clinical condition and assessing the radiation dose risk.
When assessing the reliability and reproducibility of the method proposed by Angelieri et al. [27], we were able to find contradictory information. Some authors mentioned that the method presents a substantial reliability and reproducibility as evaluated through the intraexaminer and interexaminer reliability calculation [27,52], while other authors emphasized the low reproducibility of the method [47,98], describing it as non-intuitive and requiring major training for operator calibration.
Vieira Barbosa et al. [55] mention that whenever proposing a new diagnostic method examiner’s agreement plays an important role. Methods that are considered highly reproducible are also considered reliable. Reliability is the capacity of a method to result in identical or similar outcomes in different clinical or statistical experiments. More specifically, any test or procedure considered reliable will always result in similar outcomes regardless of the time, environment, or examiner. This reliability helps reduce the occurrence of diagnostic errors.
Apart from reliability and reproducibility of a method, validation of a diagnostic method is also necessary, with this method being validated in the literature [77]. Specifically, the individual assessment of midpalatal suture maturation was compared with hand–wrist and cervical vertebrae maturation and showed strong statistical association.
4.2. Methodological Quality Assessment
Methodological quality (risk of bias) assessment is an important step before study initiation usage. Therefore, accurately judging study type is the first priority, and choosing the proper tool is also important [51]. One of the strengths of this review is that it is the first to assess the methodological quality of the articles related to this topic using the Quality assessment of the included studies using the Observational Cohort and Cross-Sectional Studies tool.
This differentiates this systematic review from another [26] in which a quality review was performed using the STROBE checklist. According to Ma et al. [51], this is not the most suitable tool for quality assessment of cross-sectional studies.
Of the seven studies included, only two [57,61] described and defined clearly the study population, mentioning in detail the demographic background, location, and time period for obtaining the samples. Seven out of 10 studies [54,55,56,57,58,60,61] mentioned how the calculations of their sample size was done. Similarly, seven out of 10 [27,53,54,55,56,57,61] studies kept the examiners blinded (Table 4).
Other points of vital importance are related to the calibration between the observers, the blinding process, and the randomization of the images used to evaluate the intra-observer agreement.
Only four studies carried out a calibration process and prior training [27,56,57,61]. Only five studies randomized the images of the second examination [27,54,55,56,57,60] and five included all the images for the second examination [53,55,56,57,59] (Table 6).

Table 6.
Distribution of maturational stages of midpalatal suture by age in the included studies.
4.3. Limitations
A limitation of this study has to do with results not being homogeneous, making it impossible to perform a meta-analysis.
The methodological quality of the studies included was assessed rigorously and many deficiencies were found, such as: lack of randomization, blinding, and sample calculation.
Another limitation has to do with the method itself. Thus, because of its qualitative nature, an extensive calibration and training program is necessary for more reliable and reproducible applications [55].
There is an urgent need for future studies to also include the evaluation of the rest of the circummaxillary sutures.
5. Conclusions
The midpalatal suture maturation method has the potential to be used for diagnostic purposes.
Before using this method, an extensive training and calibration program should be performed.
Even though age and sex play an important role in midpalatal suture obliteration, every patient should be assessed individually before choosing the best protocol for maxillary expansion.
Author Contributions
Conceptualization, A.S. and P.S.V.; methodology, A.S.; software, I.G.C.; formal analysis, P.S.V.; investigation, M.M.G.; data curation, P.S.V.; writing—original draft preparation, A.S.; writing—review and editing, P.S.V.; visualization, M.M.G.; supervision, I.G.C.; project administration, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baccetti, T.; Franchi, L.; McNamara, J.A., Jr.; Tollaro, I. Early dentofacial features of Class II malocclusion: A longitudinal study from the deciduous through the mixed dentition. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 502–509. [Google Scholar] [CrossRef]
- Haas, A.J. Palatal expansion: Just the beginning of dentofacial orthopedics. Am. J. Orthod. 1970, 57, 219–255. [Google Scholar] [CrossRef]
- A McNamara, J. An orthopedic approach to the treatment of Class III malocclusion in young patients. J. Clin. Orthod. 1987, 21, 598–608, Erratum in J. Clin. Orthod. 1987, 21, 804. [Google Scholar] [PubMed]
- Rakosi, T.; Jonas, I.; Graber, T.M. Ortodontia e Ortopedia Facial: Diagnóstico. In Análise do Modelo de Estudo; Artes Médicas Sul.: Porto Alegre, Brazil, 1999; pp. 207–209. [Google Scholar]
- Egermark-Eriksson, I.; Carlsson, G.E.; Magnusson, T.; Thilander, B. A longitudinal study on malocclusion in relation to signs and symptoms of cranio-mandibular disorders in children and adolescents. Eur. J. Orthod. 1990, 12, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, J.; Bhat, M.; Lipton, J. Prevalence and Distribution of Selected Occlusal Characteristics in the US Population, 1988–1991. J. Dent. Res. 1996, 75, 706–713. [Google Scholar] [CrossRef]
- Grünheid, T.; Larson, C.E.; Larson, B.E. Midpalatal suture density ratio: A novel predictor of skeletal response to rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 267–276. [Google Scholar] [CrossRef]
- McNamara, J.A.; Brudon, W.L. Orthodontics and Dentofacial Orthopedics; Needham Press: AnnArbor, MI, USA, 1995; p. 211e2. [Google Scholar]
- Bastos, R.T.D.R.M.; Blagitz, M.N.; Aragón, M.L.S.D.C.; Maia, L.C.; Normando, D. Periodontal side effects of rapid and slow maxillary expansion: A systematic review. Angle Orthod. 2019, 89, 651–660. [Google Scholar] [CrossRef]
- Bucci, R.; D’Antò, V.; Rongo, R.; Valletta, R.; Martina, R.; Michelotti, A. Dental and skeletal effects of palatal expansion techniques: A systematic review of the current evidence from systematic reviews and meta-analyses. J. Oral Rehabil. 2016, 43, 543–564. [Google Scholar] [CrossRef]
- Martina, R.; Cioffi, I.; Farella, M.; Leone, P.; Manzo, P.; Matarese, G.; Portelli, M.; Nucera, R.; Cordasco, G. Transverse changes determined by rapid and slow maxillary expansion—A low-dose CT-based randomized controlled trial. Orthod. Craniofac. Res. 2012, 15, 159–168. [Google Scholar] [CrossRef]
- Brunetto, M.; Andriani, J.D.S.P.; Ribeiro, G.L.U.; Locks, A.; Correa, M.; Correa, L.R. Three-dimensional assessment of buccal alveolar bone after rapid and slow maxillary expansion: A clinical trial study. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 633–644. [Google Scholar] [CrossRef]
- Vizzotto, M.B.; de Araújo, F.B.; da Silveira, H.E.D.; Boza, A.A.; Closs, L.Q. The Quad-Helix Appliance in the Primary Dentition –Orthodontic and Orthopedic Measurements. J. Clin. Pediatr. Dent. 2007, 32, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sandikcioglu, M.; Hazar, S. Skeletal and dental changes after maxillary expansion in the mixed dentition. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 321–327. [Google Scholar] [CrossRef]
- Zuccati, G.; Casci, S.; Doldo, T.; Clauser, C. Expansion of maxillary arches with crossbite: A systematic review of RCTs in the last 12 years. Eur. J. Orthod. 2011, 35, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lione, R.; Ballanti, F.; Franchi, L.; Baccetti, T.; Cozza, P. Treatment and posttreatment skeletal effects of rapid maxillary expansion studied with low-dose computed tomography in growing subjects. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, T.; Zou, W. Effects of rapid maxillary expansion on the midpalatal suture: A systematic review. Eur. J. Orthod. 2015, 37, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Camps-Perepérez, I.; Guijarro-Martínez, R.; Peiró-Guijarro, M.; Hernández-Alfaro, F. The value of cone beam computed tomography imaging in surgically assisted rapid palatal expansion: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2017, 46, 827–838. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Custódio, A.L.N. Orthodontic or surgically assisted rapid maxillary expansion. Oral Maxillofac. Surg. 2009, 13, 123–137. [Google Scholar] [CrossRef]
- Suri, L.; Taneja, P. Surgically assisted rapid palatal expansion: A literature review. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 290–302. [Google Scholar] [CrossRef]
- Lagravere, M.; Major, P.W.; Flores-Mir, C. Long-term skeletal changes with rapid maxillary expansion: A systematic review. Angle Orthod. 2005, 75, 1046–1052. [Google Scholar] [CrossRef]
- Lin, J.-H.; Li, C.; Wong, H.; Chamberland, S.; Le, A.D.; Chung, C.-H. Asymmetric Maxillary Expansion Introduced by Surgically Assisted Rapid Palatal Expansion: A Systematic Review. J. Oral Maxillofac. Surg. 2022. [CrossRef]
- Lee, K.-J.; Park, Y.-C.; Park, J.-Y.; Hwang, W.-S. Miniscrew-assisted nonsurgical palatal expansion before orthognathic surgery for a patient with severe mandibular prognathism. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Moon, W. An interview with Won Moon. By André Wilson Machado, Barry Briss, Greg J Huang, Richard Kulbersh and Sergei Godeiro Fernandes Rabelo Caldas. Dent. Press J. Orthod. 2013, 18, 12–28. [Google Scholar]
- Ventura, V.; Botelho, J.; Machado, V.; Mascarenhas, P.; Pereira, F.D.; Mendes, J.J.; Delgado, A.S.; Pereira, P.M. Miniscrew-Assisted Rapid Palatal Expansion (MARPE): An Umbrella Review. J. Clin. Med. 2022, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Acar, Y.B.; Motro, M.; Erverdi, A.N. Hounsfield Units: A new indicator showing maxillary resistance in rapid maxillary expansion cases? Angle Orthod. 2014, 85, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Angelieri, F.; Cevidanes, L.H.; Franchi, L.; Gonçalves, R.; Benavides, E.; Jr, J.A.M. Midpalatal suture maturation: Classification method for individual assessment before rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 759–769. [Google Scholar] [CrossRef]
- Thadani, M.; Shenoy, U.; Patle, B.; Kalra, A.; Goel, S.; Toshinawal, N. Midpalatal suture ossification and skeletal maturation: A com-parative computerized tomographic scan and roentgenographic study. J. Indian Acad. Oral Med. Radiol. 2010, 22, 81–87. [Google Scholar] [CrossRef]
- Baccetti, T.; Franchi, L.; McNamara, J.A., Jr. An improved version of the cervical vertebral maturation (CVM) method for the assessment of mandibular growth. Angle Orthod. 2002, 72, 316–323. [Google Scholar]
- Filho, L.C.; Neto, J.C.; Filho, O.G.D.S.; Ursi, W.J. Non-surgically assisted rapid maxillary expansion in adults. Int. J. Adult Orthod. Orthognath. Surg. 1996, 11, 57–66. [Google Scholar]
- Handelman, C.S. Nonsurgical rapid maxillary alveolar expansion in adults: A clinical evaluation. Angle Orthod. 1997, 67, 291–305. [Google Scholar] [CrossRef]
- Handelman, C.S.; Wang, L.; A BeGole, E.; Haas, A.J. Nonsurgical rapid maxillary expansion in adults: Report on 47 cases using the Haas expander. Angle Orthod. 2000, 70, 129–144. [Google Scholar] [CrossRef]
- Korbmacher, H.; Schilling, A.; Puschel, K.; Amling, M.; Kahl-Nieke, B. Age dependent three-dimensional micro-computed tomography analysis of the human midpalatal suture. J. Orofac. Orthop. 2007, 68, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Gueutier, A.; Paré, A.; Joly, A.; Laure, B.; de Pinieux, G.; Goga, D. Rapid maxillary expansion in adults: Can multislice computed tomography help choose between orthopedic or surgical treatment? Rev. Stomatol. Chir. Maxillo-Faciale Chir. Orale 2016, 117, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Thilander, B. Palatal suture closure in man from 15 to 35 years of age. Am. J. Orthod. 1977, 72, 42–52. [Google Scholar] [CrossRef]
- Knaup, B.; Yildizhan, F.; Wehrbein, H. Age-Related Changes in the Midpalatal Suture. J. Orofac. Orthop. 2004, 65, 467–474. [Google Scholar] [CrossRef]
- Persson, M.; Magnusson, B.C.; Thilander, B. Sutural closure in rabbit and man: A morphological and histochemical study. J. Anat. 1978, 125, 313–321. [Google Scholar] [PubMed]
- Wehrbein, H.; Yildizhan, F. The mid-palatal suture in young adults. A radiological-histological investigation. Eur. J. Orthod. 2001, 23, 105–114. [Google Scholar] [CrossRef]
- Ferrillo, M.; Curci, C.; Roccuzzo, A.; Migliario, M.; Invernizzi, M.; de Sire, A. Reliability of cervical vertebral maturation compared to hand-wrist for skeletal maturation assessment in growing subjects: A systematic review. J. Back Musculoskelet. Rehabil. 2021, 34, 925–936. [Google Scholar] [CrossRef]
- Luk, K.D.; Saw, L.B.; Grozman, S.; Cheung, K.M.; Samartzis, D. Assessment of skeletal maturity in scoliosis patients to determine clinical management: A new classification scheme using distal radius and ulna radiographs. Spine J. 2014, 14, 315–325. [Google Scholar] [CrossRef]
- Beit, P.; Peltomäki, T.; Schätzle, M.; Signorelli, L.; Patcas, R. Evaluating the agreement of skeletal age assessment based on hand-wrist and cervical vertebrae radiography. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 838–847. [Google Scholar] [CrossRef]
- Lamparski, D.G. Skeletal Age Assessment Utilizing Cervical Vertebrae. Master’s Thesis, Department of Orthodontics, The University of Pittsburgh, Pittsburgh, PA, USA, 1972. [Google Scholar]
- Szemraj, A.; Wojtaszek-Słomińska, A.; Racka-Pilszak, B. Is the cervical vertebral maturation (CVM) method effective enough to replace the hand-wrist maturation (HWM) method in determining skeletal maturation?—A systematic review. Eur. J. Radiol. 2018, 102, 125–128. [Google Scholar] [CrossRef]
- De Vos, W.; Casselman, J.; Swennen, G. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2009, 38, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, J.; Zhou, X.; Yu, G. In vivo methods for evaluating human midpalatal suture maturation and ossification: An updated review. Int. Orthod. 2022, 20, 100634. [Google Scholar] [CrossRef]
- Moreno, A.M.G.; Garcovich, D.; Wu, A.Z.; Lorenzo, A.A.; Martínez, L.B.; Aiuto, R. Cone Beam Computed Tomography evaluation of midpalatal suture maturation according to age and sex: A systematic review. Eur. J. Paediatr. Dent. 2022, 23, 44–50. [Google Scholar]
- Isfeld, D.; Flores-Mir, C.; Leon-Salazar, V.; Lagravère, M. Evaluation of a novel palatal suture maturation classification as assessed by cone-beam computed tomography imaging of a pre- and post expansion treatment cohort. Angle Orthod. 2019, 89, 252–261. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute, National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2017. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessmenttools (accessed on 1 October 2022).
- Tonello, D.L.; Ladewig, V.M.; Guedes, F.P.; Conti, A.C.C.F.; Almeida-Pedrin, R.R.; Capelozza-Filho, L. Midpalatal suture maturation in 11 to 15 years old subject: A tomographic study. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 42–48. [Google Scholar] [CrossRef]
- Angelieri, F.; Franchi, L.; Cevidanes, L.; Gonçalves, J.; Nieri, M.; Wolford, L.; McNamara, J. Cone beam computed tomography evaluation of midpalatal suture maturation in adults. Int. J. Oral Maxillofac. Surg. 2017, 46, 1557–1561. [Google Scholar] [CrossRef]
- Barbosa, N.M.V.; de Castro, A.C.; Conti, F.; Capelozza-Filho, L.; de Almeida-Pedrin, R.R.; Cardoso, M.D.A. Reliability and reproducibility of the method of assessment of midpalatal suture maturation: A tomographic study. Angle Orthod. 2018, 89, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, V.d.M.; Capelozza-Filho, L.; Almeida-Pedrin, R.R.; Guedes, F.P.; de Almeida, C.; de Castro, F.C. Tomographic evaluation of the maturation stage of the midpalatal suture in post adolescents. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Valdivia, L.M.; Malpartida-Carrillo, V.; Rodríguez-Cárdenas, Y.A.; Silveira, H.; Arriola-Guillén, L.E. Midpalatal suture maturation stage assessment in adolescents and young adults using cone-beam computed tomography. Prog. Orthod. 2019, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, A.S.; Kachoei, M.; Ghoghani, F.E.; Ghasemi, S.; Zarif, P. Evaluation of Midpalatal Suture Ossification Based on Age and Gender Using Cone Beam Computed Tomography (CBCT). Arch. Pharm. Pr. 2020, 11, 44–50. [Google Scholar]
- Katti, G.; Shahbaz, S.; Katti, C.; Rahman, M.S. Evaluation of Midpalatal Suture Ossification Using Cone-Beam Computed Tomography: A Digital Radiographic Study. Acta Med. 2020, 63, 188–193. [Google Scholar] [CrossRef]
- Reis, L.G.; Ribeiro, R.A.; Vitral, R.; Reis, H.N.; DeVito, K.L. Classification of the midpalatal suture maturation in individuals older than 15 years: A cone beam computed tomographic study. Surg. Radiol. Anat. 2020, 42, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, T.; Alvarado, M.J.; Concha, G.; Vicuña, D.; Oyonarte, R. Maduración de la Sutura Palatina Media En Adolescentes y Adultos Jóvenes Chilenos: Estudio Transversal. Int. J. Interdiscip. Dent. 2021, 14, 140–143. [Google Scholar] [CrossRef]
- Rungcharassaeng, K.; Caruso, J.M.; Kan, J.Y.; Kim, J.; Taylor, G. Factors affecting buccal bone changes of maxillary posterior teeth after rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 428.e1–428.e8. [Google Scholar] [CrossRef]
- Kiliç, N.; Kiki, A.; Oktay, H. A comparison of dentoalveolar inclination treated by two palatal expanders. Eur. J. Orthod. 2008, 30, 67–72. [Google Scholar] [CrossRef]
- Garib, D.G.; Henriques, J.F.C.; Janson, G.; Freitas, M.R.; Coelho, R.A. Rapid maxillary expansion—Tooth tissue-borne versus tooth-borne expanders: A computed tomography evaluation of dentoskeletal effects. Angle Orthod. 2005, 75, 548–557. [Google Scholar] [CrossRef]
- Bell, W.H.; Epker, B.N. Surgical-orthodontic expansion of the maxilla. Am. J. Orthod. 1976, 70, 517–528. [Google Scholar] [CrossRef]
- Betts, N.J.; Vanarsdall, R.L.; Barber, H.D.; Higgins-Barber, K.; Fonseca, R.J. Diagnosis and treatment of transverse maxillary deficiency. Int. J. Adult Orthod. Orthognath. Surg. 1995, 10, 75–96. [Google Scholar]
- Northway, W.M.; Meade, J.B., Jr. Surgically assisted rapid maxillary expansion: A comparison of technique, response, and stability. Angle Orthod. 1997, 67, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Wertz, R.A. Skeletal and dental changes accompanying rapid midpalatal suture opening. Am. J. Orthod. 1970, 58, 41–66. [Google Scholar] [CrossRef]
- Timms, D.J.; Moss, J.P. An histological investigation into the effects of rapid maxillary expansion on the teeth and their supporting tissues. Trans. Eur. Orthod. Soc. 1971, 263–271. [Google Scholar]
- Haas, A.J. Long-term post treatment evaluation of rapid palatal expansion. Angle Orthod. 1980, 50, 189–217. [Google Scholar]
- Greenbaum, K.R.; Zachrisson, B.U. The effect of palatal expansion therapy on the periodontal supporting tissues. Am. J. Orthod. 1982, 81, 12–21. [Google Scholar] [CrossRef]
- Proffit, W.R.; A Turvey, T.; Phillips, C. The hierarchy of stability and predictability in orthognathic surgery with rigid fixation: An update and extension. Head Face Med. 2007, 3, 21. [Google Scholar] [CrossRef]
- Bailey, L.T.J.; Cevidanes, L.H.S.; Proffit, W.R. Stability and predictability of orthognathic surgery. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 273–277. [Google Scholar] [CrossRef]
- Da Cunha, A.C.; Lee, H.; Nojima, L.I.; Nojima, M.D.C.G.; Lee, K.-J. Miniscrew-assisted rapid palatal expansion for managing arch perimeter in an adult patient. Dent. Press J. Orthod. 2017, 22, 97–108. [Google Scholar] [CrossRef]
- Brunetto, D.P.; Sant’Anna, E.F.; Machado, A.W.; Moon, W. Non-surgical treatment of transverse deficiency in adults using Microimplant-assisted Rapid Palatal Expansion (MARPE). Dent. Press J. Orthod. 2017, 22, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Park, Y.-C.; Lee, K.-J.; Cha, J.-Y.; Tahk, J.H.; Choi, Y.J. Skeletal and dentoalveolar changes after miniscrew-assisted rapid palatal expansion in young adults: A cone-beam computed tomography study. Korean J. Orthod. 2017, 47, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Angelieri, F.; Franchi, L.; Cevidanes, L.H.; McNamara, J.A., Jr. Diagnostic performance of skeletal maturity for the assessment of midpalatal suture maturation. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, M. Transpalatal distraction as a method of maxillary expansion. Br. J. Oral Maxillofac. Surg. 1999, 37, 268–272. [Google Scholar] [CrossRef]
- Epker, B.N.; Wolford, L.M. Transverse Maxillary Deficiency Dentofacial Deformities: Inte-Grated Orthodontic and Surgical Correction; Mosby: St Louis, MO, USA, 1980. [Google Scholar]
- Mossaz, C.F.; Byloff, F.K.; Richter, M. Unilateral and bilateral corticotomies for correction of maxillary transverse discrepancies. Eur. J. Orthod. 1992, 14, 110–116. [Google Scholar] [CrossRef]
- Timms, D.; Vero, D. The relationship of rapid maxillary expansion to surgery with special reference to midpalatal synostosis. Br. J. Oral Surg. 1981, 19, 180–196. [Google Scholar] [CrossRef]
- Alpern, M.C.; Yurosko, J.J. Rapid palatal expansion in adults with and without surgery. Angle Orthod. 1987, 57, 245–263. [Google Scholar] [CrossRef]
- Fishman, L.S. Radiographic evaluation of skeletal maturation. A clinically oriented method based on hand-wrist films. Angle Orthod. 1982, 52, 88–112. [Google Scholar] [CrossRef]
- Björk, A. Sutural growth of the upper face studied by the implant method. Acta Odontol. Scand. 1966, 24, 109–127. [Google Scholar] [CrossRef]
- Fishman, L.S. Chronological versus skeletal age, an evaluation of craniofacial growth. Angle Orthod. 1979, 49, 181–189. [Google Scholar] [CrossRef]
- Cole, T.J.; Rousham, E.K.; Hawley, N.L.; Cameron, N.; A Norris, S.; Pettifor, J.M. Ethnic and sex differences in skeletal maturation among the Birth to Twenty cohort in South Africa. Arch. Dis. Child. 2014, 100, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Koziel, S. Relationships among tempo of maturation, midparent height, and growth in height of adolescent boys and girls. Am. J. Hum. Biol. 2001, 13, 15–22. [Google Scholar] [CrossRef]
- Samra, D.A.; Hadad, R. Midpalatal suture: Evaluation of the morphological maturation stages via bone density. Prog. Orthod. 2018, 19, 29. [Google Scholar] [CrossRef]
- Colonna, A.; Cenedese, S.; Sartorato, F.; Spedicato, G.A.; Siciliani, G.; Lombardo, L. Association of the mid-palatal suture morphology to the age and to its density: A CBCT retrospective comparative observational study. Int. Orthod. 2021, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Filho, O.G.D.S.; Magro, A.C.; Filho, L.C. Early treatment of the Class III malocclusion with rapid maxillary expansion and maxillary protraction. Am. J. Orthod. Dentofac. Orthop. 1998, 113, 196–203. [Google Scholar] [CrossRef]
- Melsen, B.; Melsen, F. The postnatal development of the palatomaxillary region studied on human autopsy material. Am. J. Orthod. 1982, 82, 329–342. [Google Scholar] [CrossRef]
- Jafari, A.; Shetty, K.S.; Kumar, M. Study of stress distribution and displacement of various craniofacial structures following application of transverse orthopedic forces—A three-dimensional FEM study. Angle Orthod. 2003, 73, 12–20. [Google Scholar] [CrossRef]
- Magnusson, A.; Bjerklin, K.; Nilsson, P.; Marcusson, A. Surgically assisted rapid maxillary expansion: Long-term stability. Eur. J. Orthod. 2008, 31, 142–149. [Google Scholar] [CrossRef]
- Chaconas, S.J.; Caputo, A.A. Observation of orthopedic force distribution produced by maxillary orthodontic appliances. Am. J. Orthod. 1982, 82, 492–501. [Google Scholar] [CrossRef]
- Martin, M.A.; Lipani, E.; Lorenzo, A.A.; Martinez, L.B.; Aiuto, R.; Dioguardi, M.; Re, D.; Paglia, L.; Garcovich, D. The effect of maxillary protraction, with or without rapid palatal expansion, on airway dimensions: A systematic review and meta-analysis. Eur. J. Paediatr. Dent. 2020, 21, 262–270. [Google Scholar] [CrossRef]
- Stratis, A.; Zhang, G.; Jacobs, R.; Bogaerts, R.; Bosmans, H. The growing concern of radiation dose in paediatric dental and maxillo-facial CBCT: An easy guide for daily practice. Eur. Radiol. 2019, 29, 7009–7018. [Google Scholar] [CrossRef] [PubMed]
- Scarfe, W.C. Clinical recommendations regarding use of cone beam computed tomography in orthodontic treatment. Position statement by the American Academy of Oral and Maxillofacial Radiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 238–257. [Google Scholar]
- Shin, H.; Hwang, C.-J.; Lee, K.-J.; Choi, Y.J.; Han, S.-S.; Yu, H.S. Predictors of midpalatal suture expansion by miniscrew-assisted rapid palatal expansion in young adults: A preliminary study. Korean J. Orthod. 2019, 49, 360–371. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).