Radiomics Based on Nomogram Predict Pelvic Lymphnode Metastasis in Early-Stage Cervical Cancer
Abstract
1. Introduction
2. Materials and Methods
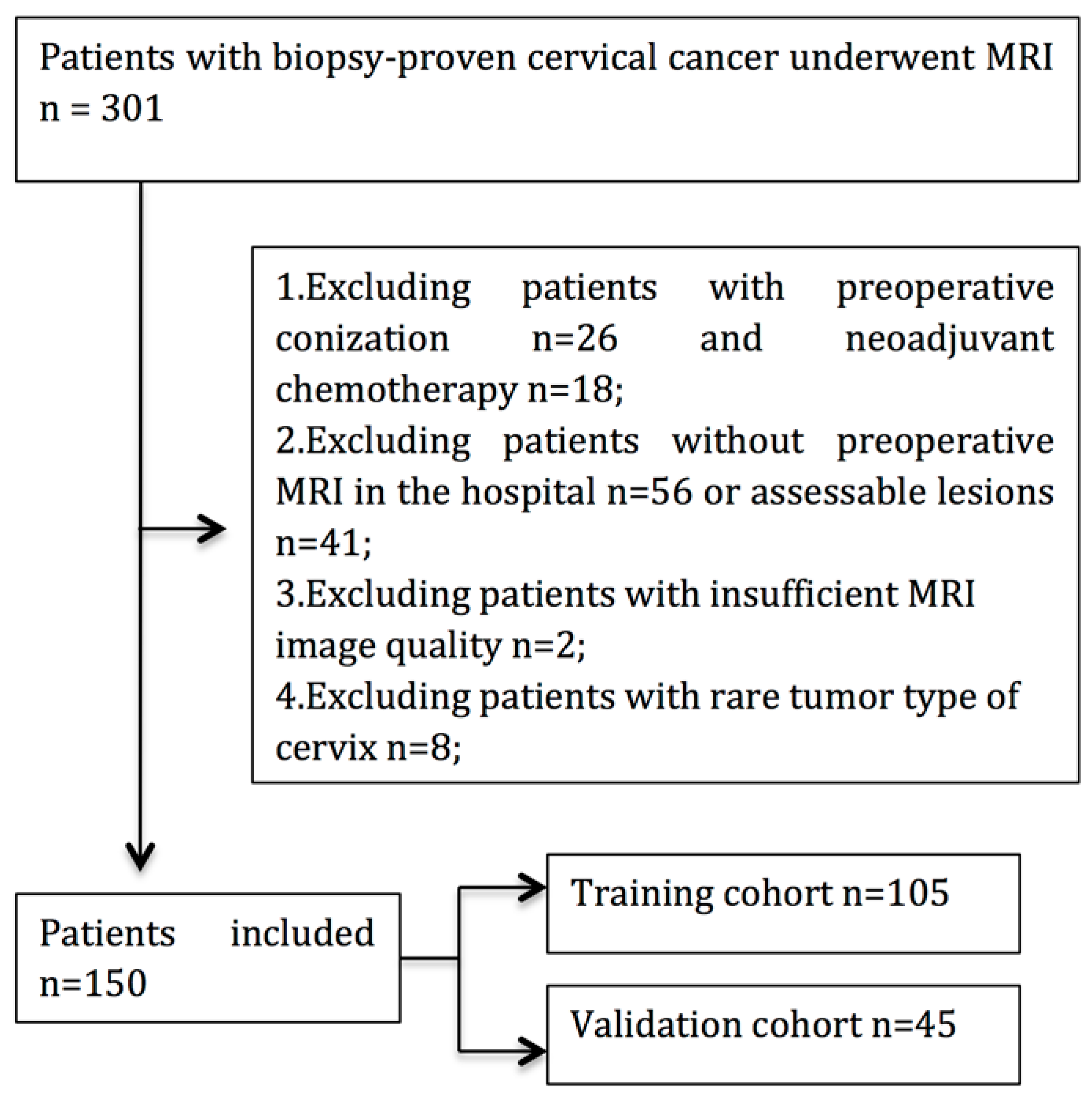
2.1. Patient
2.2. MRI Acquisition Protocol and Tumor Segmentation
2.3. Radiomic Feature Extraction
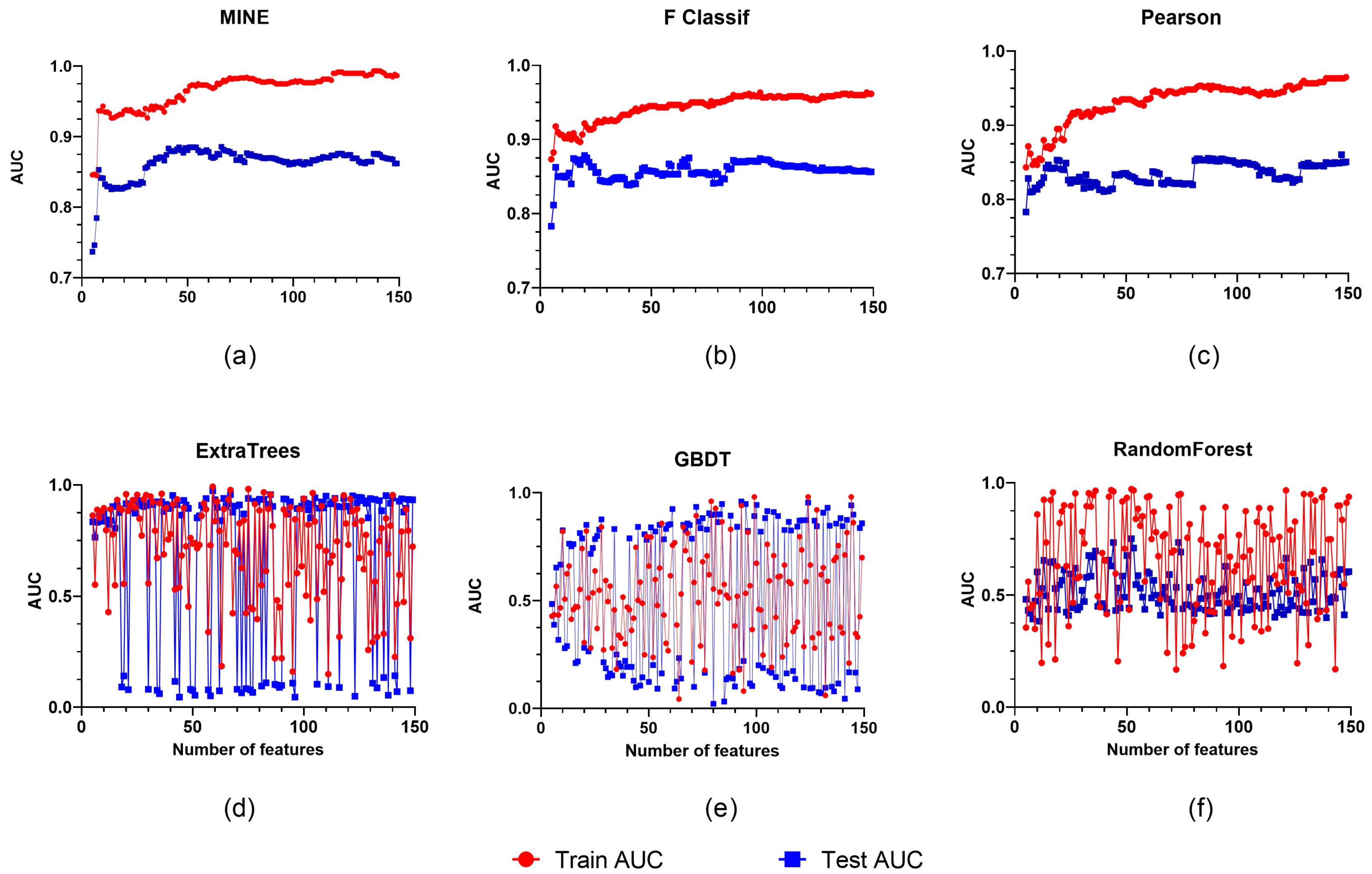
2.4. Radiomics Feature Selection and Development of the Radiomics Model
2.5. Assessment of Predictive Models
2.6. Statistical Analysis
3. Results
3.1. Patients’ Clinicopathologic Characteristics
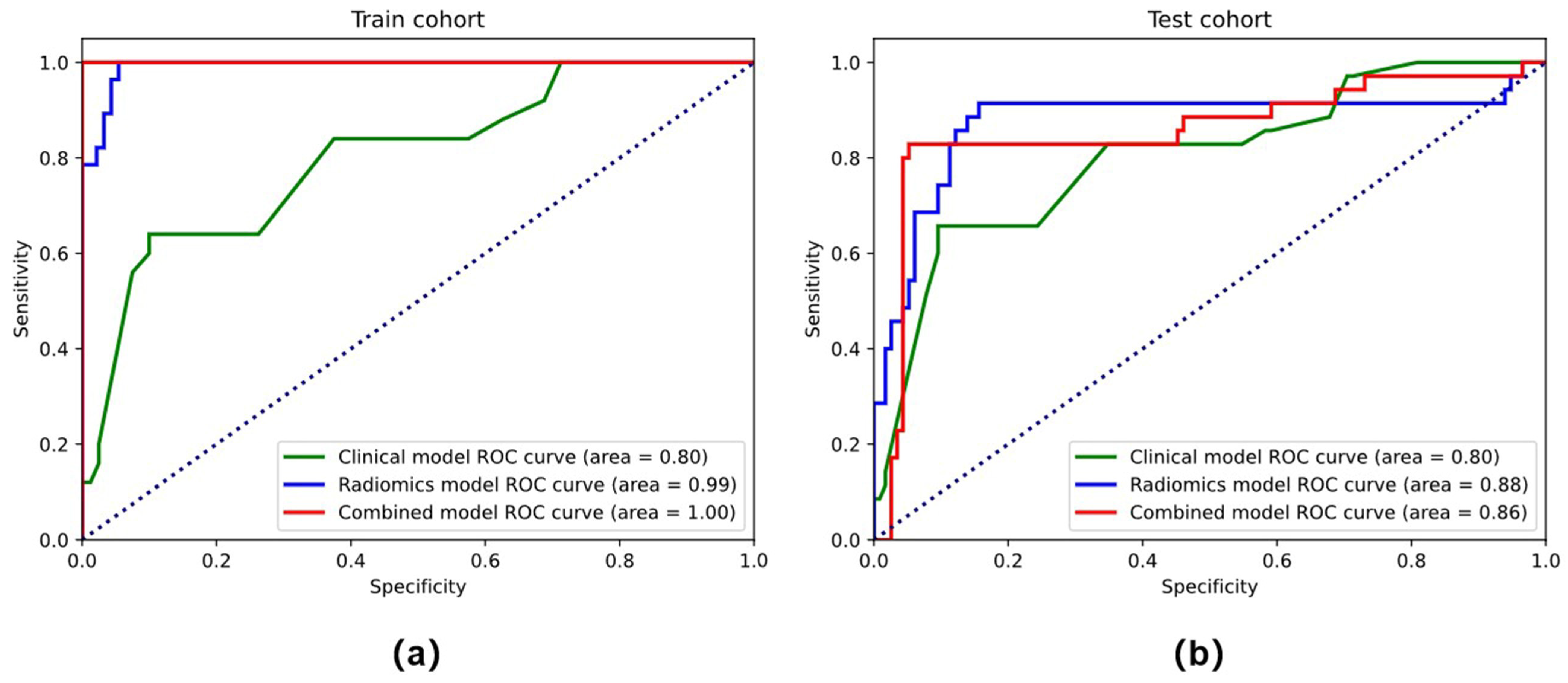
3.2. Feature Selection and Performance of the Clinical Model and Radiomics Model
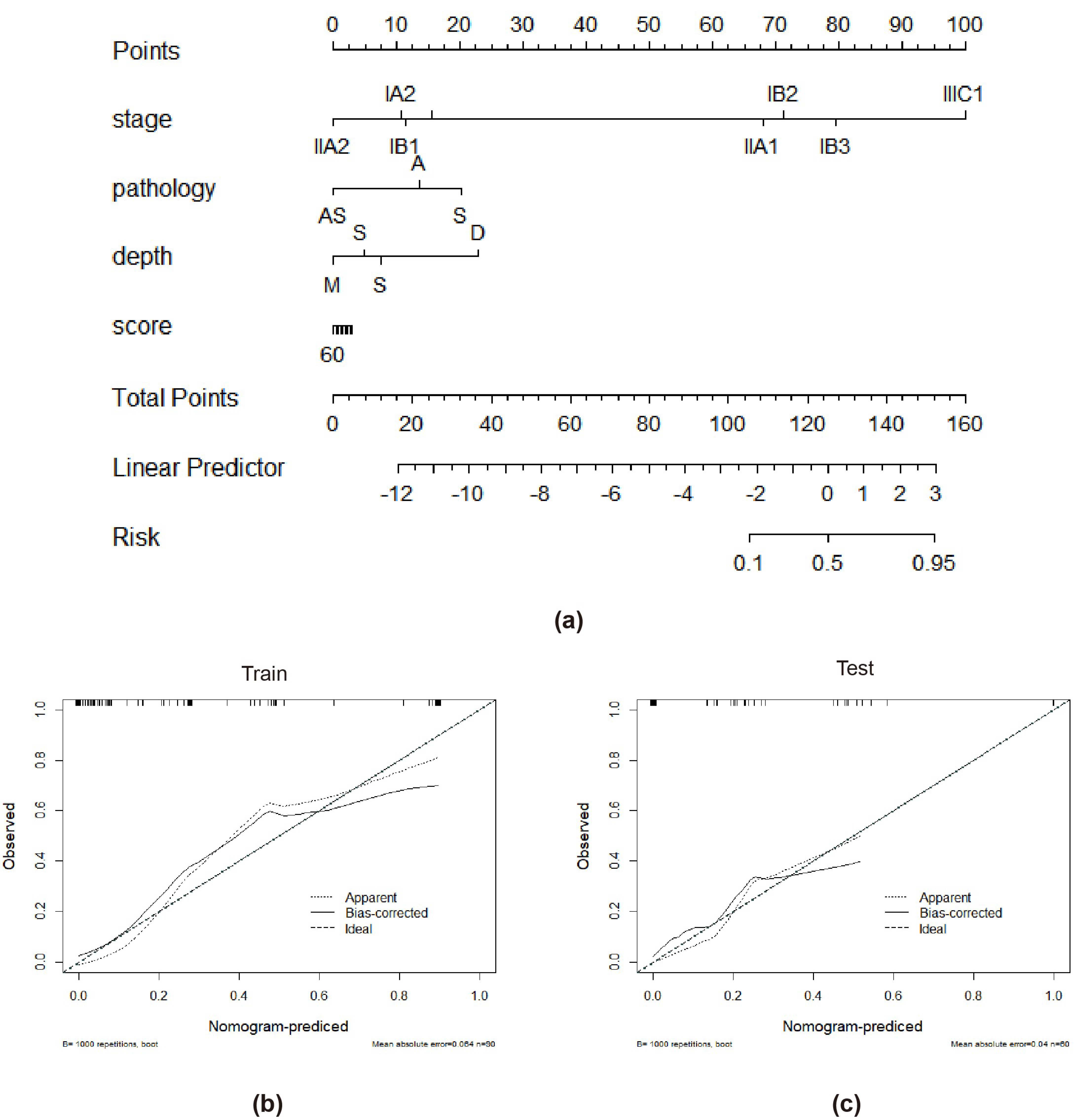
3.3. Performance of the Combined Model and the Radiomics Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.; Ngan, H.Y.; Pecorelli, S. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. S1), S43–S103. [Google Scholar] [CrossRef]
- Greggi, S.; Scaffa, C. Surgical management of early cervical cancer: The shape of future studies. Curr. Oncol. Rep. 2012, 14, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; Del Carmen, M.G.; Yang, J.; Seagle, B.L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.J.O.; et al. Survival After Minimally Invasive Radical Hysterectomy for Early-stage Cervical Cancer. JAMA Oncol. 2019, 74, 84–85. [Google Scholar] [CrossRef]
- Biewenga, P.; van der Velden, J.; Mol, B.W.; Stalpers, L.J.; Schilthuis, M.S.; van der Steeg, J.W.; Burger, M.P.; Buist, M.R. Prognostic model for survival in patients with early stage cervical cancer. Cancer 2011, 117, 768–776. [Google Scholar] [CrossRef]
- Bats, A.S.; Frati, A.; Mathevet, P.; Orliaguet, I.; Querleu, D.; Zerdoud, S.; Leblanc, E.; Gauthier, H.; Uzan, C.; Deandreis, D.; et al. Contribution of lymphoscintigraphy to intraoperative sentinel lymph node detection in early cervical cancer: Analysis of the prospective multicenter SENTICOL cohort. Gynecol. Oncol. 2015, 137, 264–269. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N.; Committee, E.G. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Diab, Y. Sentinel Lymph Nodes Mapping in Cervical Cancer a Comprehensive Review. Int. J. Gynecol. Cancer 2017, 27, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Anchora, L.P.; Gallotta, V.; Fagotti, A.; Vizza, E.; Chiantera, V.; De Iaco, P.; Ercoli, A.; Corrado, G.; Bottoni, C.; et al. Can We Define the Risk of Lymph Node Metastasis in Early-Stage Cervical Cancer Patients? A Large-Scale, Retrospective Study. Ann. Surg. Oncol. 2017, 24, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Balcacer, P.; Shergill, A.; Litkouhi, B. MRI of cervical cancer with a surgical perspective: Staging, prognostic implications and pitfalls. Abdom. Radiol. 2019, 44, 2557–2571. [Google Scholar] [CrossRef] [PubMed]
- Khiewvan, B.; Torigian, D.A.; Emamzadehfard, S.; Paydary, K.; Salavati, A.; Houshmand, S.; Shamchi, S.P.; Werner, T.J.; Aydin, A.; Roy, S.G.; et al. Update of the role of PET/CT and PET/MRI in the management of patients with cervical cancer. Hell. J. Nucl. Med. 2016, 19, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Zikan, M.; Slama, J.; Fischerova, D.; Kocian, R.; Germanova, A.; Burgetova, A.; Dusek, L.; Dundr, P.; Gregova, M.; et al. Risk of micrometastases in non-sentinel pelvic lymph nodes in cervical cancer. Gynecol. Oncol. 2016, 143, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zheng, D.; Shi, L.; Liu, M.; Wang, M.; Shi, D. Differentiating metastatic from nonmetastatic lymph nodes in cervical cancer patients using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging. Eur. Radiol. 2017, 27, 5272–5279. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yin, J. Application of Texture Analysis Based on Sagittal Fat-Suppression and Oblique Axial T2-Weighted Magnetic Resonance Imaging to Identify Lymph Node Invasion Status of Rectal Cancer. Front. Oncol. 2020, 10, 1364. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, Y.; Liu, Z.; Zhou, Z.; Lai, B.; Sun, K.; Li, L.; Huang, L.; Feng, Y.; Cao, W.; et al. Radiomics-Based Preoperative Prediction of Lymph Node Status Following Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Front. Oncol. 2020, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Feng, F.; Qiu, Y.J.; Zheng, G.H.; Ge, Y.Q.; Wang, Y.T. High-resolution MRI-based radiomics analysis to predict lymph node metastasis and tumor deposits respectively in rectal cancer. Abdom. Radiol. 2021, 46, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Berek, J.S.; Fredes, M.C.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Cree, I.A.; White, V.A.; Indave, B.I.; Lokuhetty, D. Revising the WHO classification: Female genital tract tumours. Histopathology 2020, 76, 151–156. [Google Scholar] [CrossRef]
- Chalkidou, A.; O’Doherty, M.; Marsden, P. False Discovery Rates in PET and CT Studies with Texture Features: A Systematic Review. PLoS ONE 2015, 10, e0124165. [Google Scholar] [CrossRef]
- Lecuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Darai, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: Results of the SENTICOL study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef]
- Bhosale, P.R.; Iyer, R.B.; Ramalingam, P.; Schmeler, K.M.; Wei, W.; Bassett, R.L.; Ramirez, P.T.; Frumovitz, M. Is MRI helpful in assessing the distance of the tumour from the internal os in patients with cervical cancer below FIGO Stage IB2? Clin. Radiol. 2016, 71, 515–522. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.J.; Kim, Y.W. Correlation between tumor size and surveillance of lymph node metastasis for IB and IIA cervical cancer by magnetic resonance images. Eur. J. Radiol. 2012, 81, 1945–1950. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Yang, J.; Xue, H.; Cao, D.; Huang, H.; Wu, M.; Cui, Q.; Chen, J.; Lang, J.; et al. The role of magnetic resonance imaging in pretreatment evaluation of early-stage cervical cancer. Int. J. Gynecol. Cancer 2014, 24, 1292–1298. [Google Scholar] [CrossRef]
- Lv, K.; Guo, H.M.; Lu, Y.J.; Wu, Z.X.; Zhang, K.; Han, J.K. Role of 18F-FDG PET/CT in detecting pelvic lymph-node metastases in patients with early-stage uterine cervical cancer: Comparison with MRI findings. Nucl. Med. Commun. 2014, 35, 1204–1211. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Choi, H.J.; Ju, W.; Myung, S.K.; Kim, Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: Meta-analysis. Cancer Sci. 2010, 101, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gao, T.; Yang, J.; Yan, X.; Wang, Y.; Zhou, X.; Tian, J.; Huang, L.; Zhang, M. Preoperative prediction of pelvic lymph nodes metastasis in early-stage cervical cancer using radiomics nomogram developed based on T2-weighted MRI and diffusion-weighted imaging. Eur. J. Radiol. 2019, 114, 128–135. [Google Scholar] [CrossRef]
- Kan, Y.; Dong, D.; Zhang, Y.; Jiang, W.; Zhao, N.; Han, L.; Fang, M.; Zang, Y.; Hu, C.; Tian, J.; et al. Radiomic signature as a predictive factor for lymph node metastasis in early-stage cervical cancer. J. Magn. Reason. Imaging 2019, 49, 304–310. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Zhang, R.; Dong, R.T.; Hu, Q.Y.; Yu, T.; Liu, F.; Luo, Y.H.; Dong, Y. Feasibility of an ADC-based radiomics model for predicting pelvic lymph node metastases in patients with stage IB-IIA cervical squamous cell carcinoma. Br. J. Radiol. 2019, 92, 20180986. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, C.H.; Lu, H.Y.; Chiang, H.J.; Wang, H.K.; Huang, Y.T.; Ng, S.H.; Hong, J.H.; Yen, T.C.; Lai, C.H.; et al. Deep learning for fully automated tumor segmentation and extraction of magnetic resonance radiomics features in cervical cancer. Eur. Radiol. 2020, 30, 1297–1305. [Google Scholar] [CrossRef]


| Characteristics | Training Cohort (n = 104) | Validation Cohort (n = 45) | p * | ||||
|---|---|---|---|---|---|---|---|
| PLNM(+) | PLNM(−) | PLNM(+) | PLNM(−) | ||||
| (n = 25) | (n = 80) | p | (n = 10) | (n = 35) | p | ||
| Age, years | 0.095 | 0.312 | 0.520 | ||||
| Mean | 47.12 | 46.66 | 43.30 | 46.60 | |||
| FIGO Stage (N, %) | 0.787 | 0.208 | 0.871 | ||||
| IA | 0 | 2 | 1 | 0 | |||
| IB | 15 | 52 | 5 | 22 | |||
| IIA | 10 | 26 | 4 | 13 | |||
| MTD, cm | 0.007 | 0.220 | 0.815 | ||||
| Mean | 3.95 | 2.77 | 2.97 | 3.07 | |||
| Histology (N, %) | 0.331 | 0.657 | 0.664 | ||||
| SCC | 19 | 67 | 10 | 30 | |||
| AC | 4 | 11 | 0 | 4 | |||
| ASC | 2 | 2 | 0 | 1 | |||
| Stromal Invasion | <0.001 | 0.261 | 0.448 | ||||
| Deep 1/3 | 21 | 28 | 6 | 10 | |||
| Middle 1/3 | 2 | 24 | 2 | 12 | |||
| Superficial 1/3 | 2 | 28 | 2 | 13 | |||
| LVSI status | <0.001 | 0.010 | 0.519 | ||||
| Positive | 2 | 57 | 1 | 21 | |||
| Negative | 23 | 23 | 9 | 14 | |||
| NI status | 0.627 | 0.016 | 0.185 | ||||
| Positive | 23 | 76 | 6 | 33 | |||
| Negative | 2 | 4 | 4 | 2 | |||
| Training Cohort | Testing Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | ACC (%) | SEN (%) | SPE (%) | AUC | ACC (%) | SEN (%) | SPE (%) | ||
| Clinical | stage | 0.832 | 0.816 | 0.708 | 0.920 | 0.767 | 0.771 | 0.657 | 0.886 |
| pathology | 0.476 | 0.531 | 0.08 | 0.96 | 0.527 | 0.485 | 0.08 | 0.886 | |
| diameter | 0.712 | 0.714 | 0.75 | 0.68 | 0.728 | 0.729 | 0.771 | 0.685 | |
| Stage+pathology+diameter | 0.925 | 0.816 | 0.703 | 0.920 | 0.839 | 0.742 | 0.657 | 0.828 | |
| Radiomics model | 0.975 | 0.918 | 0.920 | 0.917 | 0.852 | 0.771 | 0.829 | 0.714 | |
| Combined model | 0.988 | 0.959 | 0.920 | 1.00 | 0.922 | 0.871 | 0.857 | 0.886 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Li, D.; Du, W.; Wang, Y.; Nie, S.; Tan, Q.; Gou, Q. Radiomics Based on Nomogram Predict Pelvic Lymphnode Metastasis in Early-Stage Cervical Cancer. Diagnostics 2022, 12, 2446. https://doi.org/10.3390/diagnostics12102446
Xia X, Li D, Du W, Wang Y, Nie S, Tan Q, Gou Q. Radiomics Based on Nomogram Predict Pelvic Lymphnode Metastasis in Early-Stage Cervical Cancer. Diagnostics. 2022; 12(10):2446. https://doi.org/10.3390/diagnostics12102446
Chicago/Turabian StyleXia, Xueming, Dongdong Li, Wei Du, Yu Wang, Shihong Nie, Qiaoyue Tan, and Qiheng Gou. 2022. "Radiomics Based on Nomogram Predict Pelvic Lymphnode Metastasis in Early-Stage Cervical Cancer" Diagnostics 12, no. 10: 2446. https://doi.org/10.3390/diagnostics12102446
APA StyleXia, X., Li, D., Du, W., Wang, Y., Nie, S., Tan, Q., & Gou, Q. (2022). Radiomics Based on Nomogram Predict Pelvic Lymphnode Metastasis in Early-Stage Cervical Cancer. Diagnostics, 12(10), 2446. https://doi.org/10.3390/diagnostics12102446




