Differentiation of Intracerebral Tumor Entities with Quantitative Contrast Attenuation and Iodine Mapping in Dual-Layer Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Scans
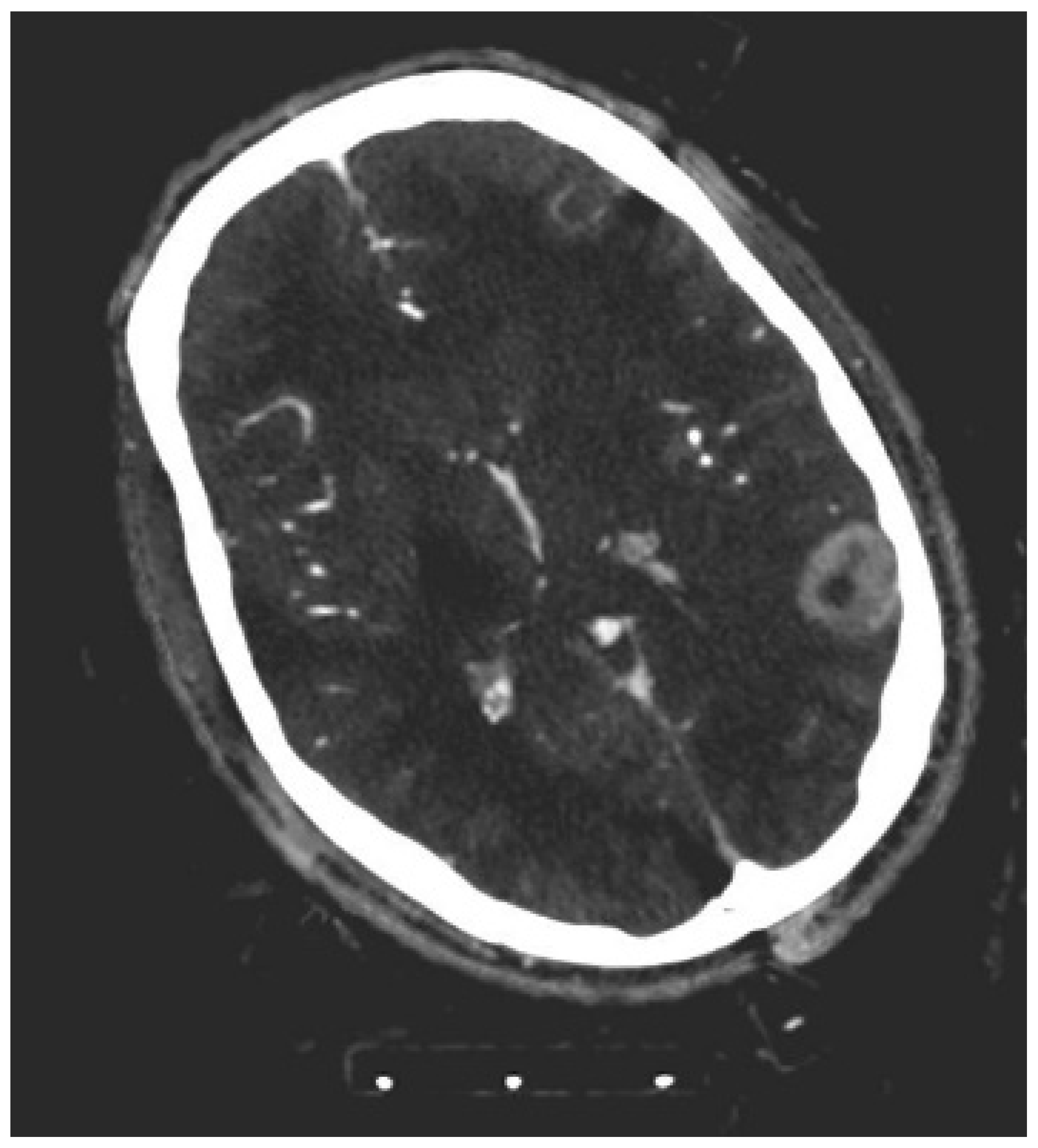
2.3. Objective Image Analysis
2.4. Stereotactic Needle Biopsy
2.5. Statistical Analysis
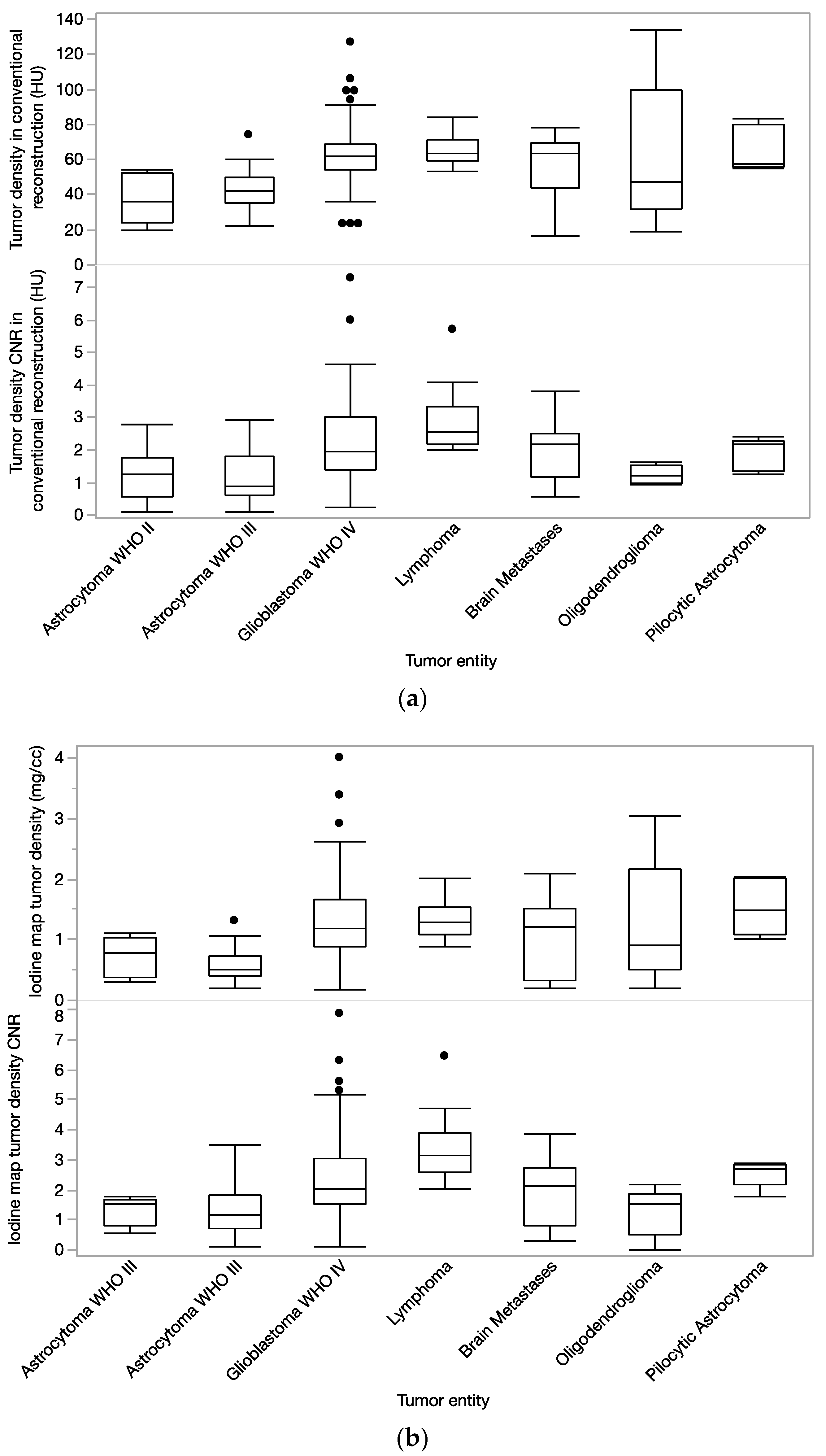
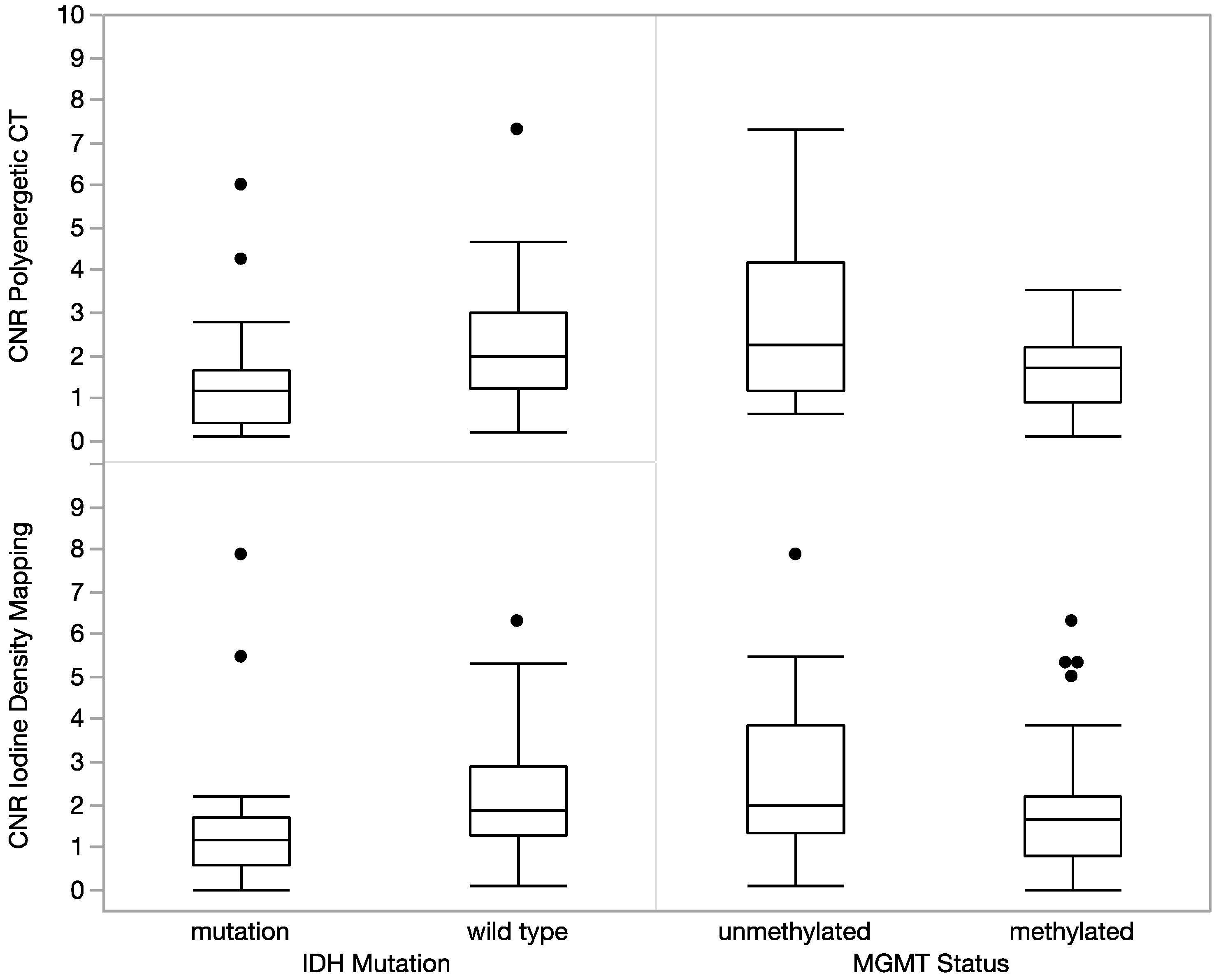
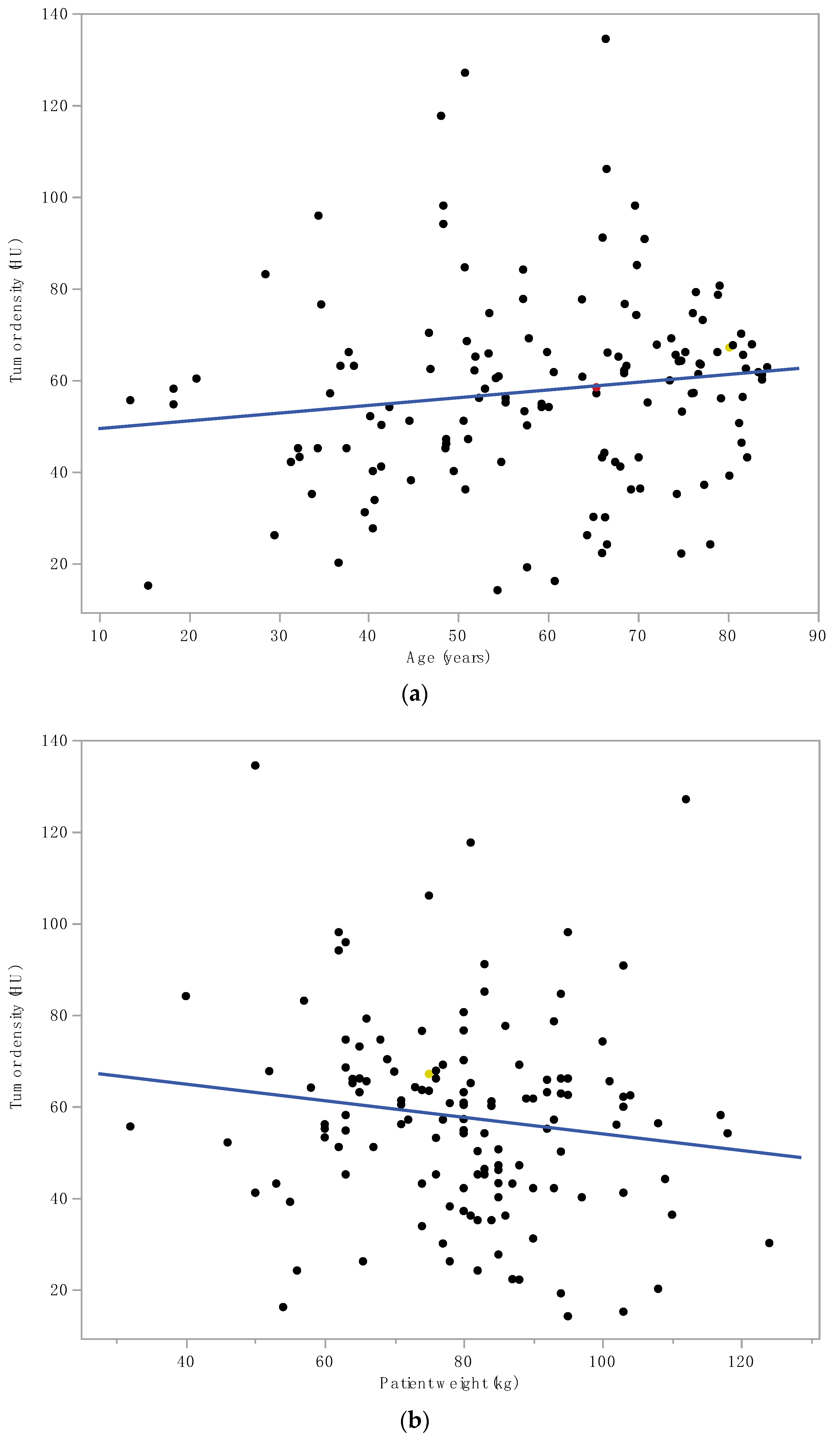
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cha, S. Neuroimaging in neuro-oncology. Neurotherapeutics 2009, 6, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.C.; Vogel, F.S.; Green, S.B.; Strike, T.A. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer 1985, 56, 1106–1111. [Google Scholar] [CrossRef]
- Okunieff, P.; Fenton, B.M.; Zhang, L.; Kern, F.G.; Wu, T.; Greg, J.R.; Ding, I. Fibroblast Growth Factors (FGFS) Increase Breast Tumor Growth Rate, Metastases, Blood Flow, and Oxygenation Without Significant Change in Vascular Density. Adv. Exp. Med. Biol. 2003, 530, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Fenton, B.M.; Paoni, S.F. Oxygenation and vascular perfusion in spontaneous and transplanted tumor models. Adv. Exp. Med. Biol. 2003, 530, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.; Wirsching, H.G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the molecular genetics of gliomas—Implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef]
- Yingying, L.; Zhe, Z.; Xiaochen, W.; Xiaomei, L.; Nan, J.; Shengjun, S. Dual-layer detector spectral CT—A new supplementary method for preoperative evaluation of glioma. Eur. J. Radiol. 2021, 138, 109649. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liu, C.; Zhao, L.; Jiang, J.; Zhang, J.; Li, S.; Zhu, W.; Wang, J. Amide proton transfer imaging allows detection of glioma grades and tumor proliferation: Comparison with Ki-67 expression and proton MR spectroscopy imaging. Am. J. Neuroradiol. 2017, 38, 1702–1709. [Google Scholar] [CrossRef]
- Paech, D.; Windschuh, J.; Oberhollenzer, J.; Dreher, C.; Sahm, F.; Meissner, J.E.; Goerke, S.; Schuenke, P.; Zaiss, M.; Regnery, S.; et al. Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro-Oncology 2018, 20, 1661–1671. [Google Scholar] [CrossRef]
- Togao, O.; Yoshiura, T.; Keupp, J.; Hiwatashi, A.; Yamashita, K.; Kikuchi, K.; Suzuki, Y.; Suzuki, S.O.; Iwaki, T.; Hata, N.; et al. Amide proton transfer imaging of adult diffuse gliomas: Correlation with histopathological grades. Neuro-Oncology 2014, 16, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ellika, S.K.; Jain, R.; Patel, S.C.; Scarpace, L.; Schultz, L.R.; Rock, J.P.; Mikkelsen, T. Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. Am. J. Neuroradiol. 2007, 28, 1981–1987. [Google Scholar] [CrossRef]
- Wu, L.M.; Li, Y.L.; Yin, Y.H.; Hou, G.Q.; Zhu, R.; Hua, X.L.; Xu, J.R.; Chen, Z.A. Usefulness of dual-energy computed tomography imaging in the differential diagnosis of sellar meningiomas and pituitary adenomas: Preliminary report. PLoS ONE 2014, 9, e90658. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Brugnara, G.; Hansen, M.B.; Nowosielski, M.; Pflüger, I.; Schell, M.; Isensee, F.; Foltyn, M.; Neuberger, U.; Kessler, T.; et al. Noninvasive characterization of tumor angiogenesis and oxygenation in bevacizumab-treated recurrent glioblastoma by using dynamic susceptibility MRI: Secondary analysis of the European Organization for Research and Treatment of Cancer 26101 Trial. Radiology 2020, 297, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Blears, E.E.; Ross, E.; Lall, R.R.; Ortega-Barnett, J. Machine learning applications for the differentiation of primary central nervous system lymphoma from glioblastoma on imaging: A systematic review and meta-analysis. Neurosurg. Focus 2018, 45, E5. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Thiele, F.; Pennig, L.; Reimer, R.P.; Shakirin, G.; Zopfs, D.; Timmer, M.; Perkuhn, M.; Borggrefe, J. Fully automated segmentation of meningiomas using deep learning on multiparametric MRI: Automated segmentation yields accuracies as good as manual interreader variabilities. In Proceedings of the European Congress of Radiology, Vienna, Austria, 27 February–3 March 2019; Volume B-1424. [Google Scholar]
- Al-Okaili, R.N.; Krejza, J.; Woo, J.H.; Wolf, R.L.; O’Rourke, D.M.; Judy, K.D.; Poptani, H.; Melhem, E.R. Intraaxial Brain Masses: MR Imaging–based Diagnostic Strategy—Initial Experience. Radiology 2007, 243, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Flohr, T.; Petersilka, M.; Henning, A.; Ulzheimer, S.; Ferda, J.; Schmidt, B. Photon-counting CT review. Phys. Medica 2020, 79, 126–136. [Google Scholar] [CrossRef]
- Hokamp, N.G.; Maintz, D.; Shapira, N.; Chang, D.H.; Noël, P.B. Technical background of a novel detector-based approach to dual-energy computed tomography. Diagn. Interv. Radiol. 2020, 26, 68–71. [Google Scholar] [CrossRef]
- Silva, A.C.; Morse, B.G.; Hara, A.K.; Paden, R.G.; Hongo, N.; Pavlicek, W. Dual-energy (spectral) CT: Applications in abdominal imaging. Radiographics 2011, 31, 1031–1046. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and multi-energy CT: Principles, technical approaches, and clinical applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Wang, S.; Kim, S.; Chawla, S.; Wolf, R.L.; Knipp, D.E.; Vossough, A.; O’Rourke, D.; Judy, R.K.D.; Poptani, H.; Melhem, E.R. Differentiation between Glioblastomas, Solitary Brain Metastases, and Primary Cerebral Lymphomas Using Diffusion Tensor and Dynamic Susceptibility Contrast-Enhanced MR Imaging. Am. J. Neuroradiol. 2011, 32, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Liu, Y.; Ward, C.; Le, N.H.; Soni, N.; Maheshwarappa, R.P.; Monga, V.; Zhang, H.; Sonka, M.; Bathla, G. Machine learning based differentiation of glioblastoma from brain metastasis using MRI derived radiomics. Sci. Rep. 2021, 11, 10478. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, V1–V100. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2009, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Bunse, L.; Wick, W.; Bunse, T. Concepts in glioma immunotherapy. Cancer Immunol. Immunother. 2016, 65, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Shakirin, G.; Thiele, F.; Baeßler, B.; Zopfs, D.; Große Hokamp, N.; Timmer, M.; Kabbasch, C.; Perkuhn, M.; Borggrefe, J. Accuracy of Radiomics-Based Feature Analysis on Multiparametric Magnetic Resonance Images for Noninvasive Meningioma Grading. World Neurosurg 2019, 132, e366–e390. [Google Scholar] [CrossRef]
- Foltyn, M.; Nieto Taborda, K.N.; Neuberger, U.; Brugnara, G.; Reinhardt, A.; Stichel, D.; Heiland, S.; Herold-Mende, C.; Unterberg, A.; Debus, J.; et al. T2/FLAIR-mismatch sign for noninvasive detection of IDH-mutant 1p/19q non-codeleted gliomas: Validity and pathophysiology. Neuro-Oncology Adv. 2020, 2, vdaa004. [Google Scholar] [CrossRef]
- Ruge, M.I.; Rueß, D.; Hellerbach, A.; Treuer, H. Letter to the Editor: Low dose rate brachytherapy for the treatment of brain metastases. J. Neurosurg. 2015, 123, 1110–1112. [Google Scholar] [CrossRef] [PubMed]

| Conventional CT Density | CNR Conventional CT Density | SDCT Iodine Density | CNR Iodine Density | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BTE A | BTE B | Wilcoxon Z-Score | Wilcoxon r-Value | Wilcoxon Z-Score | Wilcoxon r-Value | Wilcoxon Z-Score | Wilcoxon r-Value | Wilcoxon Z-Score | Wilcoxon r-Value |
| Astrocytoma II | Pilozytic Astroytoma | 2.6 | <0.01 | 1.2 | ns | 2.5 | <0.05 | 2.6 | <0.05 |
| Astrocytoma II | Lymphoma | 3.2 | <0.01 | 2.7 | <0.01 | 2.9 | <0.01 | 3.3 | <0.001 |
| Astrocytoma II | Glioblastoma | 3.1 | <0.01 | 2.2 | <0.05 | 2.1 | <0.05 | 2.0 | <0.05 |
| Astrocytoma II | Oligodendroglioma | 0.8 | ns | 0.1 | ns | 0.6 | ns | 0.1 | ns |
| Astrocytoma II | Metastases | 2.6 | <0.01 | 1.4 | ns | 0.7 | ns | 1.3 | ns |
| Astrocytoma II | Astrocytoma III | 0.8 | ns | 0.1 | ns | 1.0 | ns | 1.3 | ns |
| Astrocytoma III | Pilozytic Astroytoma | 3.0 | <0.01 | 1.7 | ns (p = 0.08) | 3.2 | <0.01 | 2.6 | <0.01 |
| Astrocytoma III | Lymphoma | 4.2 | <0.0001 | 4.1 | <0.0001 | 4.4 | <0.0001 | 4.3 | <0.0001 |
| Astrocytoma III | Metastases | 2.6 | <0.01 | 2.7 | <0.01 | 2.1 | <0.05 | 1.9 | ns (p = 0.06) |
| Astrocytoma III | Glioblastoma | 4.7 | <0.0001 | 3.7 | <0.001 | 4.6 | <0.0001 | 3.6 | <0.001 |
| Astrocytoma III | Oligodendroglioma | 1.1 | ns | 0.4 | ns | 1.5 | ns | 0.4 | ns |
| Lymphoma | Metastases | 0.6 | ns | 2.0 | <0.05 | 1.1 | ns | 2.8 | <0.01 |
| Lymphoma | Oligodendroglioma | 1.1 | ns | 3.1 | <0.01 | 1.3 | ns | 3.0 | <0.01 |
| Lymphoma | Glioblastoma | 0.8 | ns | 2.3 | <0.05 | 0.7 | ns | 2.9 | <0.01 |
| Lymphoma | Pilozytic Astroytoma | 0.7 | ns | 2.0 | <0.05 | 0.8 | ns | 1.3 | ns |
| Glioblastoma | Oligodendroglioma | 0.8 | ns | 2.3 | <0.05 | 0.7 | ns | 1.8 | ns (p = 0.06) |
| Glioblastoma | Metastases | 0.1 | ns | 0.2 | ns | 0.9 | ns | 0.5 | ns |
| Glioblastoma | Pilozytic Astroytoma | 0.2 | ns | 0.3 | ns | 1.1 | ns | 1.1 | ns |
| Pilozytic Astroytoma | Oligodendroglioma | 0.8 | ns | 1.9 | ns (p = 0.06) | 1.0 | ns | 2.3 | <0.05 |
| Pilozytic Astroytoma | Metastases | 0.3 | ns | 0.2 | ns | 1.3 | ns | 1.2 | ns |
| Oligodendroglioma | Metastases | 0.3 | ns | 1.7 | ns (p = 0.08) | 1.0 | ns | 1.2 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borggrefe, J.; Gebest, M.P.; Hauger, M.; Ruess, D.; Mpotsaris, A.; Kabbasch, C.; Pennig, L.; Laukamp, K.R.; Goertz, L.; Kroeger, J.R.; et al. Differentiation of Intracerebral Tumor Entities with Quantitative Contrast Attenuation and Iodine Mapping in Dual-Layer Computed Tomography. Diagnostics 2022, 12, 2494. https://doi.org/10.3390/diagnostics12102494
Borggrefe J, Gebest MP, Hauger M, Ruess D, Mpotsaris A, Kabbasch C, Pennig L, Laukamp KR, Goertz L, Kroeger JR, et al. Differentiation of Intracerebral Tumor Entities with Quantitative Contrast Attenuation and Iodine Mapping in Dual-Layer Computed Tomography. Diagnostics. 2022; 12(10):2494. https://doi.org/10.3390/diagnostics12102494
Chicago/Turabian StyleBorggrefe, Jan, Max Philipp Gebest, Myriam Hauger, Daniel Ruess, Anastasios Mpotsaris, Christoph Kabbasch, Lenhard Pennig, Kai Roman Laukamp, Lukas Goertz, Jan Robert Kroeger, and et al. 2022. "Differentiation of Intracerebral Tumor Entities with Quantitative Contrast Attenuation and Iodine Mapping in Dual-Layer Computed Tomography" Diagnostics 12, no. 10: 2494. https://doi.org/10.3390/diagnostics12102494
APA StyleBorggrefe, J., Gebest, M. P., Hauger, M., Ruess, D., Mpotsaris, A., Kabbasch, C., Pennig, L., Laukamp, K. R., Goertz, L., Kroeger, J. R., & Doerner, J. (2022). Differentiation of Intracerebral Tumor Entities with Quantitative Contrast Attenuation and Iodine Mapping in Dual-Layer Computed Tomography. Diagnostics, 12(10), 2494. https://doi.org/10.3390/diagnostics12102494






