Prognostic Factors for Invasiveness and Recurrence of Pituitary Adenomas: A Series of 94 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Endocrine and Radiological Screening
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Patient’s and Pituitary Adenoma’s Characteristics
3.2. Association of Patient’s Clinical and Epidemiological Characteristics with Tumor Characteristics
3.3. Immunohistopathological Markers of Pituitary Adenomas
3.3.1. Cyclin-D1
3.3.2. Ki-67 Index Levels
3.3.3. CD-56 Expression
3.3.4. E-Cadherin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melmed, S. Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol. 2011, 7, 257–266. [Google Scholar] [CrossRef]
- Ntali, G.; Wass, J.A. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 2018, 21, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Syro, L.V.; Rotondo, F.; Ramirez, A.; Di Ieva, A.; Sav, M.A.; Restrepo, L.M.; Serna, C.A.; Kovacs, K. Progress in the Diagnosis and Classification of Pituitary Adenomas. Front. Endocrinol. 2015, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.P.; Park, I.W.; Chung, Y.S. The volume of tumor mass and visual field defect in patients with pituitary macroadenoma. Korean J. Ophthalmol. 2011, 25, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, W.; Wang, X.; Yu, X.; Li, Z.; Guan, Y. Follicle-Stimulating Hormone-Secreting Pituitary Adenoma Inducing Spontaneous Ovarian Hyperstimulation Syndrome, Treatment Using In Vitro Fertilization and Embryo Transfer: A Case Report. Front. Endocrinol. 2021, 12, 621456. [Google Scholar] [CrossRef]
- Al-Shraim, M.; Asa, S.L. The 2004 World Health Organization classification of pituitary tumors: What is new? Acta Neuropathol. 2006, 111, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the pituitary gland: A summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Doglietto, F.; Trapasso, B.; Iacovazzo, D.; Giampietro, A.; Di Nardo, F.; de Waure, C.; Lauriola, L.; Mangiola, A.; Anile, C.; et al. Typical and atypical pituitary adenomas: A single-center analysis of outcome and prognosis. Neuroendocrinology 2015, 101, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Miermeister, C.P.; Petersenn, S.; Buchfelder, M.; Fahlbusch, R.; Lüdecke, D.K.; Hölsken, A.; Bergmann, M.; Ulrich Knappe, H.; Hans, V.H.; Flitsch, J.; et al. Histological criteria for atypical pituitary adenomas—Data from the German pituitary adenoma registry suggests modifications. Acta Neuropathol. Commun. 2015, 3, 50. [Google Scholar] [CrossRef]
- Trouillas, J.; Roy, P.; Sturm, N.; Dantony, E.; Cortet-Rudelli, C.; Viennet, G.; Bonneville, J.F.; Assaker, R.; Auger, C.; Brue, T.; et al. A new prognostic clinicopathological classification of pituitary adenomas: A multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013, 126, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Ezzat, S.; Asa, S.L. Biomarkers of aggressive pituitary adenomas. J. Mol. Endocrinol. 2012, 49, R69–R78. [Google Scholar] [CrossRef]
- Kontogeorgos, G. Predictive markers of pituitary adenoma behavior. Neuroendocrinology 2006, 83, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Wierinckx, A.; Auger, C.; Devauchelle, P.; Reynaud, A.; Chevallier, P.; Jan, M.; Perrin, G.; Fevre-Montange, M.; Rey, C.; Figarella-Branger, D.; et al. A diagnostic marker set for invasion, proliferation, and aggressiveness of prolactin pituitary tumors. Endocr. Relat. Cancer 2007, 14, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Cooper, O.; Bonert, V.; Mamelak, A.N.; Bannykh, S.; Melmed, S. Dural Invasion as a Marker of Aggressive Pituitary Adenomas. Neurosurgery 2022, 190, 775–783. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Acitores Cancela, A.; Vior, C.; Pascual-Corrales, E.; Rodríguez Berrocal, V. Radiological Knosp, Revised-Knosp, and Hardy–Wilson Classifications for the Prediction of Surgical Outcomes in the Endoscopic Endonasal Surgery of Pituitary Adenomas: Study of 228 Cases. Front. Oncol. 2022, 11, 807040. [Google Scholar] [CrossRef]
- Zhou, K.; Jin, H.; Luo, Y. Expression and significance of E-cadherin and β-catenins in pituitary adenoma. Int. J. Surg. Pathol. 2013, 21, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Hibberts, N.A.; Simpson, D.J.; Bicknell, J.E.; Broome, J.C.; Hoban, P.R.; Clayton, R.N.; Farrell, W.E. Analysis of cyclin D1 (CCND1) allelic imbalance and overexpression in sporadic human pituitary tumors. Clin. Cancer Res. 1999, 5, 2133–2139. [Google Scholar] [PubMed]
- Gruppetta, M.; Formosa, R.; Falzon, S.; Ariff Scicluna, S.; Falzon, E.; Degeatano, J.; Vassallo, J. Expression of cell cycle regulators and biomarkers of proliferation and regrowth in human pituitary adenomas. Pituitary 2017, 20, 358–371. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, K.H.; Kwon, J.H.; Kim, H.D.; Kim, Y.Z. Results of immunohistochemical staining of cell-cycle regulators: The prediction of recurrence of functioning pituitary adenoma. World Neurosurg. 2014, 81, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Hewedi, I.H.; Osman, W.M.; El Mahdy, M.M. Differential expression of cyclin D1 in human pituitary tumors: Relation to MIB-1 and p27/Kip1 labeling indices. J. Egypt. Natl. Cancer Inst. 2011, 23, 171–179. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.; Chong, H.; Hobbs, C.; Datta, C.; Hall, P.A. Detection of the Ki-67 antigen in fixed and wax-embedded sections with the monoclonal antibody MIB1. Histopathology 1993, 22, 355–360. [Google Scholar] [CrossRef]
- Wierzbicka-Tutka, I.; Sokołowski, G.; Bałdys-Waligórska, A.; Adamek, D.; Radwańska, E.; Gołkowski, F. PTTG and Ki-67 expression in pituitary adenomas. Przegląd Lek. 2016, 73, 53–58. [Google Scholar]
- Glebauskiene, B.; Liutkeviciene, R.; Vilkeviciute, A.; Gudinaviciene, I.; Rocyte, A.; Simonaviciute, D.; Mazetyte, R.; Kriauciuniene, L.; Zaliuniene, D. Association of Ki-67 Labelling Index and IL-17A with Pituitary Adenoma. Biomed Res. Int. 2018, 2018, 7490585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tomono, Y.; Nose, T. Expression of P27kip 1 and Ki-67 in Pituitary Adenomas: An Investigation of Marker of Adenoma Invasiveness. Acta Neurochir. 1999, 141, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.L.; Xekouki, E.; King, A.; Thomas, N.; Barazi, S.; Maratos, E.; Bullock, P.; Whitelaw, B.; Gilbert, J.; Aylwin, S. Endocrine Abstracts; Bioscientifica: Bristol, UK, 2020; Volume 70, p. AEP560. [Google Scholar] [CrossRef]
- Huuhtanen, R.L.; Blomqvist, C.P.; Böhling, T.O.; Wiklund, T.A.; Tukiainen, E.J.; Virolainen, M.; Tribukait, B.; Andersson, L.C. Expression of cyclin A in soft tissue sarcomas correlates with tumor aggressiveness. Cancer Res. 1999, 59, 2885–2890. [Google Scholar]
- Nomikos, P.; Buchfelder, M.; Fahlbusch, R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical “cure”. Eur. J. Endocrinol. 2005, 152, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.A.; Haag, T.; Trott, G.; Rech, C.G.S.L.; Ferreira, N.P.; Oliveira, M.C.; Kohek, M.B.; Pereira-Lima, J.F.S. Expression of E-cadherin, Slug and NCAM and its relationship to tumor invasiveness in patients with acromegaly. Braz. J. Med. Biol. Res. 2017, 51, e6808. [Google Scholar] [CrossRef] [PubMed]
- Gattenlöhner, S.; Stühmer, T.; Leich, E.; Reinhard, M.; Etschmann, B.; Völker, H.U.; Rosenwald, A.; Serfling, E.; Bargou, R.C.; Ertl, G.; et al. Specific detection of CD56 (NCAM) isoforms for the identification of aggressive malignant neoplasms with progressive development. Am. J. Pathol. 2009, 174, 1160–1171. [Google Scholar] [CrossRef]
- Picó, A.; Aranda-López, I.; Sesmilo, G.; Toldos-González, Ó.; Japón, M.A.; Luque, R.M.; Puig-Domingo, M. Recomendaciones sobre el diagnóstico e informe anatomopatológico de los tumores neuroendocrinos hipofisarios. Consenso de expertos de la Sociedad Española de Endocrinologia y Nutrición y de la Sociedad Española de Anatomía Patológica [Recommendations on the pathological report of pituitary tumors. A consensus of experts of the Spanish Society of Endocrinology and Nutrition and the Spanish Society of Pathology]. Rev. Esp. Patol. 2021, 54, 263–274. (In Spanish) [Google Scholar] [CrossRef]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Grieve, J.; Dorward, N.; Mendoza, N.; Nair, R.; Muquit, S.; et al. The expression of neural cell adhesion molecule and the microenvironment of pituitary neuroendocrine tumours. J. Neuroendocrinol. 2021, 33, e13052. [Google Scholar] [CrossRef] [PubMed]
- Ongaratti, B.R.; Haag, T.; D’Ávila, M.F.; Trott, G.; Ferreira, N.P.; Rech, C.G.; Pereira-Lima, J.F.; da Costa Oliveira, M. Gene and protein expression of E-cadherin and NCAM markers in non-functioning pituitary adenomas. Ann. Diagn. Pathol. 2019, 38, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt-DeMasters, B.K.; Conway, D.R.; Franklin, W.A.; Lillehei, K.O.; Kruse, C.A. Neural cell adhesion molecule expression in human pituitary adenomas. J. Neuro-Oncol. 1995, 25, 205–213. [Google Scholar] [CrossRef]
- De Jong, I.; Aylwin, S.J.B.; Olabiran, Y.; Geddes, J.F.; Monson, J.P.; Wood, D.F.; Burrin, J.M. Expression and secretion of neural cell adhesion molecules by human pituitary adenomas. Ann. Clin. Biochem. 1999, 36, 660–665. [Google Scholar] [CrossRef]
- Moreno-Bueno, G.; Cubillo, E.; Sarrió, D.; Peinado, H.; Rodríguez-Pinilla, S.M.; Villa, S.; Bolós, V.; Jordá, M.; Fabra, A.; Portillo, F.; et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006, 66, 9543–9556. [Google Scholar] [CrossRef]
- Gil, J.; Jordà, M.; Soldevila, B.; Puig-Domingo, M. Epithelial-Mesenchymal Transition in the Resistance to Somatostatin Receptor Ligands in Acromegaly. Front. Endocrinol. 2021, 12, 646210. [Google Scholar] [CrossRef]
- Chauvet, N.; Romanò, N.; Meunier, A.C.; Galibert, E.; Fontanaud, P.; Mathieu, M.N.; Osterstock, G.; Osterstock, P.; Baccino, E.; Rigau, V.; et al. Combining Cadherin Expression with Molecular Markers Discriminates Invasiveness in Growth Hormone and Prolactin Pituitary Adenomas. J. Neuroendocrinol. 2016, 28, 12352. [Google Scholar] [CrossRef]
- Yamada, S.; Ohyama, K.; Taguchi, M.; Takeshita, A.; Morita, K.; Takano, K.; Sano, T. A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery 2007, 61, 580–584; discussion 584-5. [Google Scholar] [CrossRef]
- Kawamoto, H.; Mizoue, T.; Arita, K.; Tominaga, A.; Eguchi, K.; Kurisu, K. Expression of epithelial cadherin and cavernous sinus invasion in human pituitary adenomas. J. Neurooncol. 1997, 34, 105–109. [Google Scholar] [CrossRef]
- Lipe, K.A.; Fishbein MLKerr, J.M.; Youssef, S.A.; Lillehei, K.O.; Wierman, M.E.; Kleinschmidt, B.K.; Kiseljak-Vassiliades, K. Clinical Correlation to E-cadherin and Granulation Patterns in Corticotroph Tumors. J. Endocr. Soc. 2021, 5 (Suppl.1), A519. [Google Scholar] [CrossRef]
- Fougner, S.L.; Lekva, T.; Borota, O.C.; Hald, J.K.; Bollerslev, J.; Berg, J.P. The Expression of E-Cadherin in Somatotroph Pituitary Adenomas Is Related to Tumor Size, Invasiveness, and Somatostatin Analog Response. J. Clin. Endocrinol. Metab. 2010, 95, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.J.; Fryer, A.A.; Grossman, A.B.; Wass, J.A.; Pfeifer, M.; Kros, J.M.; Clayton, R.N.; Farrell, W.E. Cyclin D1 (CCND1) genotype is associated with tumour grade in sporadic pituitary adenomas. Carcinogenesis 2001, 22, 1801–1807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kusumoto, K.; Kikuchi, M.; Fujiwara, K.; Horiguchi, K.; Kouki, T.; Kawanishi, K.; Yashiro, T. Effect of E-cadherin expression on hormone production in rat anterior pituitary lactotrophs in vitro. Acta Histochem. Et Cytochem. 2010, 43, 83–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolnes, A.J.; Øystese, K.A.B.; Olarescu, N.C.; Ringstad, G.; Berg-Johnsen, J.; Casar-Borota, O.; Bollerslev, J.; Jørgensen, A.P. FSH Levels Are Related to E-cadherin Expression and Subcellular Location in Nonfunctioning Pituitary Tumors. J. Clin. Endocrinol. Metab. 2020, 105, 2587–2594. [Google Scholar] [CrossRef] [PubMed]



| Variables | Frequency or Mean ± SD |
|---|---|
| Age (yrs) Diagnose age | 48.6 ± 13.5 46.5 ± 13.9 |
| Gender | |
| Male Female | 39/94 (41.5%) 55/94 (58.5%) |
| Tumor Diameter (cm) | 2.1 ± 1.1 |
| Tumor Size | |
| Macroadenoma (≥1 cm) Microadenoma (<1 cm) | 65/94 (69.1%) 29/94 (30.9%) |
| Adenoma subtype | |
| GH ACTH PRL Non-secreting (non-functioning) | 24/94 (25.5%) 10/94 (10.6%) 3/94 (3.2%) 57/94 (60.6%) |
| Symptoms | |
| Apoplexy Visual Deficit Headache | 6/94 (6.4%) 51/94 (54.3%) 33/94 (35.1%) |
| Invasiveness | |
| Non-invasive Invasive | 59/94 (62.8%) 35/94 (37.2%) |
| Resection | |
| Total Partial | 67/94 (71.3%) 27/94 (28.7%) |
| Resection rate | |
| 66–80% 81–95% >96% | 7/94 (7.4%) 21/94 (22.3%) 66/94 (70.2%) |
| Imaging Recurrence | 16/94 (17%) |
| Functional Recurrence | 5/37 (13.5%) |
| Re-operation | 15/94 (16%) |
| Cyclin-D1 | Ki-67 | CD-56 | E-Cadherin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1% | 1% | >1% | ||||||||||||
| N (%) | Statistical Significance (p) | Correlation (r) | N (%) | Statistical Significance (p) | Correlation (r) | N (%) | Statistical Significance (p) | Correlation (r) | N (%) | Statistical Significance (p) | Correlation (r) | |||
| Size (macro vs. microadenoma) | 56 (70.9%) vs. 23 (29.1%) | <0.001 | 0.56 | 27 (41.5%) vs. 15 (51.7%) | 6 (9.2%) vs. 5 (17.2%) | 32 (49.2%) vs. 9 (31%) | <0.001 | 0.69 | 50 (73.5%) vs. 18 (26.5%) | 0.5 | 0.07 | 42 (70%) vs. 18 (30%) | 0.4 | −0.1 |
| Extrasellar Invasion (Hardy I, II vs. III, IV) | 46 (58.2%) vs. 33 (41.8%) | <0.001 | 0.48 | 35 (59.3%) vs. 7 (20%) | 8(13.6%) vs. 3(8.6%) | 16 (27.1%) vs. 25 (71.4%) | <0.001 | 0.4 | 46 (67.6%) vs. 22 (32.4%) | 0.26 | −0.1 | 40 (66.7%) vs. 20 (33.3%) | 0.9 | −0.002 |
| Cavernous Sinus Invasion (Knosp 0, 1, 2 vs. 3, 4) | 61 (77.2%) vs. 18 (22.8%) | <0.001 | 0.39 | 40(53.3%) vs. 2(10.5%) | 9(12%) vs. 2(10.5%) | 26(34.7%) vs. 15(78.9%) | <0.001 | 0.37 | 54(79.4%) vs. 14(20.6%) | 0.7 | 0.03 | 49(81.7%) vs. 11(18.3%) | 0.7 | −0.03 |
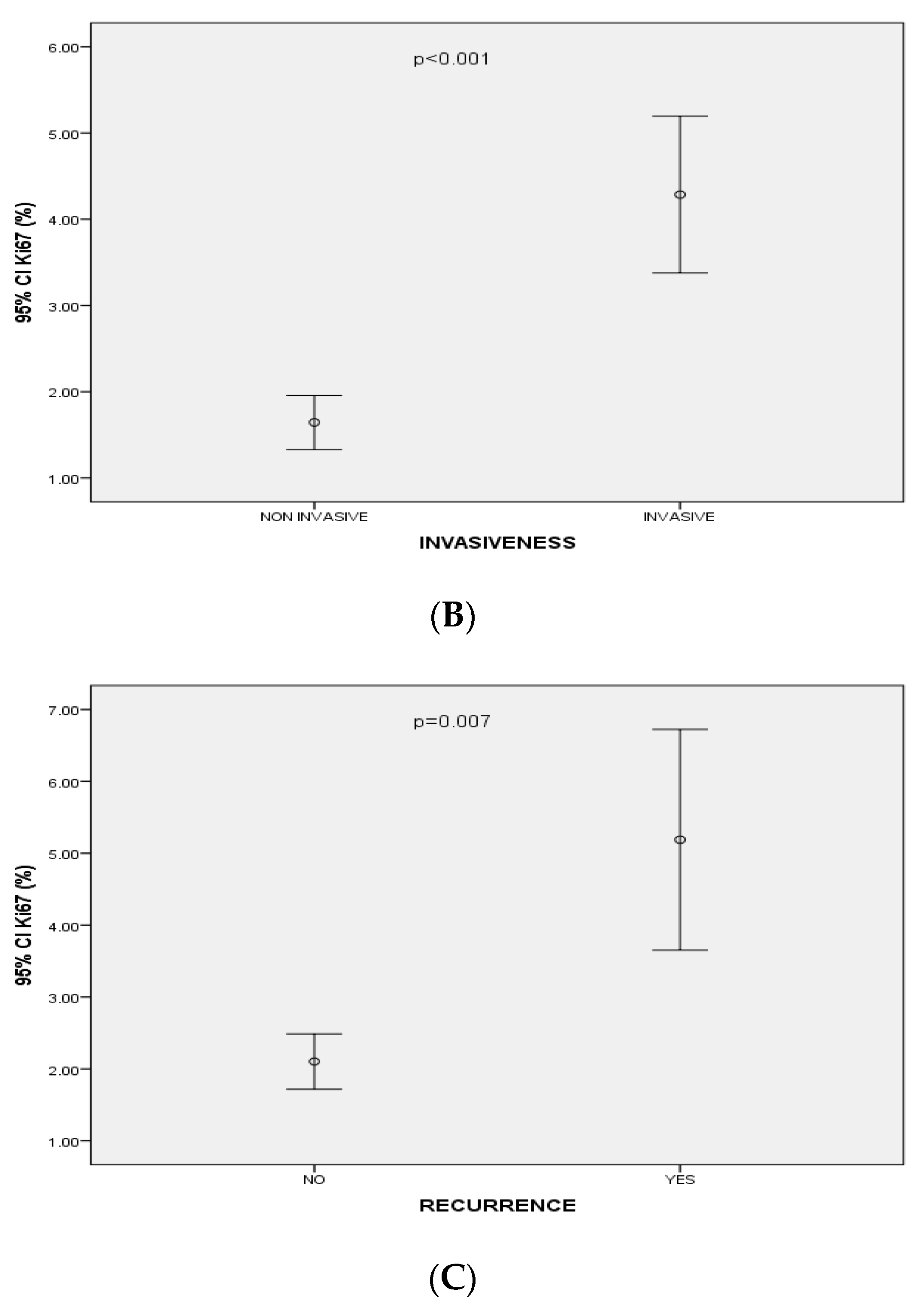
| Recurrence | 16/16 (100%) | <0.001 | 0.46 | 3 (18.8%) | 1 (6.2%) | 12 (75%) | 0.007 | 0.3 | 15/16 (93.8%) | 0.06 | 0.2 | 12/16 (75%) | 0.5 | −0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanidis, P.; Kyriakopoulos, G.; Seretis, A.M.; Korfias, S.; Theocharis, S.; Angelousi, A. Prognostic Factors for Invasiveness and Recurrence of Pituitary Adenomas: A Series of 94 Patients. Diagnostics 2022, 12, 2413. https://doi.org/10.3390/diagnostics12102413
Stefanidis P, Kyriakopoulos G, Seretis AM, Korfias S, Theocharis S, Angelousi A. Prognostic Factors for Invasiveness and Recurrence of Pituitary Adenomas: A Series of 94 Patients. Diagnostics. 2022; 12(10):2413. https://doi.org/10.3390/diagnostics12102413
Chicago/Turabian StyleStefanidis, Petros, Georgios Kyriakopoulos, Andreas Miltiadis Seretis, Stefanos Korfias, Stamatios Theocharis, and Anna Angelousi. 2022. "Prognostic Factors for Invasiveness and Recurrence of Pituitary Adenomas: A Series of 94 Patients" Diagnostics 12, no. 10: 2413. https://doi.org/10.3390/diagnostics12102413
APA StyleStefanidis, P., Kyriakopoulos, G., Seretis, A. M., Korfias, S., Theocharis, S., & Angelousi, A. (2022). Prognostic Factors for Invasiveness and Recurrence of Pituitary Adenomas: A Series of 94 Patients. Diagnostics, 12(10), 2413. https://doi.org/10.3390/diagnostics12102413







