Different Brain Phenotypes in Magnetic Resonance Imaging of Healthy Children after Prenatal Insults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Data Acquisition and Analysis
2.3. Statistical Analyses
3. Results
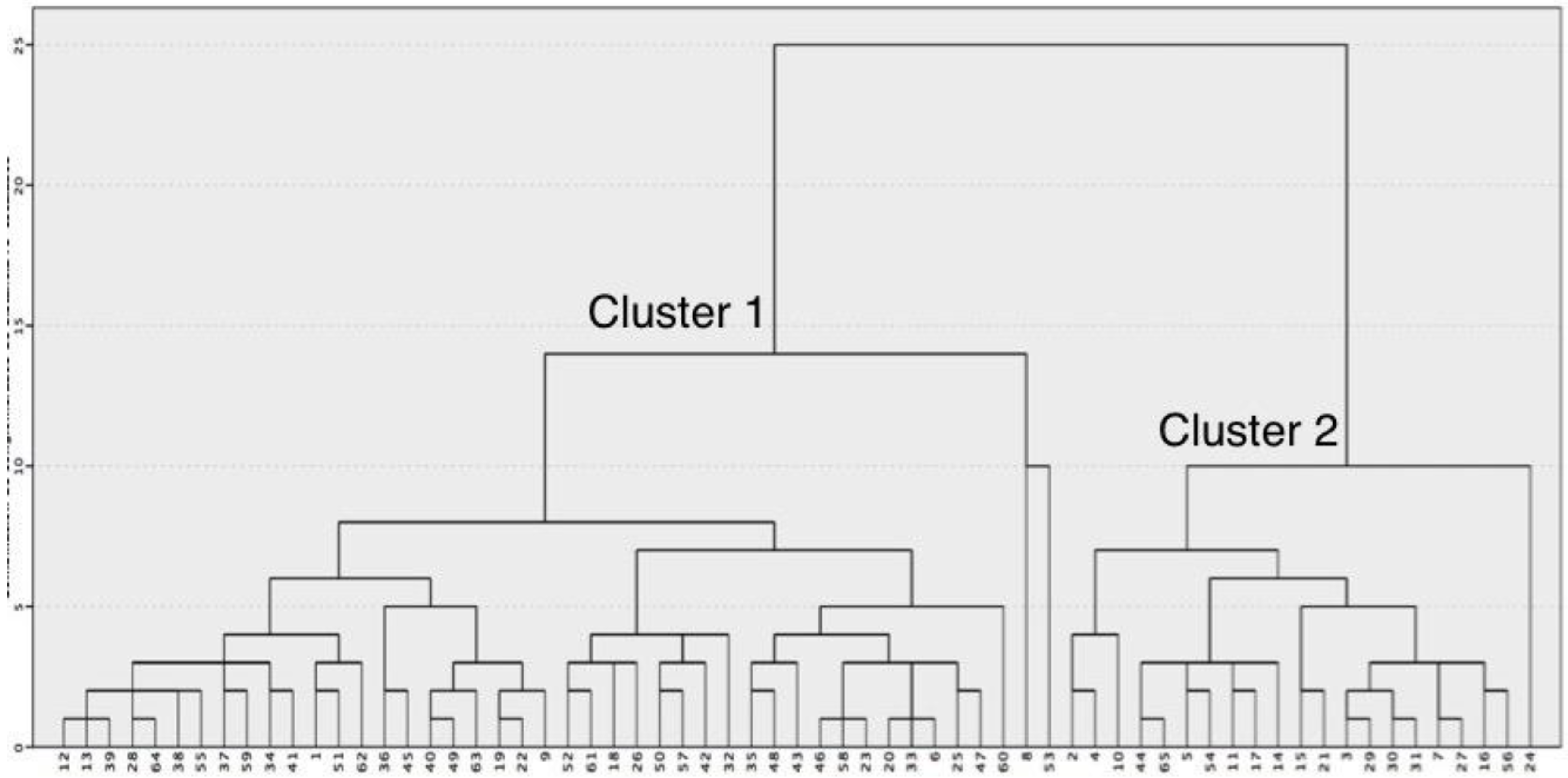
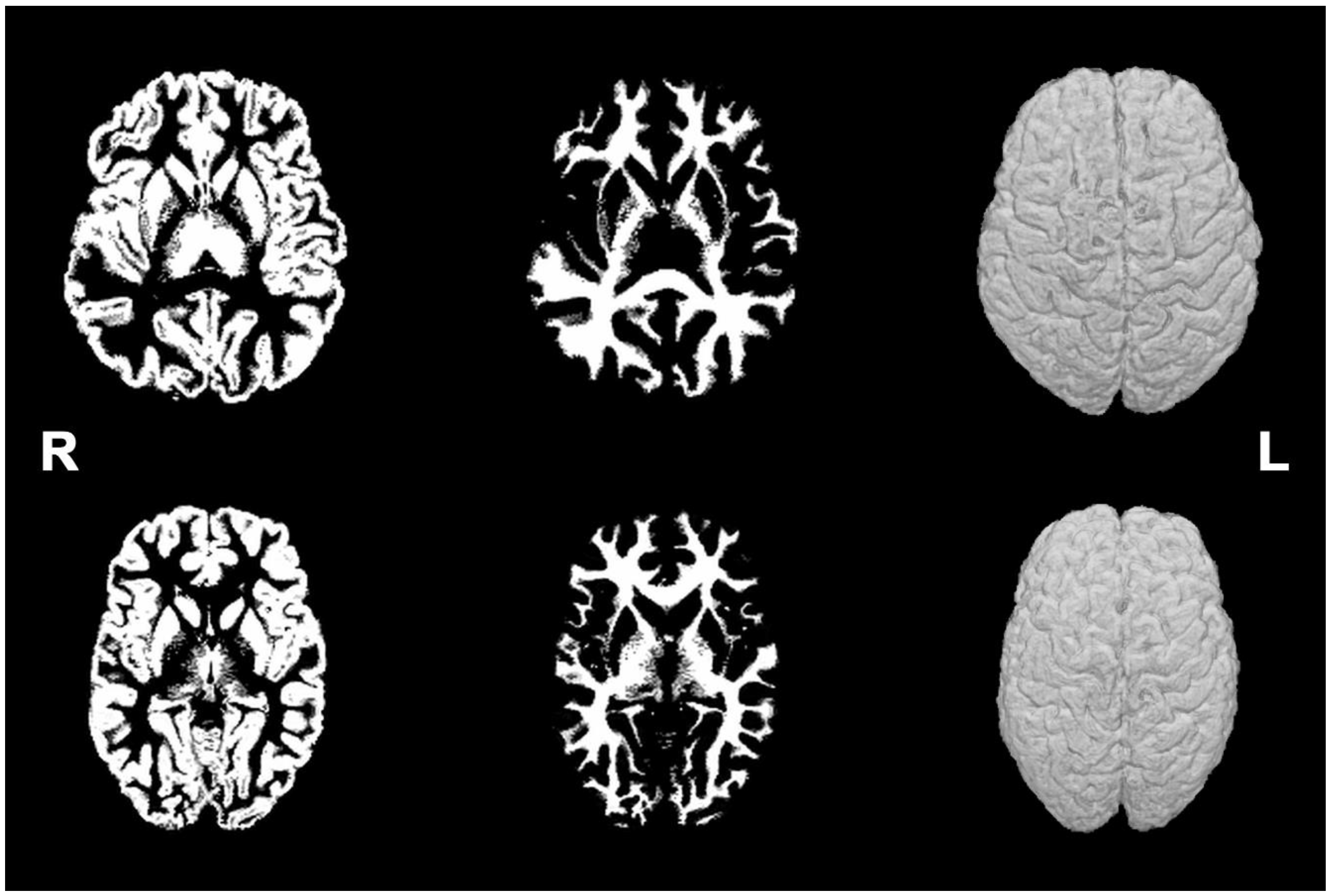
3.1. Brain MRI Phenotypes
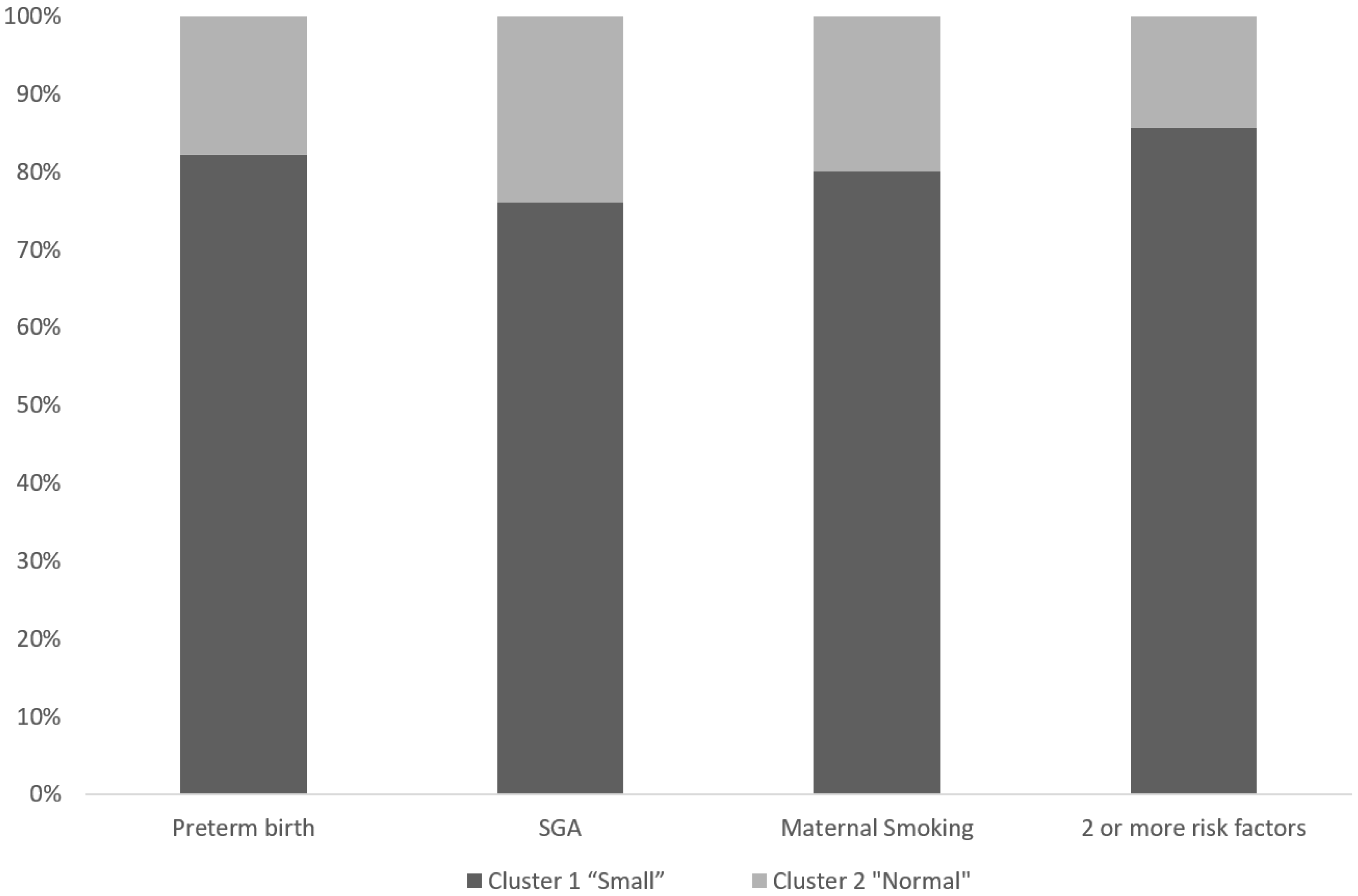
3.2. Perinatal Characteristics of Different Brain MRI Phenotypes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Barker, D. The fetal and infant origins of adult disease. BMJ Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Godfrey, K.; Gluckman, P.; Harding, J.; Owens, J.; Robinson, J. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Volpe, J.J. Overview: Normal and abnormal human brain development. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 1–5. [Google Scholar] [CrossRef]
- Lister, J.P.; Blatt, G.J.; DeBassio, W.A.; Kemper, T.L.; Tonkiss, J.; Galler, J.R.; Rosene, D.L. Effect of prenatal protein malnutrition on numbers of neurons in the principal cell layers of the adult rat hippocampal formation. Hippocampus 2005, 15, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Rehn, A.; Buuse, M.V.D.; Copolov, D.; Briscoe, T.; Lambert, G.; Rees, S. An animal model of chronic placental insufficiency: Relevance to neurodevelopmental disorders including schizophrenia. Neuroscience 2004, 129, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Wilkerson, A.; Jones, J.P.; Christopher, N.C.; Briggs, S.J. Prenatal nicotine effects on memory in rats: Pharmacological and behavioral challenges. Dev. Brain Res. 1996, 97, 207–215. [Google Scholar] [CrossRef]
- Fried, P.; Watkinson, B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicology Teratol. 2001, 23, 421–430. [Google Scholar] [CrossRef]
- Slikker, W., Jr.; Xu, Z.A.; Slotkin, T.A. Mode of action: Disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction developmental neurotoxicity of nicotine. Crit. Rev. Toxicol. 2005, 35, 703–711. [Google Scholar] [CrossRef]
- Rivkin, M.J.; Davis, P.E.; Lemaster, J.L.; Cabral, H.J.; Warfield, S.K.; Mulkern, R.V.; Robson, C.D.; Rose-Jacobs, R.; Frank, D.A. Volumetric MRI Study of Brain in Children With Intrauterine Exposure to Cocaine, Alcohol, Tobacco, and Marijuana. Pediatrics 2008, 121, 741–750. [Google Scholar] [CrossRef]
- Colella, M.; Frérot, A.; Novais, A.R.B.; Baud, O. Neonatal and Long-Term Consequences of Fetal Growth Restriction. Curr. Pediatr. Rev. 2018, 14, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.M.; Rehman Mian, M.O.; Nuyt, A.M. Long-Term Impact of Preterm Birth: Neurodevelopmental and Physical Health Outcomes. Clin. Perinatol. 2017, 44, 305–314. [Google Scholar] [CrossRef] [PubMed]
- De Bie, H.M.A.; Oostrom, K.J.; Delemarre-van de Waal, H.A. Brain development, intelligence and cognitive outcome in children born small for gestational age. Horm. Res. Paediatr. 2010, 73, 6–14. [Google Scholar] [CrossRef]
- Nosarti, C.; Nam, K.W.; Walshe, M.; Murray, R.M.; Cuddy, M.; Rifkin, L.; Allin, M.P. Preterm birth and structural brain alterations in early adulthood. Neuroimage Clin. 2014, 6, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Hüppi, P.; Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef]
- Mathur, A.M.; Neil, J.J.; Inder, T.E. Understanding Brain Injury and Neurodevelopmental Disabilities in the Preterm Infant: The Evolving Role of Advanced Magnetic Resonance Imaging. Semin. Perinatol. 2010, 34, 57–66. [Google Scholar] [CrossRef]
- Nam, K.W.; Castellanos, N.; Simmons, A.; Froudist-Walsh, S.; Allin, M.P.; Walshe, M.; Murray, R.M.; Evans, A.; Muehlboeck, J.-S.; Nosarti, C. Alterations in cortical thickness development in preterm-born individuals: Implications for high-order cognitive functions. NeuroImage 2015, 115, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Meler, E.; Iraola, A.; Eixarch, E.; Coll, O.; Francis, A.; Gratacos, E.; Gardosi, J. Customized birthweight standards for a Spanish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Robinson, H.P.; Fleming, J.E.E. A Critical Evaluation of Sonar “Crown-Rump Length” Measurements. BJOG Int. J. Obstet. Gynaecol. 1975, 82, 702–710. [Google Scholar] [CrossRef]
- Tzeng, C.-R.; Chang, Y.-C.I.; Chang, Y.-C.; Wang, C.-W.; Chen, C.-H.; Hsu, M.-I. Cluster analysis of cardiovascular and metabolic risk factors in women of reproductive age. Fertil. Steril. 2014, 101, 1404–1410.e1. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.; Cruz-Lemini, M.; Valenzuela-Alcaraz, B.; Garcia-Otero, L.; Sitges, M.; Bijnens, B.; Gratacós, E.; Crispi, F. Descriptive analysis of different phenotypes of cardiac remodeling in fetal growth restriction. Ultrasound Obstet. Gynecol. 2017, 50, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.S. “The First Thousand Days” Define a Fetal/Neonatal Neurology Program. Front. Pediatr. 2021, 9, 683138. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.S. Gene-Environment Interactions During the First Thousand Days Influence Childhood Neurological Diagnosis. Semin. Pediatr. Neurol. 2022, 42, 100970. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019, 221, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Boyce, W.T.; Sokolowski, M.B.; Robinson, G.E. Genes and environments, development and time. Proc. Natl. Acad. Sci. USA 2020, 117, 23235–23241. [Google Scholar] [CrossRef]
- Soria-Pastor, S.; Padilla, N.; Zubiaurre-Elorza, L.; Ibarretxe-Bilbao, N.; Botet, F.; Costas-Moragas, C.; Falcon, C.; Bargallo, N.; Mercader, J.M.; Junqué, C. Decreased Regional Brain Volume and Cognitive Impairment in Preterm Children at Low Risk. Pediatrics 2009, 124, e1161–e1170. [Google Scholar] [CrossRef]
- Arhan, E.; Gücüyener, K.; Soysal, Ş.; Şalvarlı, Ş.; Gürses, M.A.; Serdaroğlu, A.; Demir, E.; Ergenekon, E.; Türkyılmaz, C.; Önal, E.; et al. Regional brain volume reduction and cognitive outcomes in preterm children at low risk at 9 years of age. Childs Nerv. Syst. 2017, 33, 1317–1326. [Google Scholar] [CrossRef]
- Muller, M.; Sigurdsson, S.; Kjartansson, O.; Jonsson, P.V.; Garcia, M.; von Bonsdorff, M.B.; Gunnarsdottir, I.; Thorsdottir, I.; Harris, T.B.; van Buchem, M.; et al. Birth Size and Brain Function 75 Years Later. Pediatrics 2014, 134, 761–770. [Google Scholar] [CrossRef]
- Inder, T.E.; Warfield, S.K.; Wang, H.; Hüppi, P.S.; Volpe, J.J. Abnormal Cerebral Structure Is Present at Term in Premature Infants. Pediatrics 2005, 115, 286–294. [Google Scholar] [CrossRef]
- Nosarti, C.; Al-Asady, M.H.; Frangou, S.; Stewart, A.L.; Rifkin, L.; Murray, R.M. Adolescents who were born very preterm have decreased brain volumes. Brain 2002, 125 Pt 7, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Ment, L.R.; Vohr, B.; Pajot, S.K.; Schneider, K.C.; Katz, K.H.; Ebbitt, T.B.; Duncan, C.C.; Makuch, R.W.; Reiss, A.L. Volumetric analysis of regional cerebral development in preterm children. Pediatr. Neurol. 2004, 31, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.S.; Vohr, B.; Staib, L.H.; Cannistraci, C.J.; Dolberg, A.; Schneider, K.C.; Katz, K.H.; Westerveld, M.; Sparrow, S.; Anderson, A.W.; et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 2000, 284, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Maunu, J.; Parkkola, R.; Rikalainen, H.; Lehtonen, L.; Haataja, L.; Lapinleimu, H.; PIPARI Group. Brain and ventricles in very low birth weight infants at term: A comparison among head circumference, ultrasound, and magnetic resonance imaging. Pediatrics 2009, 123, 617–626. [Google Scholar] [CrossRef]
| Cluster 1 “Small” (N = 44) | Cluster 2 “Normal” (N = 21) | p-Value | |
|---|---|---|---|
| Total intracranial volume | 1044.53 ± 68.37 | 1200.87 ± 65.92 | <0.001 |
| Total grey matter | 644.65 ± 38.85 | 746.79 ± 39.37 | <0.001 |
| Total white matter | 383.68 ± 40.17 | 443.55 ± 36.27 | <0.001 |
| Lateral ventricle (right) | 3.02 (3.37) | 2.87 (2.87) | 0.501 |
| Lateral ventricle (left) | 3.80 (4.31) | 4.04 (3.04) | 0.319 |
| Third ventricle | 0.75 (0.28) | 0.68 (0.38) | 0.123 |
| Fourth ventricle | 1.58 ± 0.49 | 1.58 ± 0.71 | 1 |
| Frontal lobe (right) | 84.0 ± 7.58 | 99.02 ± 6.39 | <0.001 |
| Frontal lobe (left) | 83.77 ± 7.66 | 98.62 ±7.63 | <0.001 |
| Temporal lobe (right) | 50.89 ± 5.60 | 59.92 ± 6.25 | <0.001 |
| Temporal lobe (left) | 53.0 ± 6.04 | 62.19 ± 6.17 | <0.001 |
| Parietal lobe (right) | 59.79 ± 4.35 | 70.52 ± 5.19 | <0.001 |
| Parietal lobe (left) | 57.41 ± 4.67 | 67.64 ± 5.06 | <0.001 |
| Occipital lobe (right) | 24.27 ±3.15 | 27.37 ± 3.06 | <0.001 |
| Occipital lobe (left) | 23.74 ± 2.64 | 27.35 ± 3.21 | <0.001 |
| Cingulum (right) | 9.18 ±1.24 | 10.71 ± 1.43 | <0.001 |
| Cingulum (left) | 9.45 ± 1.16 | 11.34 ±1.87 | <0.001 |
| Thalamus (right) | 6.89 ± 0.71 | 7.58 ± 0.49 | <0.001 |
| Thalamus (left) | 7.13 ± 0.71 | 8.30 ± 0.83 | <0.001 |
| Caudate nucleus (right) | 3.33 ± 0.48 | 3.72 ± 0.51 | 0.004 |
| Caudate nucleus (left) | 3.36 ± 0.45 | 3.76 ± 0.48 | 0.002 |
| Putamen (right) | 5.10 ± 0.59 | 5.71 ± 0.54 | <0.001 |
| Putamen (left) | 5.17 ± 0.56 | 5.89 ± 0.65 | <0.001 |
| Pallidum (right) | 1.44 ± 0.23 | 1.54 ± 0.27 | 0.148 |
| Pallidum (left) | 1.62 ± 0.28 | 1.72 ± 0.23 | 0.162 |
| Hippocampus (right) | 3.97 ± 0.40 | 4.52 ± 0.43 | <0.001 |
| Hippocampus (left) | 3.95 ± 0.50 | 4.59 ± 0.43 | <0.001 |
| Amygdala (right) | 1.34 ± 0.16 | 1.58 ± 0.23 | <0.001 |
| Amygdala (left) | 1.34 ±0.15 | 1.55 ± 0.17 | <0.001 |
| Cerebellum grey matter (right) | 55.87 ± 5.78 | 62.46 ± 6.11 | <0.001 |
| Cerebellum grey matter (left) | 54.57 ± 5.69 | 60.81 ± 4.97 | <0.001 |
| Cerebellum white matter (right) | 11.73 ± 1.83 | 13.51 ± 1.73 | <0.001 |
| Cerebellum white matter (left) | 11.60 ± 1.53 | 133.21 ± 1.51 | <0.001 |
| Cluster 1 “Small” (N = 44) | Cluster 2 “Normal” (N = 21) | p-Value | |
|---|---|---|---|
| Age (years) | 10.5 ± 2.7 (6.1–14.1) | 10.4 ± 2.1 (6.5–14.6) | 0.89 |
| Male gender (%) | 45.5 | 66.7 | 0.186 |
| Maternal smoking (%) | 27.3 | 14.3 | 0.35 |
| Maternal education (%) | 0.815 | ||
| No studies | 9.1% | 7.1% | |
| Medium | 42.4% | 28.6% | |
| High education | 42.4% | 64.3% | |
| Gestational age at delivery (weeks) | 35.5 (9) | 40 (5) | 0.003 |
| Birthweight (gr) | 2240 ± 975 | 3053 ± 924 | 0.002 |
| Birthweight percentile | 14 (65) | 50 (83) | 0.079 |
| Preterm birth (%) | 52.3 | 23.8 | 0.036 |
| Severe preterm birth (%) | 29.5 | 9.5 | 0.12 |
| SGA (%) | 43.2 | 28.6 | 0.29 |
| Fetal growth restriction (%) | 25 | 14.3 | 0.52 |
| 2 or more risk factors * | 40.9 | 14.3 | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paules, C.; Pérez Roche, M.T.; Marin, M.A.; Fayed, N.; García-Martí, G.; Pisón, J.L.; Oros, D.; Pueyo, V. Different Brain Phenotypes in Magnetic Resonance Imaging of Healthy Children after Prenatal Insults. Diagnostics 2022, 12, 2748. https://doi.org/10.3390/diagnostics12112748
Paules C, Pérez Roche MT, Marin MA, Fayed N, García-Martí G, Pisón JL, Oros D, Pueyo V. Different Brain Phenotypes in Magnetic Resonance Imaging of Healthy Children after Prenatal Insults. Diagnostics. 2022; 12(11):2748. https://doi.org/10.3390/diagnostics12112748
Chicago/Turabian StylePaules, Cristina, María Teresa Pérez Roche, Miguel Angel Marin, Nicolás Fayed, Gracián García-Martí, Javier López Pisón, Daniel Oros, and Victoria Pueyo. 2022. "Different Brain Phenotypes in Magnetic Resonance Imaging of Healthy Children after Prenatal Insults" Diagnostics 12, no. 11: 2748. https://doi.org/10.3390/diagnostics12112748
APA StylePaules, C., Pérez Roche, M. T., Marin, M. A., Fayed, N., García-Martí, G., Pisón, J. L., Oros, D., & Pueyo, V. (2022). Different Brain Phenotypes in Magnetic Resonance Imaging of Healthy Children after Prenatal Insults. Diagnostics, 12(11), 2748. https://doi.org/10.3390/diagnostics12112748






